Abstract
Sound-producing moths have evolved a range of mechanisms to emit loud conspicuous ultrasounds directed toward mates, competitors and predators. We recently discovered a novel mechanism of sound production, i.e. stridulation of specialized scales on the wing and thorax, in the Asian corn borer moth, Ostrinia furnacalis, the male of which produces ultrasonic courtship songs in close proximity to a female (< 2 cm). The signal is very quiet, being exclusively adapted for private communication. A quiet signal is advantageous in that it prevents eavesdropping by competitors and/or predators. We argue that communication via quiet ultrasound, which has not been reported previously, is probably common in moths and other insects.
In summer, the chorus of insects is commonplace. In tympanate insects, such as crickets, katydids and cicadas, sound plays an important role in transmitting/receiving information about, for example, location and identity (sex and species).Citation1 For efficient communication over long distances, sound emitted by insects is, in general, fairly intense [>70 dB SPL (decibel sound pressure level) at a distance of 1 cm].Citation1,Citation2 Accordingly, it is not surprising that most studies to date on acoustic communication in insects have focused on loud audible sounds.
Evolution of Ultrasonic Communication in Moths
The majority of moths have ultrasound-sensitive tympanal ears, which evolved, it is believed, to counteract predacious bats that emit ultrasonic echolocation calls during foraging.Citation3,Citation4 The ears of moths within the same superfamily are morphologically similar and located on the same position of a body, e.g., the thorax in Noctuoidea or abdomen in Pyraloidea,Citation4,Citation5 suggesting that they developed independently before the divergence of superfamilies. Some moth species also evolved organs that emit ultrasound for communication in sexual and other contexts (). In contrast to the ear, various types of sound-producing organs, which differ in origin, are found within the same superfamily, suggesting that these organs evolved after acquisition of the ear.Citation3–Citation5 It is assumed that moths evolved ultrasonic sexual communication through secondary exploitation of the preexisting ultrasound-sensitive ears (sensory bias model of signal evolution).Citation3,Citation5
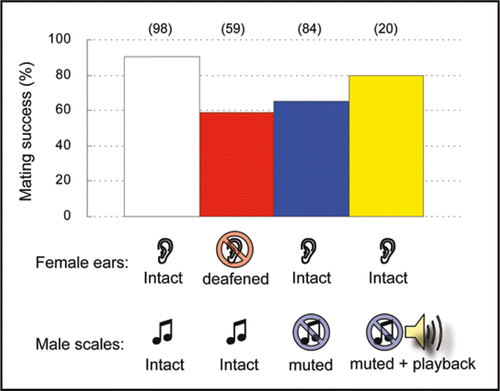
Moths that produce loud ultrasound have elaborate sound organs,Citation3 such as the tymbals, which are found on the tegula,Citation6 thoraxCitation7 or abdomen,Citation8 and the file and scrapers, which are found on the wing, legCitation9 or on the genitalia ().Citation10 In moths, three types of ultrasonic songs for sexual communication have been reported: (i) a calling song for mate attraction,Citation8–Citation13 (ii) a courtship song for mate acceptance/recognition,Citation2,Citation6,Citation14–Citation16 and (iii) a duet between sexes for the male's approach to the female after pheromonal orientation.Citation17,Citation18 In addition to sexual communication, male moths of genus Hecatesia express territorial calls with wing castanets,Citation19,Citation20 while arctiid moths of both gender emit jamming or alarm clicks from thoracic tymbals in response to echolocation calls of predacious bats.Citation21–Citation23 Other moth species are known to produce ultrasounds with abdominal,Citation24 thoracicCitation25 or wingCitation12 tymbals, although the function of these sounds are unknown. The ultrasounds described above are, without exception, characterized by the rather high sound pressure levels, ranging from 60 to 125 dB SPL at a distance of 1 cm ().
Private Ultrasonic Communication in Ostrinia furnacalis
Recently, we found that males of the Asian corn borer moth, Ostrinia furnacalis, produce ultrasonic courtship songs of extremely low intensity (46 dB SPL at 1 cm) in close proximity to a female (<2 cm).Citation2,Citation26 Electrophysiological recordings from the auditory neurons of a female revealed that only females within 3 cm of the singing male can hear the song ().Citation2 The male moths produced these sounds by rubbing specialized scales on their wings against scales on the thorax ().Citation2 Sound production via specialized scales on the body surface is a novel discovery among insects. Simple sound-producing scales on the body might have evolved more easily than other more elaborate cuticular sound-producing apparatuses, requiring major modifications of the integument.
Our behavioral experiments have clarified the mode of action of the male songs in O. furnacalis.Citation2 The male songs suppress escape behavior, or movement in general, of the female, thereby increasing the mating success of the courting male (). The female response to the male courtship song may be cognate to freezing behavior, which is induced, as a defense maneuver, in response to the echolocation calls of insectivorous bats.Citation4 This raises the intriguing possibility that the moths have not only exploited the sensory apparatus, but also the behavioral reaction “inherited” from their interaction with bats. Thus ultrasonic communication in O. furnacalis should be an excellent system for testing the receiver bias model of signal evolution.Citation5,Citation27
Advantages of Whispering Communication
In insects, loud acoustic signals are often used for intraspecific communication over long distances. However, these signals are also susceptible to eavesdropping by unintended receivers (e.g., conspecific rivals, predators and/or parasitoids).Citation28,Citation29 Indeed, communication via loud sounds is risky because predacious gleaning bats and parasitic tachinid flies, for example, locate sound-producing insects using passive hearing.Citation30,Citation31
The male courtship song of O. furnacalis has the lowest intensity (46 dB SPL at 1 cm) ever found in sound-producing moths (). What is surprising is that, within the animal world, such faint sounds can have a significant impact on mating. The utilization of quiet ultrasound is highly advantageous as it precludes eavesdropping by conspecific male competitors and natural enemies. Our discovery of extremely low intensity ultrasonic communication may reveal a world of private communication using quiet ultrasound.
Figures and Tables
Figure 1 Ultrasound levels in sound-producing moths. Sound levels (0 dB SPL = 20 µPa) at a distance of 1 cm from the moths are shown for families within the noctuoid and pyraloid superfamilies.Citation2,Citation8–Citation10,Citation12,Citation14–Citation16,Citation18,Citation20,Citation23,Citation25 Color of symbols indicates sound-producing mechanism: red, stridulation with file-scraper; black, click with tymbal; blue, percussion with alar castanet. A star indicates sound emission at a short distance. Top photographs show representative sound-producing moths. The sound level of the male song of Ostrinia furnacalis (red star) is the lowest among all sound-producing moths studied to date.Citation2
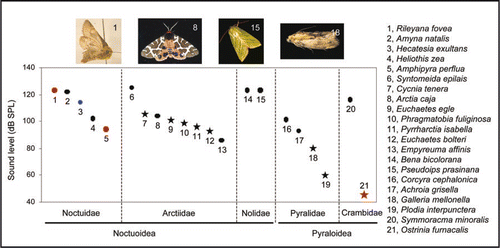
Figure 2 Male ultrasound and female audiogram in Ostrinia furnacalis. (A) Spectrum of the male ultrasound at a distance of 1 cm (blue solid line) shows main energy around 40 kHz, which corresponds to the most sensitive frequency range of female hearing (red line).Citation2 Male ultrasound at a distance of 3 cm (blue dotted line) is below the hearing threshold, indicating that the male song is not audible to females ≥3 cm distant. (B) Typical response of auditory neurons (red) to pulsed sine waves of 40 kHz (gray). (C) Oscillogram (upper) and power spectrogram (lower) of groups of ultrasonic pulses emitted by a male.
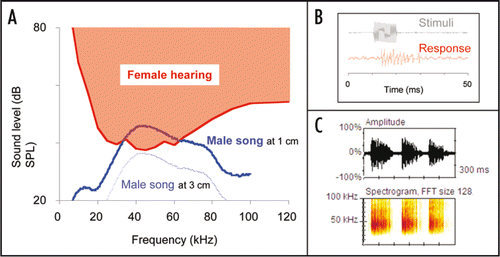
Figure 3 Sound-producing organ of male Ostrinia furnacalis. Courting male rubs specific scales on the base of forewings against scales on mesothoraces to emit ultrasonic song. Specialized sound-producing scales, which are characterized by thicker longitudinal ridges, are found only on the wing and thorax of the male (right box).Citation2
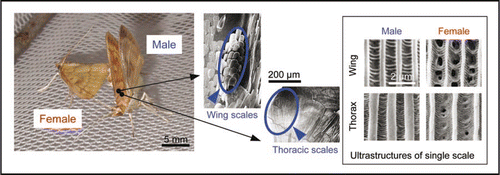
Figure 4 Effect of male ultrasound on mating success. Pairs of intact male and intact female showed high mating success (white bar). Mating success decreased when the female was deafened by puncturing the ear (red bar) or the male was muted by ablation of the sound scales (blue bar).Citation2,Citation26 Playback of male song significantly compensated for the decrease in mating success in pairs involving muted males (yellow bar). Likelihood ratio test in generalized linear model, F1, 2 = 19.5, p = 0.048). Number of pairs examined is shown in parentheses above each bar.
Acknowledgements
We would like to thank Takuji Koike, Keisuke Yoshida, Hirotaka Maruyama for their collaborations. This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (Ryo Nakano and Yukio Ishikawa), the Danish National Research Foundation (Niels Skals and Annemarie Surlykke), and the Danish Natural Science Research Council (Niels Skals and Annemarie Surlykke).
Addendum to:
References
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans 2002; Chicago and London The University of Chicago Press
- Nakano R, Skals N, Takanashi T, Surlykke A, Koike T, Yoshida K, et al. Moths produce extremely quiet ultrasonic courtship songs by rubbing specialized scales. Proc Natl Acad Sci USA 2008; 105:11812 - 11817
- Conner WE. ‘Un chant d'appel amoureaux’: acoustic communication in moths. J Exp Biol 1999; 202:1711 - 1723
- Miller LA, Surlykke A. How some insects detect and avoid being eaten by bats: tactics and countertactics of prey and predator. BioScience 2001; 51:570 - 581
- Greenfield MD. Signalers and receivers 2002; Oxford Oxford University Press
- Spangler HG. Moth hearing, defense and communication. Annu Rev Entomol 1988; 33:59 - 81
- Fullard JH. The neuroethology of sound production in tiger moths (Lepidoptera, Arctiidae) I. Rhythmicity and central control. J Comp Physiol A 1992; 170:575 - 588
- Heller K-G, Krahe R. Sound production and hearing in the pyralid moth Symmoracma minoralis. J Exp Biol 1994; 187:101 - 111
- Surlykke A, Gogala M. Stridulation and hearing in the noctuid moth Thecophora fovea (Tr.). J Comp Physiol A 1986; 159:267 - 273
- Gwynne DT, Edwards ED. Ultrasound production by genital stridulation in Syntonarcha iriastis (Lepidoptera: Pyralidae): long distance signalling by male moths?. Zool J Linn Soc 1986; 88:363 - 376
- Spangler HG, Greenfield MD, Takessian A. Ultrasonic mate calling in the lesser wax moth. Physiol Entomol 1984; 9:87 - 95
- Heller K-G, Achmann R. The ultrasonic song of the moth Amyna natalis (Lepidoptera: Noctuidae: Acontiinae). Bioacoustics 1993; 5:89 - 97
- Jang Y, Greenfield MD. Ultrasonic communication and sexual selection in wax moths: female choice based on energy and asynchrony of male signals. Anim Behav 1996; 51:1095 - 1106
- Conner WE. Ultrasound: its role in the courtship of the arctiid moth, Cycnia tenera. Experientia 1987; 43:1029 - 1031
- Simmons RB, Conner WE. Ultrasonic signals in the defense and courtship of Euchaetes egle Drury and E. bolteri Stretch (Lepidoptera: Arctiidae). J Insect Behav 1996; 9:909 - 919
- Trematerra P, Pavan G. Ultrasound production in the courtship behavior of Ephestia cautella (Walk.), E. kuehniella Z. and Plodia interpunctella (Hb.) (Lepidoptera: Pyralidae). J Stored Prod Res 1995; 31:43 - 48
- Sanderford MV, Conner WE. Acoustic courtship communication in Syntomeida epilais Wlk. (Lepidoptera: Arctiidae, Ctenuchinae). J Insect Behav 1995; 8:19 - 31
- Sanderford MV, Coro F, Conner WE. Courtship behavior in Empyreuma affinis Roths. (Lepidoptera, Arctiidae, Ctenuchinae): acoustic signals and tympanic organ response. Naturwissenschaften 1998; 85:82 - 87
- Bailey WJ. Resonant wing systems in the Australian whistling moth Hecatesia (Agarasidae, Lepidoptera). Nature 1978; 272:444 - 446
- Alcock J, Bailey WJ. Acoustical communication and the mating system of the Australian whistling moth Hecatesia exultans (Noctuidae: Agaristinae). J Zool Lond 1995; 237:337 - 352
- Miller LA. Arctiid moth clicks can degrade the accuracy of range difference discrimination in echolocating big brown bats, Eptesicus fuscus. J Comp Physiol A 1991; 168:571 - 579
- Hristov IN, Conner WE. Sound strategy: acoustic aposematism in the bat-tiger moth arms race. Naturwissenschaften 2005; 92:164 - 169
- Barber JR, Conner WE. Acoustic mimicry in a predator-prey interaction. Proc Natl Acad Sci USA 2007; 104:9331 - 9334
- Skals N, Surlykke A. Sound production by abdominal tymbal organs in two moth species: the green silver-line and the scarce silver-line (Noctuoidea: Nolidae: Chloephorinae). J Exp Biol 1999; 202:2937 - 2949
- Kay RE. Acoustic signaling and its possible relationship to assembling and navigation in moth Heliothis zea. J Insect Physiol 1969; 15:989 - 1001
- Nakano R, Ishikawa Y, Tatsuki S, Surlykke A, Skals N, Takanashi T. Ultrasonic courtship song in the Asian corn borer moth, Ostrinia furnacalis. Naturwissenschaften 2006; 93:292 - 296
- Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trend Ecol Evol 1998; 13:415 - 420
- Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Qua Rev Biol 1998; 73:415 - 438
- Mason AC, Oshinsky ML, Hoy RR. Hyperacute directional hearing in a microscale auditory system. Nature 2001; 410:686 - 690
- Bailey WJ, Haythornthwaite S. Risks of calling by the field cricket Teleogryllus oceanicus; potential predation by Australian long-eared bats. J Zool Lond 1998; 244:505 - 513
- Zuk M, Rotenberry JT, Tinghitella RM. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett 2006; 2:521 - 524