Abstract
The localization of plasma membrane calcium ATPase (PMCA) isoforms in specified membrane compartments is crucial for their function in local Ca2+ handling. PMCA2w/b is present in the apical membrane whereas alternative splice variants PMCA2x/b and 2z/b reside in the basolateral membrane in polarized epithelial cells. Here we found that the apical scaffolding protein NHERF2 greatly enhances the apical concentration of PMCA2w/b by tethering the pump to the underlying actin cytoskeleton. The interaction requires the C-terminal PDZ binding sequence in PMCA2b and results in increased membrane retention and decreased lateral mobility of the pump. In contrast, PMCA2x/b remains exclusively basolateral even when NHERF2 is overexpressed. Our results suggest that the alternatively spliced intracellular loop in PMCA2 imposes dominant membrane targeting information. NHERF2-mediated recruitment may be an effective means for polarized cells to regulate the abundance of PMCA2w/b in the apical membrane to meet an increased demand for local Ca2+ extrusion.
Plasma membrane Ca2+-ATPases (PMCAs) are the major high-affinity Ca2+ efflux system of eukaryotic cells. They are required not only for setting basal intracellular [Ca2+]i levels but are also intricately involved in localized Ca2+ signaling and vectorial Ca2+ transport, e.g., in the kidney, intestine and mammary gland.Citation1 The need for specialized Ca2+ handling in different cells and tissues is reflected by the fact that mammals express over 20 distinct PMCA isoforms from four separate genes and via alternative RNA splicing. These PMCAs differ in their localization, regulation and kinetics of response to an incoming Ca2+ signal. The proper targeting of specific PMCAs is essential for physiological function, as illustrated in the inner ear where the absence of PMCA2 in the apical stereocilia of cochlear hair cells results in hearing loss even when PMCA1 is still normally expressed in the lateral membrane of these cells.Citation2–Citation4 Recent work has shown that alternative splicing affects the targeting of PMCA2 to different membrane compartments: splice variants differing in the size of their first intracellular loop are differentially targeted to the apical and basolateral membrane of cochlear hair cells or polarized kidney epithelial cells.Citation5 In human PMCA2, this splice (at a site called splice site A) results in variants 2w, 2x and 2z containing 45, 14 and 0 additional amino acid residues, respectively, in the first intracellular loop (). The w-variant goes to the apical membrane whereas the x- and z-variants are mainly found in the basolateral membrane.Citation6 All PMCAs are also affected by alternative splicing at site C, which results in major variants “a” and “b” differing in their C-terminal tails (). All b-variants carry at their C-terminus a PDZ domain interacting motif, which enables them to bind to a variety of scaffolding and signaling proteins such as PSD95, CASK and Na+/H+ regulatory factor 2 (NHERF2).Citation1 NHERF2 is a scaffolding protein that recruits membrane proteins to the apical membrane by tethering them to the apical cytoskeleton via its ezrin/radixin/moesin (ERM) domain.Citation7,Citation8 Earlier studies showed that PMCA2b interacts specifically with NHERF2 via its C-terminal ETSL sequence.Citation9 However, the relative role of targeting information in the first intracellular loop versus that in the C-tail of the PMCA has not yet been investigated.
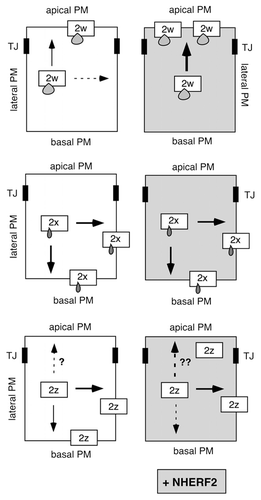
In our recent study, we found that co-expression of GFP-tagged PMCA2w/b with NHERF2 in polarized MDCK (Madin Darby canine kidney) cells resulted in a remarkable (two-fold) increase in the ratio of apical to basolateral localization of the PMCA, as determined by quantitative confocal microscopy. This enhanced apical localization was not observed with a PMCA2w/b mutant lacking the six C-terminal PDZ binding residues (GFP-PMCA2w/bΔ6), showing the dependence of NHERF2-mediated recruitment of the pump on an intact PDZ-domain interaction. Interestingly, although it can also interact with NHERF2, GFP-PMCA2x/b always remained basolateral, even when the two proteins were co-expressed. GFP-PMCA2z/b displayed variable localization: when expressed alone, it was mostly basolateral, but upon co-expression with NHERF2 a substantial fraction of the pump was recruited to the apical membrane. We interpreted this to indicate that PMCA2z/b is relatively flexible in its membrane localization and may be “pulled” to the apical membrane by NHERF2. In contrast, the short 14-residue insert in the first loop of splice variant PMCA2x/b results in retention of the pump in the basolateral membrane such that it cannot be re-directed to the apical domain even in the presence of abundant NHERF2 ().
Our original interpretation of the results with PMCA2z/b may need to be revised, however. We recently discovered that the GFP-PMCA2z/b used in our study carries a single point mutation that results in a change of a leucine to a proline residue (Leu-258>Pro). Although this mutant is expressed as full-length protein (as demonstrated on western blots), it is possible that the altered residue affects the membrane targeting and retention of this pump. Leu-258 is located in the first cytosolic loop about 40 residues upstream of the splice insert, and its non-conservative change to a proline may impact the structure of this region. We also note that Leu-258 is highly conserved among all PMCAs and may thus play a critical structural and/or functional role. Whether the wild-type PMCA2z/b still shows targeting “flexibility” and can be recruited to the apical membrane by NHERF2 thus remains to be re-evaluated (). Regardless, however, the finding that PMCA2x/b is firmly retained in the basolateral membrane whereas PMCA2w/b can be further enriched in the apical membrane by NHERF2 suggests that the alternatively spliced first intracellular loop of the pump contains dominant targeting information.
How could this work mechanistically? Results reported by Grati et al.Citation5 suggest that the size of the insert at splice site A makes an important contribution to the outcome of the targeting. When the 45-residue w-insert was shortened by more than 14 residues, PMCA2 was found in both the apical and basolateral membrane in transfected rat hair cells. When the insert was longer than 30 residues (shortened by less than 14 residues), the pump was almost exclusively present in the apical membrane. Even a scrambled amino acid sequence unrelated to the original PMCA2 w-insert was effective in sending the pump to the apical membrane as long as the overall size was >30 residues. This makes it unlikely that the w-insert engages in specific protein interactions (e.g., with an apical sorting/retention protein) to “drive” the protein to the apical membrane. Rather, the bulkiness of the insert may play a general structural role, e.g., by masking a basolateral sorting/retention signal. However, the details of how this works at the molecular level remain to be determined.
The above findings are based on work using PMCA2w/a, which is the major splice form in the apical membrane of hair cells.Citation5 The “a” splice variant lacks the PDZ-binding motif () and other less characterized sequences at the C-terminus. Although PMCA2w/a is highly enriched in the stereocilia, fluorescence recovery after photobleaching (FRAP) experiments show that about 60% of the pump molecules diffuse freely on the cell surface.Citation10 These results suggest that a fraction of the PMCA2w/a molecules may interact with components other than PDZ scaffolds and the rest of the PMCA molecules remain highly mobile in this specialized compartment of hair cells.
Our recent report showed that one of the effects of NHERF2 on the apical PMCA2w/b is to reduce its lateral membrane mobility and to decrease recycling. By FRAP, we found that co-expression of NHERF2 reduced the mobile fraction of the PMCA from almost 90% to less than 50%. As expected, the C-terminally truncated GFP-PMCA2w/bΔ6 unable to interact with NHERF2 showed unaltered (high) mobility in the membrane. In agreement with NHERF2-mediated immobilization of the pump (by anchoring it to the underlying apical membrane cytoskeleton), endocytosis of PMCA2w/b was significantly reduced in the presence of NHERF2 (as determined by reversible surface biotinylation). Interestingly the rate of endocytosis of the truncated PMCA2w/bΔ6 was much higher than that of the full-length PMCA2w/b even in the absence of co-expressed NHERF2. This suggests that PDZ domain-mediated interactions with endogenous NHERF(s) or other scaffolding proteins contribute to the increased stability of PMCA2w/b in the membrane. NHERF2-mediated recruitment of PMCA2w/b may provide an effective means for polarized cells to regulate the abundance of this pump in the apical membrane to meet an increased demand for Ca2+ extrusion. This is relevant, e.g., in the lactating mammary gland where apical PMCA2 is required to provide milk calcium.Citation11
Figures and Tables
Figure 1 Scheme of PMCA2 and its major alternative splice variants. The membrane-spanning domains are numbered 1–10. Alternative splicing at site A results in the insertion of 45 (w), 14 (x) or 0 (z) amino acid (aa) residues in the first cytosolic loop. Splicing at site C generates variants a and b, which differ in their C-terminal tails. Only the b-variant contains a C-terminal PDZ domain-binding sequence (PDZ) that can interact with the apical scaffolding protein NHER F2. N, N-terminus; C, C-terminus.
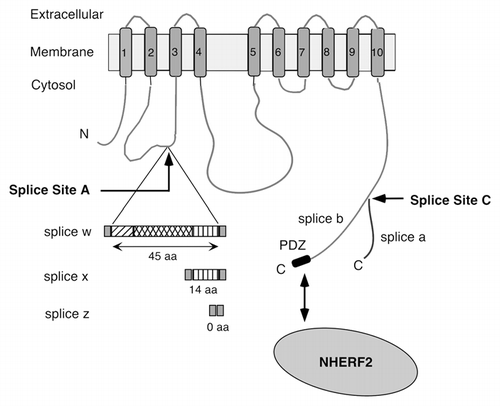
Figure 2 Scheme illustrating the effect of NHERF2 on the distribution of PMCA2b splice site A variants in epithelial cells. Arrows indicate the direction of targeting of PMCA2w/b (top), PMCA2x/b (middle) and PMCA2z/b (bottom) in polarized MDCK cells, in the absence (left parts) and presence (right parts) of co-expressed NHERF2. A stippled arrow indicates a minor fraction of the PMCA is distributed in that direction. The three splice site A variants differ only in the size of the insert in their first intracellular loop, as illustrated schematically by small “drops” on the boxes representing the 2w, 2x and 2z variants. Note that upon NHER F2 expression, PMCA2w/b is greatly enhanced in the apical membrane (as indicated by the thick black arrow), whereas PMCA2x/b is unaffected and remains basal and lateral. The effect of NHER F2 on the distribution of PMCA2z/b is not yet clear, as indicated by the broken arrows with question marks. TJ, tight junction.
Acknowledgements
This work was supported by grants from the NIH (NS51769) and OTKA (CK 80283, ETT 215/2009).
Addendum to:
References
- Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi Á. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann NY Acad Sci 2007; 1099:226 - 236
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, et al. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 1998; 273:18693 - 18696
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nature Genet 1998; 19:390 - 394
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+ ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 2001; 21:5066 - 5078
- Grati M, Aggarwal N, Strehler EE, Wenthold RJ. Molecular determinants for differential membrane trafficking of PMCA1 and PMCA2 in mammalian hair cells. J Cell Sci 2006; 119:2995 - 3007
- Chicka MC, Strehler EE. Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 2003; 278:18464 - 18470
- Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. Regulation of ion transport by the NHERF family of PDZ proteins. Physiol 2004; 19:362 - 369
- Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, et al. NHERF family and NHE3 regulation. J Physiol 2005; 567:3 - 11
- DeMarco SJ, Chicka MC, Strehler EE. Plasma membrane Ca2+-ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J Biol Chem 2002; 277:10506 - 10511
- Grati M, Schneider ME, Lipkow K, Strehler EE, Wenthold RJ, Kachar B. Rapid turnover of stereocilia membrane proteins: evidence from the trafficking and mobility of plasma membrane Ca2+-ATPase 2. J Neurosci 2006; 26:6386 - 6395
- Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem 2004; 279:42369 - 42373