Abstract
The correct positioning of postmitotic neurons in the developing neocortex and other laminated brain structures requires the activation of a Reelin-lipoprotein receptor-Dab1 signaling cascade. The large glycoprotein Reelin is secreted by Cajal-Retzius pioneer neurons and bound by the apolipoprotein E receptor family members Apoer2 and Vldl receptor on responsive neurons and radial glia. This leads to the tyrosine phosphorylation of the cytoplasmic protein Disabled-1 (Dab1) by non-receptor tyrosine kinases of the Src family. Various signaling pathways downstream of Dab1 connect Reelin to the actin and microtubule cytoskeleton. Despite this knowledge, a comprehensive view linking the different cell-biological and biochemical actions of Reelin to its diverse physiological roles not only during neurodevelopment but also in the maintenance and functioning of the adult brain is still lacking. In this review, we discuss our finding that Reelin activates Rho GTPases in neurons in the light of other recent studies, which demonstrate a role of Reelin in Golgi organization, and suggest additional roles of Cdc42 activation by Reelin in radial glial cells of the developing cortex.
Introduction
Reelin, a conserved extracellular glycoprotein, controls the migration and laminar arrangement of neurons during the development of the neocortex, hippocampus, cerebellum and spinal cord. When newborn postmitotic neurons have reached their final position, Reelin promotes the maturation of dendrites and dendritic spines. In reeler mice, which have a naturally occurring mutation that makes them Reelin-deficient, cortical neurons migrate abnormally, resulting in an inversion of the cortical laminar organization, with later-born neurons remaining in the deeper layers of the cortex (reviewed in ref. Citation1–Citation3).
Reelin binding to the lipoprotein receptors very low-density lipoprotein receptor (Vldlr) and the apolipoprotein E receptor 2 (Apoer2) induces the Src family kinase (SFK)-mediated tyrosine phosphorylation of the adaptor protein Disabled-1 (Dab1).Citation4–Citation7 Hence, disruption of Dab1, absence of both lipoprotein receptors Apoer2 and Vldlr or simultaneous inactivation of the Src family kinases (SFKs) Src and Fyn leads to a reeler-like phenotype.Citation8–Citation11 The phosphorylation of Dab1 activates, besides other signaling pathways, class I phosphatidylinositol-3-kinase (PI3K).Citation12–Citation14 However, despite growing insights into the signaling cascades activated by Reelin in responsive neurons, the cellular mechanisms of its action on neuronal positioning and development are still poorly understood.Citation15
Rho GTPases as Phosphatidylinositol-3-Kinase Effectors during Neurodevelopment
The large number of PI3K effectors creates a complex signaling web downstream of PI3K activation.Citation16 Among the key players linking PI3K activity to specific cellular responsesCitation17 are small GTPases of the Rho family, which regulate the cytoskeletal and membrane rearrangements required for cell movement.Citation18,Citation19 Small GTPases act as molecular switches, cycling between an active, GTP-bound and an inactive, GDP-bound form.Citation20,Citation21 The members of the Rho-family Rac, RhoA and Cdc42 are essential regulators of many cellular processes during neural development.Citation22,Citation23
Rho GTPases Link Reelin Signaling to the Neuronal Cytoskeleton
In order to investigate cellular effects of Reelin, we employed time-lapse microsopy of cultured primary neurons and found an increased motility of distal neurites.Citation24 Using this cellular effect as a readout we identified the Rho GTPase Cdc42 as a novel cellular effector of Reelin signaling. Our results indicate that Reelin and Rho GTPase-mediated signaling cascades interact during neuromorphogenesis to increase filopodia formation and growth cone motility (). The activation of Cdc42 by Reelin requires signaling through Apoer2, Dab1 and PI3K (). In addition, Reelin-induced localized Rac1 activation might participate in Reelin's effects on growth cone motility. Furthermore, Reelin increases neuronal transport vesicle formation and redirects neuronal vesicle transport into small neurites, the prospective dendrites (). Cdc42, but not RhoA or Rac1, localizes in part to the Golgi apparatus, together with its targets N-WASP, IQGAP and the Golgi vesicle coat protein and coatomer (reviewed in ref. Citation26 and Citation27). Cdc42- and N-WASP-controlled actin polymerization regulates Golgi-to-ER transportCitation28 and thus is a central event of the multiple steps of vesicle trafficking. The differential subcellular localization and activity of Cdc42 and the Par complex are critical for axon specification.Citation29,Citation30 By activating Cdc42 via Apoer2 at the tips of all neurites in stage-II neurons, Reelin seems to interfere with axondendrite differentiation.Citation24
Reelin, Neuronal Polarization and Golgi Organization
Of note, a recent study demonstrated that Reelin-Dab1 signaling antagonizes the action of the protein kinase LKB1 on neuronal polarization.Citation31 LKB1, whose effect on cell polarization is controlled by its interaction with the pseudokinase STRAD,Citation32 was shown to form a complex involving the serine/threonine kinase Stk25 and the Golgi matrix protein GM130, which regulates Golgi morphology and axon specification in neurons. Interestingly, the kinase activity of Stk25 was dispensable for mediating these effects.Citation31 Thus, Reelin seems to have prominent roles in the regulation of the Golgi apparatus and the direction of neuronal vesicle transport, thereby participating in the axon determination of maturing neurons. Importantly, it has been shown that the Golgi protein GM130 can also form a complex that includes Cdc42.Citation33 Future studies will have to address how Cdc42 activation by Reelin relates to the antagonizing effect of Reelin on LKB1-STRAD-Stk25-GM130 signaling.
Reelin and Neuropeptidergic Signaling Converge on the Level of Rho GTPases
In the adult brain, Reelin is mainly localized to GABA-containing peptidergic interneurons.Citation34 The functional relevance of this colocalization, which implies a cosecretion under physiological or pathophysiological conditions, is largely unknown. We could demonstrate that Reelin functionally interacts with the peptidergic VIP/PACAP38-receptor system to increase axonal branch formation.Citation24 As VIP-mediated Rho-kinase inhibitionCitation35,Citation36 induces the elongation of dendrites and axons by stabilizing microtubules,Citation37 these results demonstrate that Reelin and a neuropeptidergic system can cooperate to promote neuronal development by inducing axonal branching. In addition, these findings pinpoint the influence of the tubulin cytoskeleton in mediating Reelin's effect on neuromorphogenesis, which is also targeted by other effectors of the Reelin-Dab1 signaling cascade, including the Tau kinase GSK3beta,Citation12 Lis1,Citation38 and Map1b.Citation39
Putative Functions of Cdc42 in Reelin-Dependent Spine Morphogenesis and Synapse Formation
In addition to its prominent role in controlling neuronal positioning in developing brain strucutres, Reelin is also involved in the development of dendritesCitation40,Citation41 and promotes spine morphogenesis and synapse formation.Citation42,Citation43 Given the known function of Rho GTPases in the growth and remodeling of dendrites and synaptogenesis (reviewed in ref. Citation22, Citation44 and Citation45), which require extensive remodeling of the neuronal cytoskeleton, it is tempting to speculate that Cdc42 cooperates with other Reelin effector molecules such as the adapter proteins Crk und CrkL,Citation46 Akt and mTor signalingCitation14 to regulate dendrite morphogenesis. An additional level of complexity is added to this signaling network by the interaction of Reelin and Notch signaling,Citation47 which cooperates with Rho GTPases in the specification of dendrite morphology (reviewed in ref. Citation48). Defects in dendrite and spine morphology and a reduction in synapse number are observed in many neuropsychiatric diseases,Citation49 and both have been connected to alterations in Reelin signaling as well as to defects in Rho GTPase function (reviewed in ref. Citation22 and Citation50). We propose that the activation of Cdc42 by ReelinCitation24 links the observation that defects in Rho GTPase function and Reelin signaling both contribute to morphological defects leading to neurological and psychiatric disorders.
Role of Cdc42 Activation in Radial glia
A recent report demonstrated that Cdc42 is required for maintaining the polarized morphology of the cortical radial glial scaffold.Citation51 Live imaging of radial glial cells in embryonic cortical slice cultures revealed dynamic inter-radial glial interactions involving transient filopodia-like radial glial protrusions and highly motile radial glial leading edges resembling neuronal growth cones. By in utero electroporation of a dominant-negative Cdc42-GFP construct under control of a radial glial-specific Blbp promoter it was demonstrated that polarized Cdc42 activation was necessary for maintaining radial glial morphology and dynamics. Crossing mice carrying floxed Cdc42 alleles with hGFAP-Cre transgenic mice expressing Cre recombinase under control of the human GFAP promoter resulted in the conditional ablation of Cdc42 in radial progenitor cells and led to cortical layering defects and neuronal ectopias. Defects in axonogenesis were also observed.Citation51 This phenotype resembles but is not identical to that of mice lacking essential components of the Reelin signaling cascade, probably because Cdc42 integrates the input of additional ligand-receptor signaling systems. Moreover, it should be noted that the use of radial glial-specific promoters will not prevent neuronal Cdc42 inactivation, since the vast majority of neurons is derived from radial glial progenitors.Citation52 To clearly distinguish the separate effects of radial glial vs. neuronal Cdc42 ablation and their relative contributions to the observed lamination defect, the comparison with mice lacking Cdc42 specifically in neurons, e.g., by using a Dcx-Cre transgenic mouse line, might be helpful. However, in light of the data generated by Yokota and colleagues, it seems likely that at least some of the effects of Cdc42 on radial glial morphology and dynamics are modulated by Reelin, whose receptors and intracellular effectors have been described to be expressed in radial glial cells of cortical structures.Citation53,Citation54
Perspective
At first sight it might seem surprising that so many different cellular effects of Reelin are modulated by a single Rho GTPase. However, one has to bear in mind that Rho-GTPases are embedded in different effector-domain complexes depending on the contextual situation. Selectivity can be achieved by engaging different guanine nucleotide-exchange factors (GEFs). GEFs are responsible for the activation of Rho-family GTPases in response to various extracellular stimuli, and regulate diverse downstream cellular responses in neurons such as migration, morphogenesis and axonal pathfinding.Citation55 Dbl-related GEFs represent the largest family of direct activators of Rho GTPases in humans. In addition, atypical Rho-GEFs that contain Dock homology regions (DHR-1 and DHR-2) are expressed in a variety of tissues, including the nervous system and achieve spatial and temporal restriction of Rho GTPase-signaling.Citation56 It remains to be determined which of these GEFs are activated by Reelin and lipoprotein receptor-mediated signaling and how they preferentially couple to different downstream effector pathways, thereby mediating Reelin's different effects on the actin and microtubule cytoskeleton via Rho GTPases. The fine tuning and signal integration of these partially convergent or divergent pathways downstream of the Rho GTPase effectors is fundamental for correct neuronal development.Citation57
Abbreviations
| Akt | = | v-AKT murine thymoma viral oncogene homolog |
| Apoer2 | = | apolipoprotein E receptor 2 |
| Blbp | = | brain lipid-binding protein |
| Cdc42 | = | cell division cycle 42 |
| Crk | = | v-CRK avian sarcoma virus CT10 oncogene homolog |
| CrkL | = | Crk-like |
| Dab1 | = | disabled-1 |
| Dcx | = | doublecortin |
| DHR | = | dock homology region |
| ER | = | endoplasmic reticulum |
| GABA | = | gamma-aminobutyric acid |
| GEF | = | guanine nucleotide-exchange factor |
| GFP | = | green fluorescent protein |
| GM130 | = | golgi matrix protein of 130 kDa |
| GSK3 | = | glycogen synthase kinase 3 |
| GTP | = | guanosine triphosphate |
| hGFAP | = | human glial fibrillary acidic protein |
| IQGAP | = | IQ motif-containing GTPase-activating protein |
| LIMK | = | LIM domain kinase |
| Lis1 | = | lissencephaly 1 |
| LKB1 | = | liver kinase B1 |
| Map1b | = | microtubule-associated protein 1b |
| n-WASP | = | neuronal wiskott-aldrich syndrome protein |
| PACAP | = | pituitary adenylate cyclase-activating polypeptide |
| PAK | = | p21-activated kinase |
| PI3K | = | phosphatidylinositol-3-kinase |
| Rac | = | ras-related C3 botulinum toxin substrate |
| RhoA | = | Ras homolog gene family member A |
| SFK | = | Src family kinases |
| Stk25 | = | serine/threonine protein kinase 25 |
| STRAD | = | STE20-related adaptor protein |
| VIP | = | vasoactive intestinal peptide |
| Vldlr | = | very low-density lipoprotein receptor |
| WAVE | = | WASP family verprolin-homologous protein |
Figures and Tables
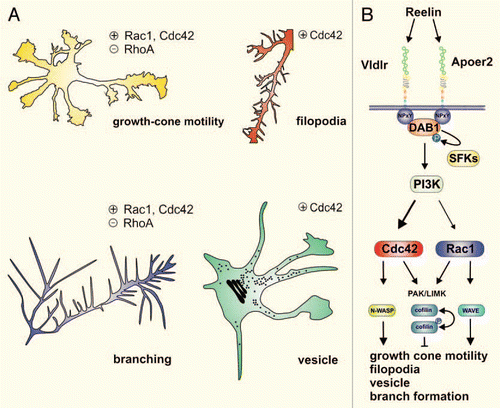
Figure 1 Reelin influences neuromorphogenesis by activating Rho GTPases. (A) Growth cone motility and neurite branch formation are activated (+) by Rac1 and Cdc42 and negatively regulated (−) by RhoA. Reelin participates in the regulation of growth cone motility and branching by regulating Rho GTPase activity (see B). Filopodia formation and the formation of neuronal transport vesicles, both known to be mediated by Cdc42, are triggered by Reelin.Citation24 (B) Binding of the extracellular matrix protein Reelin to its transmembrane receptors Apoer2 and Vldlr triggers Dab1 tyrosine phosphorylation by Src family kinases (SFK). This leads to the activation of several downstream signals, including phosphatidylinositol-3-kinase (PI3K), which activates Cdc42 via an unknown intermediate effector. There is evidence that Reelin also might locally activate Rac1. N-WASP and WAVE link Cdc42 and Rac1 activity to changes of the actin cytoskeleton, leading to increased growth cone motility, filopodia and vesicle formation and dendritic branching (see A). Cdc42 and Rac1 also contribute to activation of the PAK/LIMK pathway, which inhibits actin filament disassembly by phosphorylating and thereby inhibiting the actin-depolymerizing factor ADF/cofilinCitation25 leading to growth inhibition or stabilization of filopodia. The fine tuning of these context-dependent convergent or divergent pathways downstream of Rho GTPases is fundamental for correct neuronal development.Citation24
Acknowledgements
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG) and the Forschungskommission der Medizinischen Fakultät Freiburg. We wish to thank Michael Frotscher for continuous support and Lutz Hein for critical reading of the manuscript.
References
- Frotscher M. Role for Reelin in stabilizing cortical architecture. Trends Neurosci 2010; 33:407 - 414
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 2001; 24:1005 - 1039
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci 2003; 4:496 - 505
- Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol 2003; 13:9 - 17
- Bock HH, Herz J. Reelin activates Src family tyrosine kinases in neurons. Curr Biol 2003; 13:18 - 26
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron 1999; 24:471 - 479
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 1999; 24:481 - 489
- Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J 1997; 16:121 - 132
- Kuo G, Arnaud L, Kronstad-O'Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci 2005; 25:8578 - 8586
- Sheldon M, Rice DS, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 1997; 389:730 - 733
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 1999; 97:689 - 701
- Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem 2002; 277:49958 - 49964
- Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, et al. Phosphatidylinositol-3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem 2003; 278:38772 - 38779
- Jossin Y, Goffinet AM. Reelin Signals through Phosphatidylinositol-3-kinase and Akt To Control Cortical Development and through mTor To Regulate Dendritic Growth. Mol Cell Biol 2007; 27:7113 - 7124
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci 2008; 31:113 - 119
- Cantrell DA. Phosphoinositide-3-kinase signalling pathways. J Cell Sci 2001; 114:1439 - 1445
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 2006; 34:647 - 662
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 2010; 11:633 - 643
- Wymann MP, Marone R. Phosphoinositide-3-kinase in disease: timing, location and scaffolding. Curr Opin Cell Biol 2005; 17:141 - 149
- Hakoshima T, Shimizu T, Maesaki R. Structural basis of the Rho GTPase signaling. J Biochem 2003; 134:327 - 331
- Mackay DJ, Hall A. Rho GTPases. J Biol Chem 1998; 273:20685 - 20688
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev 2005; 19:1 - 49
- Kawauchi T, Hoshino M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. Dev Neurosci 2008; 30:36 - 46
- Leemhuis J, Bouche E, Frotscher M, Henle F, Hein L, Herz J, et al. Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation. J Neurosci 2010; 30:14759 - 14772
- Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci 2009; 29:288 - 299
- Erickson JW, Cerione RA. Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol 2001; 13:153 - 157
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 2006; 16:522 - 529
- Egea G, Lazaro-Dieguez F, Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol 2006; 18:168 - 178
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, et al. Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci 2007; 27:13117 - 13129
- Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 2004; 7:923 - 929
- Matsuki T, Matthews RT, Cooper JA, van der Brug MP, Cookson MR, Hardy JA, et al. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell 2010; 143:826 - 836
- Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science 2009; 326:1707 - 1711
- Kodani A, Kristensen I, Huang L, Sutterlin C. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell 2009; 20:1192 - 1200
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, et al. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA 1998; 95:3221 - 3226
- Henle F, Fischer C, Meyer DK, Leemhuis J. Vasoactive intestinal peptide and PACAP38 control N-methyl-D-aspartic acid-induced dendrite motility by modifying the activities of Rho GTPases and phosphatidylinositol-3-kinases. J Biol Chem 2006; 281:24955 - 24969
- Meyer DK, Fischer C, Becker U, Gottsching I, Boutillier S, Baermann C, et al. Pituitary adenylyl cyclase-activating polypeptide 38 reduces astroglial proliferation by inhibiting the GTPase RhoA. J Biol Chem 2005; 280:25258 - 25266
- Leemhuis J, Henle F, Meyer DK. VIP induces the elongation of dendrites and axons in cultured hippocampal neurons: role of microtubules. Peptides 2007; 28:1700 - 1705
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, et al. Interaction of reelin signaling and Lis1 in brain development. Nat Genet 2003; 35:270 - 276
- Gonzalez-Billault C, Del Rio JA, Urena JM, Jimenez-Mateos EM, Barallobre MJ, Pascual M, et al. A role of MAP1B in reelin-dependent neuronal migration. Cereb Cortex 2005; 15:1134 - 1145
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 2004; 41:71 - 84
- Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci 2006; 26:1767 - 1775
- Niu S, Yabut O, D'Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci 2008; 28:10339 - 10348
- Borrell V, Del Rio JA, Alcantara S, Derer M, Martinez A, D'Arcangelo G, et al. Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J Neurosci 1999; 19:1345 - 1358
- Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist 2005; 11:187 - 191
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 2006; 16:95 - 101
- Matsuki T, Pramatarova A, Howell BW. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci 2008; 121:1869 - 1875
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, Radtke F, et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 2008; 60:273 - 284
- Redmond L, Ghosh A. The role of Notch and Rho GTPase signaling in the control of dendritic development. Curr Opin Neurobiol 2001; 11:111 - 117
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 2000; 10:981 - 991
- Fatemi SH. Reelin glycoprotein: structure, biology and roles in health and disease. Mol Psychiatry 2005; 10:251 - 257
- Yokota Y, Eom TY, Stanco A, Kim WY, Rao S, Snider WD, et al. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development 2010; 137:4101 - 4110
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 2004; 41:881 - 890
- Hartfuss E, Forster E, Bock HH, Hack MA, Leprince P, Luque JM, et al. Reelin signaling directly affects radial glia morphology and biochemical maturation. Development 2003; 130:4597 - 4609
- Förster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, et al. Reelin, Disabled 1 and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci USA 2002; 99:13178 - 13183
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167 - 180
- Cote JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol 2007; 17:383 - 393
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 2004; 44:779 - 793