Abstract
A number of recent studies have investigated posterior development in several different arthropods. As previously found in spiders, it has been discovered that Delta-Notch signaling is required for the development of posterior segments in an insect, the cockroach Periplaneta americana. Furthermore analysis of Wnt8 function in the spider Achaearanea tepidariorum and the beetle Tribolium castaneum demonstrates that this Wnt ligand is required for the establishment of the growth zone and development of posterior segments in both these arthropods. Taken together these studies provide an interesting insight into the architecture of the genetic network that regulated posterior development in the common ancestor of the arthropods.
Introduction
During embryogenesis in the fruit fly, Drosophila melanogaster, all segments are specified almost simultaneously. This is called the long germ mode of development. In contrast, most other arthropods exhibit short germ development, during which posterior segments are added sequentially from a posteriorly located growth zone.Citation1 Short germ development appears to be an ancestral feature of arthropods.Citation2 Indeed it appears that the genetic regulation underlying posterior development in Drosophila is highly derived.Citation3 All animals are of course a composite of both retained ancestral traits and derived features, therefore comparisons of different short germ arthropods has the potential to reveal the ancestral regulatory network for posterior development in these animals.
A number of recent studies by Bolognesi and colleagues, Pueyo and colleagues and us have investigated posterior development in two insects, the red flour beetle Tribolium castaneum and the cockroach Periplaneta americana, and a chelicerate, the common house spider Achaearanea tepidariorum.Citation4–Citation6 From these and many informative previous studies a picture is now emerging of some of the genes and pathways that may have been responsible for the regulation of posterior development in the common ancestor of arthropods.
Delta-Notch Signaling in Posterior Segmentation
Although the Delta-Notch signaling pathway is required for segmentation in vertebrates, this pathway is not involved in segmentation in Drosophila.Citation1,Citation3 However, functional analysis in the spider Cupiennius salei surprisingly revealed that Delta-Notch signaling is required for generating the posterior segments in this chelicerate.Citation7 Knockdown of Delta (Dl), Notch (N) and other components of this pathway using embryonic-RNAi in Cupiennius disrupted expression of hairy (h) and manifested as malformed opisthosomal segments.Citation7,Citation8 In addition, parental-RNAi (pRNAi) knockdown of Delta-Notch components in a different spider Achaearanea tepidariorum, also resulted in truncated embryos, missing all opisthosomal segments, which shows that this pathway is also involved in the early steps of growth zone formation as well as segmentation.Citation9
This work in chelicerates naturally led to the question of whether Delta-Notch signaling is also involved in posterior development in other arthropods. The expression of Delta-Notch components in the myriapod Strigamia maritima (a centipede) and the insect Tribolium is consistent with a role in posterior development and segmentation, but there is as yet no functional evidence for a role of Delta-Notch in this process in these arthropods.Citation10,Citation11
However a recent study of the cockroach Periplaneta americana has provided the first functional evidence for the involvement of Delta-Notch signaling in posterior development and segmentation in an insect.Citation6 Pueyo and colleagues showed that Delta-Notch signaling is required for the establishment of the growth zone and for the subsequent sequential generation of segments from this tissue. Manipulation of this pathway gave rise to truncated embryos missing all abdominal segments in the most severe cases, which is consistent with a disrupted growth zone. In less severe phenotypes, embryos exhibited some abdominal tissue, but hedgehog (hh) stripes formed abnormally or were missing, indeed they were able to show that the expression of hh in nascent segments required dynamic expression of DL and h.Citation6
Together the data from spiders and the cockroach suggests that the common ancestor of arthropods employed Delta-Notch signaling for the development of a posterior growth zone and generation of posterior segments arising from this tissue. In contrast, Drosophila does not add segments sequentially from a posterior growth zone and this may explain why Delta-Notch signaling is not involved in segmentation in this long germ insect.
Wnt Signaling in Posterior Development
It was previously shown that Wnt signaling is involved in posterior development in short germ insects. Knockdown of armadillo (arm) and pangolin in the cricket Gryllus and the milkweed bug Oncopeltus respectively resulted in truncated embryos with missing abdominal segments.Citation12,Citation13 Again, although in Drosophila Wnt signaling is not required for the sequential addition of posterior segments, wg has a segment polarity function and WntD/8 is involved in dorso-ventral patterning.Citation14,Citation15
Recent detailed analysis of the function of Wnt signaling in Tribolium has now given further insights into the role of Wnt signaling in posterior development in insects.Citation4 Bolognesi and colleagues tested the function of the four Wnt ligands expressed in the growth zone in Tribolium, Wnt1/wg, Wnt5, WntD/8 and WntA.Citation4,Citation16 They found that loss of wg resulted in shorter embryos with smaller segments. In contrast WntD/8 knockdown resulted in embryos with a smaller growth zone and narrow malformed abdominal segments, or in extreme cases truncated embryos with the growth zone and abdomen completely missing. Interestingly, the extreme WntD/8 phenotypes were more frequent in WntD/8 and wg double knockdown embryos, suggesting there is some functional redundancy of Wnt ligands in the growth zone. WntA and Wnt5 RNAi knockdown in Tribolium had no obvious effect on posterior development.
In contrast to insects relatively little was known about the role of individual Wnt ligands in posterior development in other arthropods. Therefore, we recently investigated the role of Wnt8 in the spider Achaearanea.Citation5 Knockdown of Wnt8 using pRNAi in this spider resulted in posterior defects similar to those when WntD/8 is knocked down in Tribolium. Achaearanea Wnt8 pRNAi phenotypes ranged from embryos with smaller opisthosomal segments to truncated embryos missing all their opisthosomal segments.Citation5 Our results suggest Wnt8 is required for establishing and maintaining the growth zone during opisthosomal development through the maintenance of an undifferentiated pool of cells. Furthermore, our analysis indicates that the mechanism of this function of Wnt8 is through the direct or indirect regulation of dynamic expression of Dl in the growth zone, which is required for the correct expression of caudal (cad) and twist. Removal of Wnt8 appears to arrest dynamic Dl expression and thus gives rise to extensive ectopic Dl expression throughout the posterior. This results in premature differentiation of growth zone cells and therefore truncated embryos. Intriguingly a similar role has been suggested for Wnt8 during somitogenesis in vertebrates.Citation17
Knock down of Wnt8 in Achaearanea also resulted in fusion of limb buds along the dorso-ventral (D-V) axis suggesting that Wnt8 is also involved in patterning the spider embryo along the D-V axis. A role in D-V patterning has also been described for Wnt8 in vertebrates.Citation5 Intriguingly, although Drosophila does not require Wnt8 for posterior development this insect may have retained an ancestral role of Wnt8 along the D-V axis.Citation15
These analyses of the function of Wnt8 in a beetle and spider add to previous studies of Wnt signaling in insects and show that not only was Wnt signaling integral to the regulation of posterior development in the ancestral arthropod, but that the Wnt8 and Wnt1/wg ligands likely played key roles. Additional analysis of these and other Wnt signaling components in spiders, beetles and other arthropods should prove rewarding in further dissecting the role of this pathway in posterior development.
Caudal and Posterior Development
It is clear that cad/cdx genes are key factors in the posterior development of many animals suggesting that these genes had an ancient role in this process.Citation18,Citation19 In spiders it appears that cad is expressed zygotically throughout the addition of posterior segments from the growth zone,Citation5,Citation20 but its expression is not consistent with a role in anterior patterning as found in other arthropods.Citation19,Citation21 Experiments in spiders have revealed that the Delta-Notch and Wnt8 signaling pathways are both involved in the regulation of cad expression in the posterior, although whether this is direct or indirect is not known. There is evidence that cad expression is also regulated by Wnt signaling in short germband insects like the cricket and the beetle. Knockdown of arm in Gryllus results in a loss of cad expression, although the specific Wnt ligand involved has not yet been determined, and although cad expression in Tribolium is regulated by the torso pathway, this may be mediated by Wnt signaling.Citation21,Citation22 Therefore it is possible that Wnt signaling and perhaps Delta-Notch played an important role in the regulation of zygotic cad expression in the arthropod common ancestor.
An Ancestral Regulatory Network for Posterior Development?
Although lineage specific differences have evolved, comparative studies using a growing number of arthropods are beginning to reveal a common core of factors and pathways involved in the regulation of posterior development in these animals (). This suggests that the common ancestor of arthropods employed the Delta-Notch pathway, Wnt signaling possibly through Wnt8, and cad for the establishment of a posterior growth zone and the sequential generation of segments from this structure (). A picture of how these factors interact is also beginning to emerge, for example it appears that Wnt signaling is required for zygotic cad expression in different arthropods (). However, much work remains to be done in dissecting the precise regulatory interactions between these factors and other components involved in posterior development.
It is striking that these three core factors in posterior development in arthropods are also involved in posterior development in vertebrates.Citation18,Citation23–Citation25 This suggests that this regulatory network was present in the common ancestor of arthropods and vertebrates. However, to address the question of whether the common ancestor of bilaterally symmetrical animals, Urbilateria,Citation26 used this network for the generation of segments as well as posterior development requires further experimental efforts in vertebrates and arthropods, and functional studies in other animal phyla like annelids will be particularly illuminating with respect to these questions.
Figures and Tables
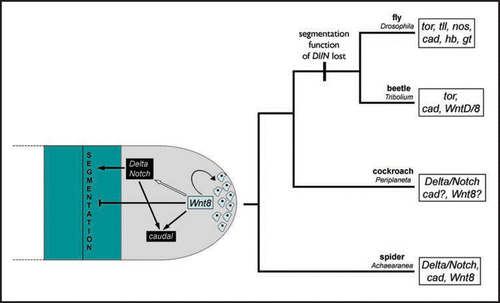
Figure 1 Model of the ancestral regulatory network for posterior development in arthropods and its evolution in insects and spiders. The left-hand illustration, at the base of the tree, shows the possible regulatory network of Wnt8, Delta-Notch (Dl/N) and caudal (cad) in a schematic, generalized growth zone of the hypothetical ancestral arthropod. The last two segments (blue highlighted area) and the unsegmented growth zone (grey highlighted area) are shown with the posterior to the right. Dashed lines indicate more anterior segments. Wnt8 is thought to establish and maintain a pool of undifferentiated cells at the very posterior of the growth zone (curved arrow pointing to the cells in blue) and may block segmentation (blunt arrow). At the same time, Wnt8 may be responsible for the dynamic expression of Delta and Notch because it initiates the clearance of expression of these genes from the very posterior of the growth zone (white arrow), which is needed for the correct induction of the segmentation process. Wnt8 and the Delta-Notch signaling may also be directly or indirectly responsible for the regulation of cad (black arrows). While Delta and Notch play an important role in the segmentation mechanism in spiders and insects like the cockroach, the function of these genes in segmentation may have been lost in the lineage leading to other insects like flies and possibly beetles (indicated by the vertical bar at the branch to beetles and flies).
References
- Damen WG. Evolutionary conservation and divergence of the segmentation process in arthropods. Dev Dyn 2007; 236:1379 - 1391
- Davis GK, Patel NH. Short, long and beyond: molecular and embryological approaches to insect segmentation. Annu Rev Entomol 2002; 47:669 - 699
- Peel AD, Chipman AD, Akam M. Arthropod segmentation: beyond the Drosophila paradigm. Nat Rev Genet 2005; 6:905 - 916
- Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol 2008; 18:1624 - 1629
- McGregor AP, Pechmann M, Schwager EE, Feitosa NM, Kruck S, Aranda M, et al. Wnt8 is required for growth-zone establishment and development of opisthosomal segments in a spider. Curr Biol 2008; 18:1619 - 1623
- Pueyo JI, Lanfear R, Couso JP. Ancestral Notch-mediated segmentation revealed in the cockroach Periplaneta americana. Proc Natl Acad Sci USA 2008; 105:16614 - 16619
- Stollewerk A, Schoppmeier M, Damen WG. Involvement of Notch and Delta genes in spider segmentation. Nature 2003; 423:863 - 865
- Schoppmeier M, Damen WG. Suppressor of Hairless and Presenilin phenotypes imply involvement of canonical Notch-signalling in segmentation of the spider Cupiennius salei. Dev Biol 2005; 280:211 - 224
- Oda H, Nishimura O, Hirao Y, Tarui H, Agata K, Akiyama-Oda Y. Progressive activation of Delta-Notch signaling from around the blastopore is required to set up a functional caudal lobe in the spider Achaearanea tepidariorum. Development 2007; 134:2195 - 2205
- Aranda M, Marques-Souza H, Bayer T, Tautz D. The role of the segmentation gene hairy in Tribolium. Dev Genes Evol 2008; 218:465 - 477
- Chipman A, Akam M. The segmentation cascade in the centipede Strigamia maritima: Involvement of the Notch pathway and pair-rule gene homologues. Dev Biol 2008; 319:160 - 169
- Angelini DR, Kaufman TC. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev Biol 2005; 283:409 - 423
- Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, et al. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev 2004; 121:119 - 130
- Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J 1987; 6:1765 - 1773
- Ganguly A, Jiang J, Ip YT. Drosophila WntD is a target and an inhibitor of the Dorsal/Twist/Snail network in the gastrulating embryo. Development 2005; 132:3419 - 3429
- Bolognesi R, Beermann A, Farzana L, Wittkopp N, Lutz R, Balavoine G, et al. Tribolium Wnts: evidence for a larger repertoire in insects with overlapping expression patterns that suggest multiple redundant functions in embryogenesis. Dev Genes Evol 2008; 218:193 - 202
- Thorpe CJ, Weidinger G, Moon RT. Wnt/beta-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development 2005; 132:1763 - 1772
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol 2005; 279:125 - 141
- Copf T, Schroder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci USA 2004; 101:17711 - 17715
- Akiyama-Oda Y, Oda H. Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development 2003; 130:1735 - 1747
- Shinmyo Y, Mito T, Matsushita T, Sarashina I, Miyawaki K, Ohuchi H, et al. caudal is required for gnathal and thoracic patterning and for posterior elongation in the intermediate-germband cricket Gryllus bimaculatus. Mech Dev 2005; 122:231 - 239
- Schoppmeier M, Schroder R. Maternal torso signaling controls body axis elongation in a short germ insect. Curr Biol 2005; 15:2131 - 2136
- Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, et al. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science 2006; 314:1595 - 1598
- Cinquin O. Understanding the somitogenesis clock: what's missing?. Mech Dev 2007; 124:501 - 517
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 2001; 1:103 - 114
- DeRobertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature 1996; 380:37 - 40