Abstract
Protein domain architecture can be used to construct supramolecular structures, to carry out specific functions and to mediate signaling in prokaryotic and eukaryotic cells. The Rgd1p protein of budding yeast contains two domains with different functions in the cell: the F-BAR and RhoGAP domains. The F-BAR domain has been shown to interact with membrane phospholipids and is thought to induce or sense membrane curvature. The RhoGAP domain activates the GTP hydrolysis of two Rho GTPases, thereby regulating different cellular pathways. Specific molecular interactions with the F-BAR and RhoGAP domains, cell signaling and interplay between these domains may allow the Rgd1p protein to act in several different biological processes, all of which are required for polarized growth in yeast.
Key words:
The establishment of cell polarity in eukaryotes involves asymmetric organization of the cytoskeleton and secretory pathway. In the yeast Saccharomyces cerevisiae, polarization results in the budding of daughter cells and the asymmetric segregation of cell components.Citation1 Polarized growth occurs at discrete regions of the cell surface, the presumptive bud site (late G1), the tip of small buds (S phase to G2 phase), the entire bud surface (G2/M phase to anaphase) and the bud neck of cells with large buds (late anaphase to telophase). Overall mother-bud polarity is maintained until late anaphase, when the growth machinery is redirected to the bud neck to promote cytokinesis and cell separation. Local polarity results from the fine-tuning of overall polarity by several factors, including GTPases and their regulators.
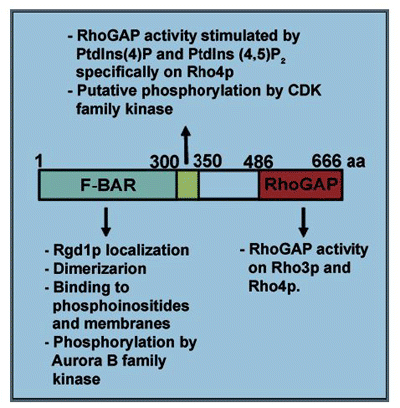
In S. cerevisiae, Rgd1p, a GTPase-activating protein (GAP), activates hydrolysis by the Rho3p and Rho4p GTPases, thereby negatively regulating the action of these enzymes in polarized growth. Consistent with the distribution of Rho3p and Rho4p, Rgd1p is found mostly in areas of polarized growth during cell cycle progression. Rgd1p localizes to the bud tip and bud cortex during polarized growth and to a ring at the site of cytokinesis. The C-terminal Rho GTPase-activating protein (RhoGAP) domain of Rgd1p is dispensable for localization to these sites. All the essential information for targeting Rgd1p is located in the F-BAR-containing region at the N-terminus (see for domain organization).Citation2 The F-BAR (extended Fer-CIP4 homology/FCH-BAR) domain has been shown to generate and bind to tubular membrane structures.Citation3 The distribution of Rgd1p suggests that F-BAR may interact with plasma membranes, specifically through binding to PtdIns(4,5)P2.Citation2 Fluorescence microscopy analysis with the PtdIns(4,5)P2-binding domain of the Boi1 proteinCitation4 has shown that Rgd1p and PtdIns(4,5) P2 colocalize at the plasma membrane in the bud tip and bud neck. However, Rgd1p may act at membranes other than the plasma membrane. The distribution of Rgd1p is altered in a strain with impaired PtdIns(4)P biosynthesis at Golgi membranes.Citation2 This suggests that Rgd1p may also be recruited to the Golgi apparatus via the F-BAR domain, leading to Rgd1p delivery to the plasma membrane via the secretory pathway. Alternatively, it may promote vesicle budding at the Golgi membrane, through the F-BAR domain.
The F-BAR domain is thought to be largely α-helical and to engage in coiled-coil interactions to form a banana-shaped dimer.Citation5 Using a two-hybrid approach, we showed that the Rgd1p protein did indeed form dimers via the F-BAR region. We also identified a phosphorylation site for a kinase of the Aurora B family within the F-BAR domain. The kinases of this family regulate kinetochore-microtubule attachment and help to maintain chromosomes in a condensed state during anaphase and early telophase in S. cerevisiae.Citation6 Another site, close to the F-BAR region, was identified by mass spectrometry within a consensus sequence for CDK phosphorylation. These features are consistent with Rgd1p function being controlled through the cell cycle. In budding yeast, cell cycle-dependent cell morphogenesis is regulated by the essential CDK Cdc28p. It has recently been reported that Rga2p, one of four GTPase-activating proteins for Cdc42p in S. cerevisiae, is phosphorylated by the cyclin/CDK complex.Citation7 We suggest that, as for Rga2p, Rgd1p phosphorylation is required for appropriate temporal and spatial function in yeast cells.
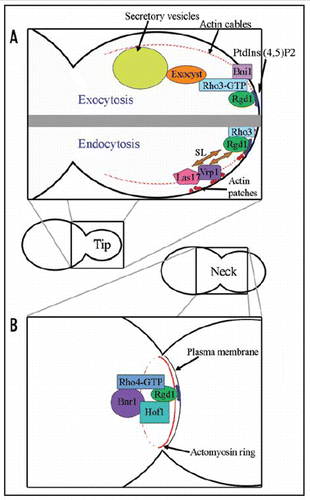
One of the key features of the RhoGAP domain of Rgd1p is its ability to activate two different RhoGTPases in S. cerevisiae,Citation8 Rho3p and Rho4p. Both these enzymes are involved in polarized growth events, but at different locations and different times in the cell cycle. Rho3p is mostly localized to the plasma membrane of daughter cells, whereas Rho4p is found around the contractile ring during cytokinesis. The specificity of molecular interactions may make it possible for the modular organization of Rgd1p (F-BAR and RhoGAP) to be used in different pathways and subcellular regions (). For example, we demonstrated the stimulation of GAP activity by phosphoinositides specifically for Rho4p/Rgd1p.Citation2 Similarly, the protein and lipid composition of membranes may influence the distribution of Rgd1p at the bud tip or bud neck, thereby also affecting the effects of this protein on the Rho3p and Rho4p GTPases.
Rho3p was initially shown to affect the organization of cortical actin patches and actin cables.Citation9 Rho4p must also be involved in these processes as the deletion of RHO4 exacerbates the actin cytoskeleton defect observed in rho3Δ mutants. A polarized array of actin cables in the cell cortex is the primary structural determinant of polarity. Motors, such as class V myosins, use this array to transport secretory vesicles and organelles to sites of growth. In budding yeast, Rho3p and Rho4p activate the Bni1p and Bnr1p formins, which nucleate actin filaments, thus directing the assembly of actin cables.Citation10 Cortical actin patches enhance and maintain this polarity, probably through endocytic recycling, allowing the reuse of materials and preventing continuous growth at specific locations. The dynamic organization of targeting and recycling provides flexibility for the precise control of morphogenesis. The F-BAR domain, like N-BAR domains, is involved in the invagination of the plasma membrane during processes such as endocytosis.Citation11 The F-BAR domain of Rgd1p may act as a membrane-targeting module during endocytosis in S. cerevisiae. Consistent with a role for endocytosis, additional copies of the LAS17 and VRP1 genes encoding the homologs of the human WASP and WIP proteins involved in endocytosis, restore correct actin organization in a strain lacking RHO3.Citation12 Las17p binds strongly to Vrp1p and activates the Arp2/3 protein complex, which nucleates branched actin filaments. The synthetic lethality of RGD1 inactivation with the las17Δ and vrp1Δ mutations suggests that the RhoGAP protein plays a role in endocytosis together with Las17p and Vrp1p.Citation13 Rho3p also interacts with the exocyst subunit Exo70.Citation14 The exocyst defines a hub integrating signals from many small GTPases (Cdc42, Rho1, Sec4 and Rho3) during polarized growth in yeast. A specific mutation in the Rho3 effector domain (rho3E51V) abolishing interaction with Exo70p causes the accumulation of post-Golgi vesicles in yeast cells. These data suggest that Rho3 plays a role in vesicle transport and tethering to the plasma membrane and positively regulates exocytosis. Rho3p may maintain polarized growth and cell wall integrity during subsequent bud growth, through exocytosis, endocytosis and actin organization.
Rho4p interacts with the formin Bnr1p and regulates the interaction between Bnr1p and Hof1p, two proteins involved in cytokinesis and localized at the bud neck, in a GTP-Rho4p-dependent manner.Citation15 Genetic analysis revealed that both the rgd1Δ and hof1Δ deletions are synthetic lethal with vrp1 and myo1 inactivation.Citation12 We also confirmed, in two-hybrid assays, that Rgd1p interacts with Hof1p. Thus, Rgd1p plays a role in cytokinesis, in the cellular mechanism involving Hof1p. Cytokinesis in S. cerevisiae involves coordination between actomyosin ring contraction and septum formation and/or targeted membrane deposition. The actomyosin ring is a contractile structure composed of actin filaments and Myo1p, the only known class II myosin. It forms beneath the plasma membrane at the mother-bud neck. Rgd1p and Hof1p form rings positioned at or close to the bud neck. Hof1p is required early in mitosis for the assembly of a functional actomyosin ring, but is specifically degraded late in mitosis, remaining absent throughout the entire G1 phase of the cell cycle. Hof1p downregulation is required at the end of mitosis for efficient contraction of the actomyosin ring and cell separation during cytokinesis.Citation16 Concomitant with ring contraction, membrane vesicles are added at the cleavage site to facilitate the necessary expansion of the cell membrane and cell wall. Rgd1p, through its GAP activity on the Rho4p GTPase, should coordinate the regulation of cell contraction between mother and daughter cells. Rapid signaling to release Hof1p for degradation might involve the stimulation of GAP activity by PtdIns(4,5)P2. The role of Rgd1p in cytokinesis may also involve septum formation, as RGD1 inactivation is synthetic lethal with BNI4 deletion. Bni4p is required for the assembly of the chitin ring, and is also involved in septum formation and the maintenance of bud neck integrity.Citation17
The function of the Rho3p and Rho4p GTPases and the regulation of the processes in which they are involved must be coordinated in space and time to facilitate yeast cell growth. Rgd1p, through its F-BAR and RhoGAP domains, interacts with specific lipids and proteins at different sites of polarized growth (see for models of Rgd1p action). In this way, a protein with a single organization of its internal domains can integrate different cell signals and take part in different polarized growth and morphogenesis processes during the cell cycle in S. cerevisiae.
Figures and Tables
Acknowledgements
This research was supported by Bordeaux 2 University and the CNRS.
References
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol 2004; 20:559 - 591
- Prouzet-Mauleon V, Lefebvre F, Thoraval D, Crouzet M, Doignon F. Phosphoinositides affect both the cellular distribution and activity of the F-BAR-containing RhoGAP Rgd1p in yeast. J Biol Chem 2008; 283:33249 - 33257
- Takano K, Toyooka K, Suetsugu S. EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J 2008; 27:2817 - 2828
- Hallett MA, Lo HS, Bender A. Probing the importance and potential roles of the binding of the PH-domain protein Boi1 to acidic phospholipids. BMC Cell Biol 2002; 3:16
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, et al. Structural basis of membrane invagination by F-BAR domains. Cell 2008; 132:807 - 817
- Zhang X. Aurora kinases. Curr Biol 2008; 18:146 - 148
- McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR 3rd, Gygi SP, et al. Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol 2007; 9:506 - 515
- Doignon F, Weinachter C, Roumanie O, Crouzet M. The yeast Rgd1p is a GTPase-activating protein of the Rho3 and Rho4 proteins. FEBS Lett 1999; 459:458 - 462
- Matsui Y, Toh EA. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol Cell Biol 1992; 12:5690 - 5699
- Dong Y, Pruyne D, Bretscher A. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J Cell Biol 2003; 161:1081 - 1092
- Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 2006; 172:269 - 279
- Roumanie O, Peypouquet MF, Thoraval D, Doignon F, Crouzet M. Functional interactions between the VRP1-LAS17 and RHO3-RHO4 genes involved in actin cytoskeleton organization in Saccharomyces cerevisiae. Curr Genet 2002; 40:317 - 325
- Roumanie O, Peypouquet MF, Bonneu M, Thoraval D, Doignon F, Crouzet M. Evidence for the genetic interaction between the actin-binding protein Vrp1 and the RhoGAP Rgd1 mediated through Rho3p and Rho4p in Saccharomyces cerevisiae. Mol Microbiol 2000; 36:1403 - 1414
- Robinson NG, Guo L, Imai J, Toh EA, Matsui Y, Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol 1999; 19:3580 - 3587
- Kamei T, Tanaka K, Hihara T, Umikawa M, Imamura H, Kikyo M, et al. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J Biol Chem 1998; 273:28341 - 28345
- Blondel M, Bach S, Bamps S, Dobbelaere J, Wiget P, Longaretti C, et al. Degradation of Hof1 by SCF(Grr1) is important for actomyosin contraction during cytokinesis in yeast. EMBO J 2005; 24:1440 - 1452
- Kozubowski L, Panek H, Rosenthal A, Bloecher A, DeMarini DJ, Tatchell K. A Bni4-Glc7 phosphatase complex that recruits chitin synthase to the site of bud emergence. Mol Biol Cell 2003; 14:26 - 39