Abstract
Animals must be capable of sensing hazardous temperatures to avoid physical injury. Recent progress has revealed the molecular mechanisms underlying this capability. This essential function requires a subset of the Transient Receptor Potential (TRP) channel family in both mammals and Drosophila. We recently showed that a Drosophila TRP channel, dubbed Painless, possesses heat sensitivity that is essential for avoidance of noxious heat. The temperature threshold for Painless activation is consistent with the temperatures that cause avoidance behaviors in vivo, indicating that Painless acts as a primary noxious heat detector in Drosophila. In this review, we summarize the properties of temperature-sensitive TRP channels, including Painless, in fruit flies.
Temperature-Sensitive TRP Channels are Physiological Thermosensors
The ability to sense environmental temperature is necessary to discriminate between permissive habitats and hazardous environments that cause injury. Recently, it has been revealed that several members of the TRP ion channel super family are activated by temperature changes. The temperature sensitivity of molecules is usually evaluated with their Q10 values, which are defined as ratios of reactions at 10-degree increments. Many enzymes and channels have Q10 values less than 3,Citation1 whereas the thermosensitive TRP channels (thermoTRPs) have extremely high values greater than 10. A number of reports clearly demonstrate that thermal activation of these TRP channels contributes to various temperature-dependent responses in vivo, such as thermosensation, thermotaxis and regulation of cellular/tissue functions at physiological body temperature. So far, nine TRP channels have been reported to respond to a physiological range of temperatures in mammals.Citation2 TRPV1 and TRPV2 expressed in nociceptive neurons are activated by heat (>43°C and >52°C, respectively), and TRPV1-null mice have defects in sensing noxious heat.Citation3 TRPV3 and TRPV4 are predominantly expressed in skin keratinocytes rather than in sensory neurons, and gene knock-out of each channel causes abnormal thermotaxis in vivo.Citation4,Citation5 TRPM8, which senses cold temperatures (<27°C),Citation6,Citation7 is expressed in nociceptive and non-nociceptive neurons and its loss impairs cold sensitivity.Citation8–Citation12 TRPA1 is expressed in nociceptive neuronsCitation13 and acts as a sensor for various harmful stimuli, whereas its responsiveness to noxious cold stimuli is controversial even after the analysis of mice lacking the channel.Citation14–Citation17 Other thermoTRPs, TRPM2, TRPM4 and TRPM5 are not expressed in sensory neurons, and are reportedly involved in several functions at physiological body temperatures including insulin secretion, taste sensation and immune response.Citation18–Citation20 Thus, the thermoTRPs are now considered to be primary elements for temperature-dependent responses.
TRP Channel-Dependent Thermosensation in Fruit Flies
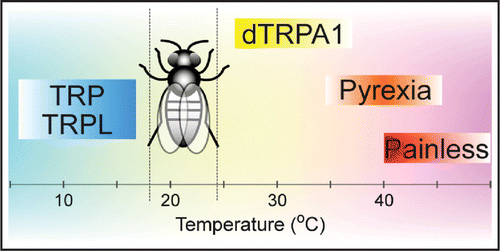
In fruit flies, some TRP channels are important for temperature detection like in mammals. In Drosophila melanogaster, thirteen genes encode TRP channels. Five of those channels belong to the TRPA and the TRPC subfamilies, which contribute to temperature-related behaviors (). dTRPA1 was first identified as a heat-activated channel (>27°C) in vitro,Citation21 and its knockdown by RNAiCitation22 or geneknockoutCitation23 revealed the importance of the channel for thermotaxis from warm temperature to a preferred range (18–24°C). dTRPA1 proteins are expressed in anterior cell neurons, two pairs of neurons at the brain's anterior.Citation24 Pyrexia is a noxious heat-sensitive TRPA channel with high potassium permeability, and it may protect flies from high temperature stress.Citation25 Pyrexia-expressing sensory neurons are widely distributed throughout the body. Another TRPA subtype, dubbed Painless, was found to be essential for avoidance of noxious heat above 40°C in Drosophila larvae and adults.Citation26,Citation27 The protein was found at the tips of the dendritic arbor in a small subset of multidendritic sensory neurons which resemble our peripheral afferent neurons. More recently, TRP and TRPL (belonging to the TRPC subfamily), which are critical for vision in Drosophila, were reported to be involved in cold avoidance.Citation28 This evidence strongly demonstrates that certain TRP channels share a common role in temperature sensation between distantly related species. Interestingly, in flies, four channels are classified in the TRPA subfamily and three of them are known to contribute to thermosensation. Therefore, the TRPA subfamily may play a central role in temperature detection and related behaviors in insects.
Noxious Heat Sensitivity of Painless
Drosophila melanogaster prefer temperatures in the range of 18–24°C. They quickly escape from noxious temperatures above 40°C. Wild-type larvae display stereotypical ‘rolling behavior’, which is defined as a vigorous rolling movement lateral to the body axis, within one second of being touched with a heated probe.Citation26 Genetic screening to obtain mutants that have abnormal response against noxious heat identified painless mutants. These mutant larvae required much longer times to escape from a heated probe, indicating that the painless gene is essential for heat avoidance. The mRNA and the protein appeared to be localized in the tips of multidendritic sensory neurons of the peripheral nervous system, which resemble peripheral sensory neurons in mammals. The firing of these neurons increased upon heating over 38°C in wild type neurons, whereas such increases were not seen in the painless mutant. The Painless protein is predicted to have ankyrin repeats in the N-terminal region, and six transmembrane domains with one putative pore-forming region between transmembrane domains 5 and 6, all of which are common structures for the TRP ion channel super family. Since two Drosophila TRPA channels, dTRPA1 and Pyrexia, have been shown to have temperature sensitivity both in vitro and in vivo, it has been inferred that Painless might also be a heat-sensitive TRP channel. Indeed, we first revealed that the channel actually functions as a thermosensor.Citation29 Painless expressed in human embryonic kidney (HEK) 293 cells showed transient inward currents upon heating at negative membrane potentials, and the temperature thresholds for activation (42–44°C) were consistent with the temperatures that cause avoidance behavior in vivo.Citation26 Painless displayed heat responses in a cell-free excised membrane patch, indicating that Painless may sense heat directly, without utilizing intracellular signaling molecules. These in vitro analyses clearly demonstrate that in Drosophila, Painless itself is a primary heat detector, the activation of which raises neural activity against dangerous heat.
Functional Regulation of Painless by Ca2+
A unique property of Painless is the extremely high and selective permeability of Ca2+, which is almost 42 times higher than that of sodium.Citation29 Intriguingly, the various activation properties of the channel are affected by Ca2+, all of which seem to be correlated with the flies' physiology. This interpretation is supported by the following observations. First, Ca2+ enables Painless to be heat-sensitive. Painless failed to respond to heat in the absence of intracellular and extracellular Ca2+, whereas 200 nM intracellular Ca2+ restored the heat-dependent activation. The mammalian thermoTRPs such as TRPM4, TRPM5 and TRPA1 are activated by intracellular Ca2+,Citation18,Citation30–Citation32 whereas Painless requires Ca2+ as a co-agonist for heat-evoked activation. A similar concept has been reported in TRPM8, in that intracellular Ca2+ supports robust icilin-evoked responses.Citation33 Intracellular Ca2+ (100–200 nM) was sufficient for Painless activation, a concentration close to the reported value in the terminal and dorsal organs of the larval head.Citation34 Second, Ca2+ accelerates Painless activation. Channel activation is rapid in the presence (but not in the absence) of physiological levels of intracellular Ca2+, which reflects the fly larva's rapid rolling response when challenged by a heated probe.Citation26 Third, Ca2+ sensitizes Painless to heat. The temperature threshold for activation was ∼42.6°C in the presence of intracellular Ca2+ regardless of the presence of extracellular Ca2+, which was significantly lower than that in the presence of extracellular Ca2+ alone (∼44.1°C). Quick avoidance from a heated probe occurred around 42°C in fly larvae.Citation26 Therefore, the in vivo temperature threshold seems close to that obtained in the presence of intracellular Ca2+. Fourth, Ca2+ sensitizes Painless upon repetitive heating. Significant reduction in the temperature thresholds by more than 1°C was observed in the presence of extracellular and intracellular Ca2+, but not in the presence of intracellular Ca2+ alone upon repeated heating, suggesting that extracellular Ca2+ and/or Ca2+ influx play a role in the sensitization. This is reasonable because flies are able to escape from a hazardous heat source in less time after a second exposure. Sensitization upon repetitive heating is a common feature in TRPV1, TRPV2 and TRPV3,Citation35,Citation36 although the underlying mechanism is still unknown, including the requirement for Ca2+.
Ca2+-dependent regulation is partly ascribed to the properties of the N-terminal region of Painless.Citation29 Specific amino acid substitution (N363A), which is located in the ankyrin repeat domain, displayed small heat-evoked currents, increased temperature thresholds, and higher intracellular Ca2+ requirement with a reduced Hill coefficient. These results suggest that N363 is a key residue in Ca2+ sensitivity of Painless. Interestingly, the amino acid sequence, which includes N363, has partial similarity to the EF-hand-like domain in mammalian TRPA1. The EF-hand-like motif is reported to be responsible for Ca2+-evoked activation of human TRPA1 which was impaired by an amino acid substitution.Citation31,Citation32 However, the function of a Ca2+-regulatory site in Painless seems different from that of the EF-hand-like motif. TRPA1 requires Ca2+ at a micromolar range for its activation, whereas Painless was not activated by intracellular Ca2+ alone and requires much less Ca2+ for its modulation. Furthermore, mutation of N356, S357 or D366 did not affect the properties of Painless, while the corresponding amino acids in the EF-hand-like motif are critical for Ca2+-dependent TRPA1 activation. Such Ca2+-dependency upon thermal activation is unique to Painless.
Is Painless a Polymodal Receptor?
Our investigation demonstrated that Painless did not respond to typical stimuli known to activate mammalian thermoTRPs.Citation29 This is somewhat surprising because most mammalian thermoTRPs are activated by various stimuli in addition to temperature,Citation37–Citation39 and Painless has also been reported to be involved in sensitivity to allyl isothiocyanate, a wasabi ingredient, sugar or mechanical stimuli in vivo.Citation26,Citation40,Citation41 Expression of painless was seen in a subset of sensory neurons among gustatory bristles located in the labial palpus, the legs, the wings, and the internal pharyngeal sensory structures in adults, all of which have been known to contribute to gustatory detection.Citation40 Expression of painless was also observed in mechanically-sensitive Johnston's organ and the central nervous system including the mushroom body,Citation26 where Painless could be activated by mechanical stimuli or endogenous ligands, respectively. Therefore, Painless possibly senses multiple stimuli other than heat, which might require endogenous accessory proteins, heteromeric channel formation, splice variants and/or phosphorylation. GPCR signaling might also regulate Painless function since the Gq-PLC pathway has been implicated in modulation of dTRPA1-dependent thermosensation.Citation23 These observations must be confirmed under more physiological conditions. It should be noted that heat activation of Painless was inhibited by ruthenium red and camphor,Citation29 which was reminiscent of the properties of mammalian TRPA1. This feature suggests some common mechanisms for channel activation within mammalian and Drosophila TRPA channels.
Thus far, no chemical or other physical stimuli have been available for activation of invertebrate thermoTRPs. Identification of new activators would be useful to control insects' behavior as the TRP channels primarily contribute to sensing mechanisms in invertebrates as well as in vertebrates. Camphor, a wood derivative from camphor laurel, has been used as a repellent for pests and proved to be effective for mosquitoes.Citation42 It would, therefore be intriguing to test the effects of camphor at the behavioral level, in spite of its inhibitory effect on Painless. Since activation of Painless evokes ‘avoidance’ in larvae and adult flies,Citation26,Citation27 this channel is a good target for the development of new insect repellents.
Future Tasks: Analyzing the Mechanism of Thermosensation
Identification of the thermoTRPs sheds light on molecular mechanisms for temperature sensation. However, compared to the recent remarkable progress in mammalian research fields, understanding of the molecular properties and the physiological roles of thermoTRPs in ectotherms and invertebrates is quite limited, although the temperature sensation must be more important for them than for endotherms. Recent studies, including our own, provide clear evidence that fruit flies utilize specific channels to detect warm to hot temperatures, which emphasizes the significance of the evolutionarily conserved property of the TRP channels. In contrast, little is known about the mechanisms for cold sensation in Drosophila. They avoid undesirable cold temperatures (<18°C) and some specific neurons appear to respond to cold exposure.Citation34 Therefore, Drosophila must have specific cold sensor(s) like TRPM8 in mammals. In addition to TRP and TRPL channels,Citation28 it has been reported that histamine signaling molecules including a histamine-gated chloride channel, and a cyclic AMP-PKA pathway are involved in temperature preference and cold tolerance in Drosophila.Citation43,Citation44 Furthermore, a recent report demonstrates that TRPA1 modulation via a Gq-PLC pathway participates in discriminating cooler temperatures in an optimal range (18–24°C).Citation23
We have clarified the activation properties of Painless, and they are better understood than any other insect channel. Given that TRP channels play a primary role in sensation, elucidation of the channel properties in various insects would be beneficial to our understanding and facilitate control of their physiological responses. Genetic and behavioral analyses could reveal the basic mechanisms for temperature sensation in fruit flies, which may help us understand the complicated thermoregulatory system in mammals. Comparison of the function of the thermoTRPs among various species may also help clarify how specific channels are activated by temperature changes.
Abbreviations
| TRP | = | transient receptor potential |
| HEK293 cells | = | human embryonic kidney 293 cells |
Figures and Tables
Figure 1 TRP channels and thermosensation in Drosophila. Flies utilize TRP channels to sense environmental temperatures. dTRPA1 is activated above 27°C and contributes to avoidance of warmer temperatures. Pyrexia is activated around 40°C and has a higher potassium permeability. Channel activation prevents paralysis during high temperature stress. Painless is activated above 40°C, and is essential for avoiding hazardous temperatures. These channels belong to the TRPA subfamily and posses temperature sensitivity. TRP and TRPL channels, both of which belong to the TRPC subfamily, are involved in cool temperature avoidance. However, the temperature sensitivity of these channels is unknown. The preferred range of Drosophila melanogaster (18–24°C) is indicated by dotted lines.
Acknowledgements
This work was supported by grants from the Japan Society for the Promotion of Science in Japan (Takaaki Sokabe) and from the Ministry of Education, Culture, Sports, Science and Technology in Japan (Makoto Tominaga).
References
- Hille B. Ion Channels of Excitable Membrane 2001; 3rd Ed Sunderland Sinauer Associates, Inc
- Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: Mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol 2007; 17:490 - 497
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306 - 313
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005; 307:1468 - 1472
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 2005; 25:1304 - 1310
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52 - 58
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108:705 - 715
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007; 448:204 - 208
- Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007; 54:379 - 386
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 2007; 54:371 - 378
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 2007; 27:14147 - 14157
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 2008; 28:566 - 575
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112:819 - 829
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50:277 - 289
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124:1269 - 1282
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427:260 - 265
- Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res 2007; 1160:39 - 46
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005; 438:1022 - 1025
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 2006; 25:1804 - 1815
- Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, et al. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 2007; 41:51 - 61
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, et al. Opposite thermosensor in fruitfly and mouse. Nature 2003; 423:822 - 823
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev 2005; 19:419 - 424
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci 2008; 11:871 - 873
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008; 454:217 - 220
- Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet 2005; 37:305 - 310
- Tracey WD Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell 2003; 113:261 - 273
- Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, et al. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav 2006; 5:602 - 613
- Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci USA 2008; 105:14668 - 14673
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci 2008; 28:9929 - 9938
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, et al. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 2006; 25:467 - 478
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 2007; 10:277 - 279
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 2007; 282:13180 - 13189
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004; 43:859 - 869
- Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci 2003; 6:267 - 273
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002; 418:181 - 186
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999; 398:436 - 441
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006; 68:619 - 647
- Tominaga M. Liedtke WB. The Role of TRP Channels in Thermosensation. TRP ion channel function in sensory transduction and cellular signaling cascades 2007; New York CRC Press 271 - 286
- Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci 2006;
- Al-Anzi B, Tracey WD Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol 2006; 16:1034 - 1040
- Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat Neurosci 2008; 11:676 - 682
- Gillij YG, Gleiser RM, Zygadlo JA. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol 2007;
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, et al. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 2006; 26:7245 - 7256
- Hong ST, Bang S, Hyun S, Kang J, Jeong K, Paik D, et al. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature 2008; 454:771 - 775