Abstract
Extension of the plasma membrane is one of the first steps in cell migration. Understanding how cells “choose” between various types of membrane protrusion enhances our knowledge of both normal and cancer cell physiology. The EGF receptor is a paradigm for understanding how transmembrane receptor tyrosine kinases regulate intracellular signaling following ligand stimulation. Evidence from the past decade indicates that EGF receptors also form macromolecular complexes with integrin receptors leading to EGF receptor transactivation during cell adhesion. However, relatively little is known about how these complexes form and impact cell migration. Our recent work characterized a molecular complex between EGF receptor and β3 integrin which recognizes RGD motifs in extracellular matrix proteins. Complex formation requires a dileucine motif (679-LL) in the intracellular juxtamembrane region of the EGF receptor that also controls whether or not the receptor undergoes Src kinase-dependent phosphorylation at Tyr-845. In contrast to wild-type receptors, mutant EGF receptors defective for Tyr-845 phosphorylation form complexes with β1 integrin that also binds RGD motifs. In addition, we have discovered that EGF receptor antagonizes small GTPase RhoA by mediating membrane recruitment of its regulatory GAP p190RhoGAP. In this addendum we discuss a potential new role for Src-dependent EGF receptor transactivation in integrin/EGF receptor complex formation. We also discuss how our study fits with previous observations linking p190RhoGAP to RhoA-dependent cytoskeletal rearrangements involved in cell migration, and provide new data that the EGF receptor is compartmentalized to relatively immature zyxin-poor focal adhesions which are the likely site of p190RhoGAP signaling.
Allosteric Mechanisms for EGFR Activation
EGF receptors (EGFR) form molecular complexes with multiple integrin extracellular matrix receptors leading to EGFR transactivation and fine-tuning of adhesion-induced cell signaling responses.Citation1-Citation3 How EGFR activation is achieved remains an active area of investigation. On the one hand, adhesion-activated integrins may sequester EGFRs leading to receptor oligomerization and activation independent of soluble ligand. Alternatively or perhaps in concert with increased receptor density, adhesion-induced Src kinase activity may have a critical role in EGFR transactivation. Src kinase phosphorylates EGFR residue Tyr-845 which is located in the activation loop of the EGFR catalytic domain.Citation4 The corresponding tyrosine residues in other receptor tyrosine kinases are autophosphorylated following ligand stimulation and phenylalanine substitutions significantly impair kinase signaling and downstream signaling. In contrast, EGFR Tyr-845 phosphorylation is not required for ligand-induced EGFR activation but may instead represent a primary mechanism for EGFR transactivation.Citation4 Our recently published results have identified a specific dileucine motif (679-LL) (see Box 1) located in the EGFR juxtamembrane (JM) domain that is absolutely required for Tyr-845 phosphorylation during cell adhesion.Citation5 These results were obtained by adhering cells to the extracellular matrix protein fibronectin (FN) containing an RGD sequence (Arg-Gly-Asp) recognized by several integrin receptors. Our studies were focused on RGD-directed β1 integrin and β3 integrin receptors.
EGFR 679-LL was originally identified as a lysosomal sorting signal following ligand-stimulated EGFR downregulation.Citation6 A 679-AA substitution does not affect ligand-induced internalization but promotes EGFR recycling and enhanced activation of a subset of EGFR tyrosine kinase substrates in recycling endosomes.Citation7 Subsequently, this motif was shown to have a critical role in ligand-induced EGFR activation. An important study by Zhang et al. showed that EGFR is activated by an allosteric mechanism involving formation of an asymmetric dimer in which one kinase domain induces an active conformation in the other.Citation8 This occurs through intra-molecular interactions between the C-terminal lobe of a “activator” kinase domain and the N-terminal lobe of a “acceptor” kinase domain leading to its catalytic activation.Citation8 In this model 679-LL resides in the asymmetric dimer interface adjacent to a JM segment that latches the “acceptor” domain to the activator domain to achieve full activation of EGFR.Citation9-Citation11 We speculate that integrin/EGFR complex formation triggers conformational changes in the activation loop that make Tyr-845 accessible to Src kinase. 679-AA does not block integrin complex formation per se, but interferes with Tyr-845 exposure preventing EGFR transactivation by Src kinase.
Our data are consistent with a model in which the 679-LL motif is necessary to form a stable β3 integrin-EGFR complex. However, EGFRs form complexes with different integrin receptors in other cell models.Citation12 How then is integrin-EGFR complex formation regulated? Based on the resistance of EGFR (679-AA) to Src-dependent Tyr-845 phosphorylation, we hypothesize that Src kinase has a critical role in assembling and/or stabilizing β3 integrin-EGFR complexes. β1 and β3 integrins both activate focal adhesion kinase (FAK) leading to activation of Src kinase.Citation13 However, β3 integrins also promote Src activation via direct interactions between Src and β3 integrin cytoplasmic tails.Citation14 Our data therefore raise two interesting possibilities that are not mutually exclusive. First, Tyr-845 phosphorylation may be preferentially mediated by Src kinase activated via direct interaction with β3 integrin cytoplasmic tail. Second, β3 integrin-EGFR complexes may be stabilized by interactions between Src and its EGFR substrate Tyr-845. Thus alternative integrin-Src activation events may determine the integrin constituents of the integrin-EGFR complex.
Integrin-EGFR Complex Formation and p190RhoGAP Activation
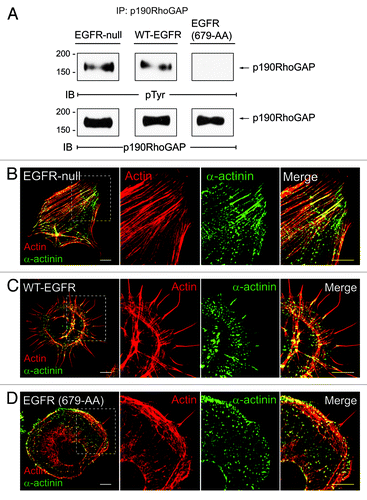
The small GTPase RhoA is a well-known regulator of actin cytoskeletal rearrangements associated with formation of focal adhesions (FAs), actin stress fibers, and filopodia and lamellipodia membrane protrusions.Citation15-Citation17 Previous reports have shown that RhoA is antagonized by p190RhoGAP, the most abundant GAP for RhoA in mammalian cells, during the early stages of cell adhesion.Citation16 To become fully active p190RhoGAP must be tyrosine phosphorylated by Src kinase, form a complex with p120RasGAP, and undergo recruitment to the cell periphery.Citation16,Citation18,Citation19 Different membrane targeting mechanisms have an important role in determining the effect of local changes in GTP Rho activity. Our recent study showed that EGFR mediates p190RhoGAP activation by promoting its membrane recruitment.Citation5 In this context, p120RasGAP serves as a bridge, physically linking EGFR and p190RhoGAP. We found that adhesion-activated EGFRs form a complex with p120RasGAP, EGFR-p120RasGAP complex formation is associated with a reduction in RhoA activity, and p190RhoGAP is recruited to the cell periphery where it co-localizes with EGFR.Citation5 Two lines of evidence indicate the β3 integrin/EGFR axis is primarily responsible for p190RhoGAP activation. First, a D119A inhibitory mutation in β3 integrin that blocks RGD binding inhibits EGFR-p120RasGAP complex formation.Citation5 Second, EGFR (679-AA) which forms a complex with β1 integrin does not recruit p120RasGAP.Citation5 We also provide new data that wild-type EGFR promotes p190RhoGAP tyrosine phosphorylation necessary for p120RasGAP-p190RhoGAP complex formation (). Similar to cells reconstituted with wild-type EGFR, p190RhoGAP is tyrosine phosphorylated () and also antagonizes RhoA activity in newly adherent EGFR-null cells.Citation5 Although the mechanism for p190RhoGAP activation in the EGFR-null cells is not known, previous studies indicate that p120RasGAP-p190RhoGAP membrane recruitment is mediated by FAK following FN engagement.Citation20 Our data suggest the mechanism of p190RhoGAP membrane recruitment has a dramatic effect on cell morphology.Citation5 The EGFR-null cells form prominent vertical stress fibers (), compared with cells reconstituted with wild-type EGFR that form abundant filopodial extensions (). Remarkably, EGFR (679-AA) expression abolishes adhesion-induced p190RhoGAP tyrosine phosphorylation (). Furthermore, RhoA inhibition which usually accompanies cell adhesion, is compromised in cells expressing EGFR (679-AA),Citation5 and this dominant-inhibitory phenotype is associated with formation of broad lamellipodial membrane protrusions (). We cannot assess whether complex formation between β1 integrin and EGFR (679-AA) represents a physiological signaling unit or an experimental artifact due to the introduction of the dialanine substitution. However, these results raise the possibility that EGFR may inhibit certain adhesion-induced responses. Overall our results have uncovered an unexpected role for EGFR in p190RhoGAP activation during formation of membrane protrusions involved in cell migration.Citation5 They also underscore the critical role p190RhoGAP membrane recruitment has on allowing cells to switch between different types of cytoskeletal rearrangements.
Figure 1. (A) EGFR modulates FN-dependent phosphorylation of p190RhoGAP. FN-dependent adhesion assays were performed as previously described.Citation5 Briefly, cells were adhered to FN for 1 h and p190RhoGAP immune complexes were analyzed by SDS-PAGE followed by immunoblotting with phosphotyrosine or p190RhoGAP antibodies. p190RhoGAP undergoes tyrosine phosphorylation in EGFR-null fibroblasts (left), and cells reconstituted with wild-type EGFR (middle), but not in cells expressing EGFR (679-AA) (right). (B–D) EGFR modulates ?-actin in distribution. Confocal projections of EGFR-null cells (B) and cells with WT-EGFR (C) or EGFR (679-AA) (D) adhered to FN for 20 min co-stained with phalloidan to visualize actin (red) and α-actinin antibody (green). Magnified images of individual and merged channels for boxed areas are shown to right. Scale bars, 10 μM.
p190RhoGAP Activity and Focal Adhesions
Upon engagement of extracellular matrix, integrins cluster and recruit various anchor/adaptor and signaling proteins to help form FAs.Citation21 Adaptor proteins such as p130Cas, vinculin, α-actinin and zyxin provide links to the actin cytoskeleton, allowing for the formation of tension necessary to change cell morphology.Citation21 The RhoA antagonist p190RhoGAP is also localized to FAs during cell adhesion.Citation20 FAs are formed by sequential recruitment of different anchor proteins that are involved in binding the actin cytoskeleton to the membrane.Citation22,Citation23 Our recent studies showed that EGFR expression is associated with altered distribution of vinculin, one of the first proteins recruited to newly formed FAs, when cells are adhered to FN.Citation5,Citation17 Here we extend these studies by examining additional FA proteins, the actin binding protein α-actinin and the anchor protein zyxin, which serve as markers for mid and late stage FAs, respectively.Citation17 α-actinin is concentrated in elongated FAs at stress fiber tips in EGFR-null mouse fibroblasts (). Similar to vinculin, α-actinin is enriched at the base of filopodial actin filaments in cells with wild-type EGFR ().Citation5 Cells with EGFR (679-AA) display a distinct α-actinin staining pattern characterized by small puncta at the cell periphery along with periodic staining in the dense cortical actin region and the sparse actin network in the lamellum. The late stage FA marker zyxin is recruited to FAs at stress fiber tips in EGFR-null mouse fibroblasts (). In contrast, cells expressing either wild-type or mutant EGFR were characterized by a statistically significant reduction in zyxin incorporation at peripheral adhesion complexes compared with EGFR-null cells ().
Figure 2. (A–C) EGFR is compartmentalized to zyxin-poor focal adhesions. Confocal imaging was performed as previously described.Citation5 EGFR-null cells (A) and cells with wild-type EGFR (B) or EGFR (679-AA) (C) were adhered to FN for 20 min and co-stained with phalloidan (red) and zyxin antibody (green). Magnified images of individual and merged channels for boxed areas are shown to right. Asterisks indicate zyxin positive FAs. Scale bars, 10 μM. (D) Bars represent the average number of zyxin-positive adhesion complexes 20 min post-adhesion to FN. White bar, EGFR-null cells; light gray bark cells with WT-EGFR; dark gray bar, cells with EGFR (679-AA). Asterisks indicate differences between cells that were statistically significant (p < 0.001) as determined by Student’s t-test. These results show a significant reduction in zyxin-positive FAs in cells expressing either form of EGFR.
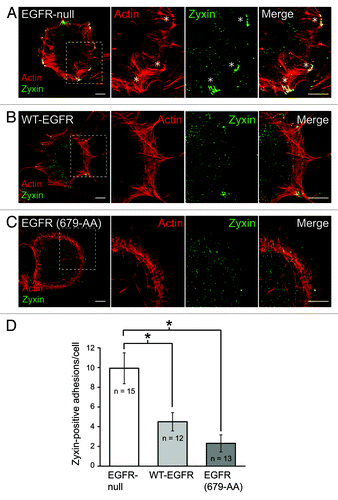
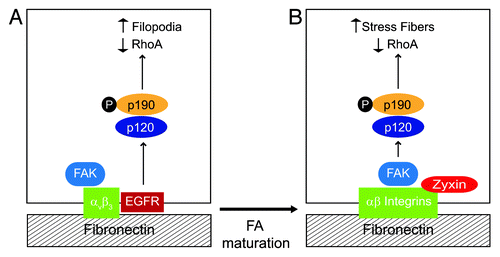
Altogether our data suggest EGFR is compartmentalized to relatively immature FAs during cell adhesion (). As FAs mature they grow in size and incorporate additional constituent molecules.Citation24 Immature FAs are smaller in size and relatively short-lived. This may explain why EGFRs form complexes with β3 integrin since αvβ3 is relatively enriched in zyxin-negative FAs compared with other RGD directed integrin receptorsCitation23 (). β3 integrin/EGFR compartmentalization could serve two purposes. First, it may confine EGFR-dependent p190RhoGAP activation to relatively immature FAs. Second, compartmentalization may contribute to the reduction in stress fiber formation. Zyxin is recruited to FAs under increased tension.Citation25 Zyxin also senses mechanical load and enhances actin polymerization at FAs contributing to stress fiber growth.Citation25-Citation27 Membrane p190RhoGAP recruitment to mature FAs is probably mediated by FAK in EGFR-null cellsCitation11 (). Thus EGFR may reduce stress fiber formation by driving a switch in how p190RhoGAP is activated.
Figure 3. EGFR regulates RhoA-mediated cytoskeletal rearrangements by reconfiguring p190RhoGAP activation. To become fully active the RhoA antagonist p190RhoGAP must be tyrosine phosphorylated by Src kinase, form a complex with p120RasGAP, and undergo recruitment to the cell periphery. Our data suggest that EGFR has an important role in determining the effect of local changes in GTP-Rho activity by regulating p190RhoGAP membrane recruitment. (A) Simplified schematic depicting EGFR-expressing cell adhered to fibronectin. β3 integrin ligation activates FAK and also promotes complex formation with EGFR. β3 integrin-EGFR complexes localize to relatively immature, zyxin-poor FAs. EGFR recruits p120RasGAP which serves as a bridge to p190RasGAP leading to formation of filopodia. (B) EGFR-null cell adhered to fibronectin. RGD-directed αβ integrins activate FAK which serves as a scaffold to recruit p120RasGAP to mature zyxin-positive FAs. p120RasGAP facilitates a bridge between FAK and p190RasGAP leading to formation of actin stress fibers.
Perspectives
Our work raises several interesting questions for future investigation. The first question relates to how EGFR chooses to interact with different RGD directed integrin binding partners. Our data suggest integrin/Src cross-talk may have a significant role in this selection process. It has also been shown that the p130Cas signaling adaptor is required for EGFR complex formation with integrins recognizing RGD motifs,Citation12 and we suspect additional adaptor proteins have yet to be identified. Integrin/EGFR complex formation and its impact on adhesion-induced signaling have traditionally been viewed as distinct processes. We propose that these processes are intimately linked and that regulation is bidirectional. The ability of EGFR to switch integrin binding partners is probably important in allowing cells to parse and adjust to extracellular cues in a physiological context. Data from this addendum suggest that EGFR/integrin complexes are resident in immature FAs which have a higher rate of turnover compared with mature FAs.Citation21 Because of their constant turnover immature FAs can rapidly sample and adjust to the extracellular environment. Rapid turnover is also necessary for cell migration. Thus immature FAs represent an ideal platform to integrate information from extracellular cues to regulate formation of membrane protrusions. We also suspect interactions with different RGD-containing extracellular matrix proteins may influence formation of distinct integrin/EGFR complexes. As in vivo environments are seldom homogeneous in matrix composition, cells undoubtedly integrate signals from many distinct EGFR/integrin complexes simultaneously. How these signals are interpreted and their hierarchy in relation to membrane protrusion outcomes is an interesting future direction. We believe these questions will further establish a pivotal role for the EGFR in allowing cells to switch between different mechanisms of membrane protrusion and hence regulate cell migration in response to a dynamic microenvironment.
Box 1. Glossary.
679-LL: Dileucine motif in EGFR juxtamembrane domain required for Src kinase-mediated Tyr-845 phosphorylation and β3 integrin complex formation; 679-AA substitutions block these activities.
α-actinin: Actin filament cross-linking and bundling protein; commonly used marker for mid-stage focal adhesions.
Fibronectin (FN): High-molecular weight glycoprotein of the extracellular matrix with an RGD motif that binds to membrane-spanning integrin receptors.
Focal adhesions (FA): Large, dynamic protein complexes at cell surface connecting actin cytoskeleton to extracellular matrix.
Focal adhesion kinase (FAK): Cytosolic protein tyrosine kinase recruited to early-stage cell-matrix adhesions which mediates many downstream responses.
p120RasGAP: A negative regulator of Ras signaling that also forms a complex with p190RhoGAP contributing to GTPase RhoA inhibition during cells adhesion; may bind directly to activated growth factor receptors via an SH2 domain.
p190RhoGAP: The most prominent GAP for GTPase RhoA in mammalian cells; probably linked to activated growth factor receptors through p120RasGAp; antagonizes RhoA during cell adhesion.
RGD (Arg–Gly–Asp): Conserved motif found in a variety of adhesive proteins present in extracellular matrices including fibronectin, vitronectin, and osteopontin; β1 and β3 integrins are RGD-directed adhesion receptors.
RhoA: Small GTPase known to regulate actin cytoskeletal rearrangements associated with formation of focal adhesions, actin stress fibers, and filopodia and lamellipodia membrane protrusions.
Tyrosine-845: Tyrosine residue residing in the EGFR activation loop which is phosphorylated by Src kinase; not required for ligand-mediated EGFR activation.
Src kinase: Non-receptor tyrosine kinase responsible for phosphorylating Tyrosine 845 on the EGFR; present in integrin/EGFR complex.
Vinculin: Cytosolic protein involved in attaching actin cytoskeleton to plasma membrane; commonly used marker for early-stage focal adhesions.
Zyxin: Cytosolic protein involved in actin polymerization at focal adhesions; commonly used marker for mature focal adhesions.
| Abbreviations: | ||
| EGFR | = | EGF receptor |
| FA | = | focal adhesion |
| FAK | = | focal adhesion kinase |
| FN | = | fibronectin |
| JM | = | juxtamembrane |
Acknowledgments
Supported by T32 HL007653 (N.B.), RO1 GM081498 (C.R.C.), and a pilot project grant from development funds of the Case Comprehensive Cancer Center Support Grant P30 CA043703 (C.R.C.).
References
- Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, et al. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans 2004; 32:438 - 42; http://dx.doi.org/10.1042/BST0320438; PMID: 15157155
- Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J 2009; 418:491 - 506; http://dx.doi.org/10.1042/BJ20081948; PMID: 19228122
- Alexi X, Berditchevski F, Odintsova E. The effect of cell-ECM adhesion on signalling via the ErbB family of growth factor receptors. Biochem Soc Trans 2011; 39:568 - 73; http://dx.doi.org/10.1042/BST0390568; PMID: 21428941
- Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol 1997; 9:187 - 92; http://dx.doi.org/10.1016/S0955-0674(97)80062-2; PMID: 9069259
- Balanis N, Yoshigi M, Wendt MK, Schiemann WP, Carlin CR. β3 integrin-EGF receptor cross-talk activates p190RhoGAP in mouse mammary gland epithelial cells. Mol Biol Cell 2011; 22:4288 - 301; http://dx.doi.org/10.1091/mbc.E10-08-0700; PMID: 21937717
- Kil SJ, Hobert ME, Carlin C. A leucine-based determinant in the EGF receptor juxtamembrane domain is required for the efficient transport of ligand-receptor complexes to lysosomes. J Biol Chem 1999; 274:3141 - 50; http://dx.doi.org/10.1074/jbc.274.5.3141; PMID: 9915853
- Kostenko O, Tsacoumangos A, Crooks D, Kil SJ, Carlin C. Gab1 signaling is regulated by EGF receptor sorting in early endosomes. Oncogene 2006; 25:6604 - 17; http://dx.doi.org/10.1038/sj.onc.1209675; PMID: 16715136
- Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006; 125:1137 - 49; http://dx.doi.org/10.1016/j.cell.2006.05.013; PMID: 16777603
- Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, et al. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell 2009; 137:1293 - 307; http://dx.doi.org/10.1016/j.cell.2009.04.025; PMID: 19563760
- Endres NF, Engel K, Das R, Kovacs E, Kuriyan J. Regulation of the catalytic activity of the EGF receptor. Curr Opin Struct Biol 2011; 21:777 - 84; http://dx.doi.org/10.1016/j.sbi.2011.07.007; PMID: 21868214
- Red Brewer M, Choi SH, Alvarado D, Moravcevic K, Pozzi A, Lemmon MA, et al. The juxtamembrane region of the EGF receptor functions as an activation domain. Mol Cell 2009; 34:641 - 51; http://dx.doi.org/10.1016/j.molcel.2009.04.034; PMID: 19560417
- Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem 2002; 277:9405 - 14; http://dx.doi.org/10.1074/jbc.M109101200; PMID: 11756413
- Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011; 63:610 - 5; http://dx.doi.org/10.1016/j.addr.2010.11.001; PMID: 21118706
- Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A 2003; 100:13298 - 302; http://dx.doi.org/10.1073/pnas.2336149100; PMID: 14593208
- Ridley AJ. Life at the leading edge. Cell 2011; 145:1012 - 22; http://dx.doi.org/10.1016/j.cell.2011.06.010; PMID: 21703446
- Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell 2001; 12:2711 - 20; PMID: 11553710
- Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509 - 14; http://dx.doi.org/10.1126/science.279.5350.509; PMID: 9438836
- Bradley WD, Hern´ndez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell 2006; 17:4827 - 36; http://dx.doi.org/10.1091/mbc.E06-02-0132; PMID: 16971514
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci 1999; 112:947 - 56; PMID: 10036244
- Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci 2009; 122:1852 - 62; http://dx.doi.org/10.1242/jcs.046870; PMID: 19435801
- Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M. Turnover of focal adhesions and cancer cell migration. Int J Cell Biol 2012; 2012:310616; http://dx.doi.org/10.1155/2012/310616; PMID: 22319531
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 2003; 116:4605 - 13; http://dx.doi.org/10.1242/jcs.00792; PMID: 14576354
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 2004; 32:416 - 20; http://dx.doi.org/10.1042/BST0320416; PMID: 15157150
- Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol 2012; 196:363 - 74; http://dx.doi.org/10.1083/jcb.201107042; PMID: 22291038
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol 2005; 171:209 - 15; http://dx.doi.org/10.1083/jcb.200505018; PMID: 16247023
- Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol 2008; 1:192 - 5; http://dx.doi.org/10.4161/cib.1.2.7001; PMID: 19513257
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature 2010; 468:580 - 4; http://dx.doi.org/10.1038/nature09621; PMID: 21107430