Abstract
Background: Post-pubertal acne is an inflammatory form, whose cause is unknown. Contrasting data are available on correlation between acne and smoking habit.
Objectives: To verify the frequency of NIA among women, a possible correlation with cigarette smoking, possible differences in sebum composition in a group of smoker ladies with acne compared to healthy smokers and non smokers.
Method and results: 1046 randomly selected women (25–50 years old) took part in the study. Personal details were acquired. In 60 selected female subjects we analysed sebum composition for α-tocopherol, squalene and squalene monohydroperoxide. We found a high prevalence of NIA among women (74.6%), a strong correlation with smoking habit (p < 0.0001), as well as an increase in the grade of sebum peroxidation (p < 0.05) with a reduction in Vitamin E (p = 0.02), in the subjects with acne compared to the controls.
Conclusions: The clinical evidences and the experimental data showed a straight correlation between smoking habit and post-pubertal acne in which the non-inflammatory form (NIA) is the most frequent. In the more severe cases we could consider NIA as a new entity (smoker’s acne)
Introduction
Acne is a common dermatosis, usually considered an adolescent disease.Citation1 However, recent epidemiological studies have shown that it affects a significant percentage (12–14%) of women between 25 and 50 years of age.Citation2 Post-pubertal acne is described as an inflammatory mild-moderate form, whose cause is still unknown and whose incidence is increasing.Citation3 However, in the clinical practice of our ambulatory for acne treatment, we observed a form characterized predominantly by retentional lesions (micro and macrocomedones), with few inflammatory lesions (papules and pustules), which seemed to be particularly frequent among adult female smokers, which we conventionally named atypical post-adolecent acne (APAA).
On the other hand, there are few available contrasting data on a possible correlation between acne and smoking. It is commonly accepted that smoking provokes important alterations on the skin microcirculation, on keratinocytes and on the collagen and elastin synthesis. Nicotinic receptors are expressed on keratinocytes, fibroblasts and blood vessels. Nicotine induces vasoconstriction associated with local hyperaemia. It inhibits inflammation through effects on central and peripheral nervous system and through direct effect on immune cells. It delays wound healing and accelerates skin aging.Citation4 This could contribute to define the “smoker’s face” described by several authors.Citation5 Another important role, while less investigated, could be played by the relative deficiency in antioxidants caused by smoking, which could lead to alterations in sebum composition, as described by Pelle et al. who demonstrated that peroxidation is induced in human skin by cigarette smoke and subsequently inhibited by the presence of antioxidants.Citation6
The aim of the study was to verify the frequency of APAA among women and to identify a possible correlation with cigarette smoking. We also tried to find possible differences in sebum composition in a group of female smokers with acne compared to healthy smokers and non-smokers.
Our study consists in a clinical and in a laboratory part. Clinically, our aim was to verify the frequency of APAA among women in a random selection of 1046 female subjects and to identify possible correlations with cigarette smoking. From an experimental point of view, we evaluated squalene, squalene monohydroperoxide and vitamin E in sebum collectected from a group of female smokers with acne compared to healthy smokers and non-smokers.
Our results showed a strong correlation between cigarette smoking and APAA as well as an increase in the grade of sebum peroxidation, in parallel to a reduction in vitamin E, in the subjects with acne compared to the controls.
Patients
For this study, 1046 women aged between 25–50 years were randomly interviewed among mothers or accompanying females of children attending the Paediatric Dermatological Ambulatory of San Gallicano Institute between January and May 2006. These patients were predominantly referred to the ambulatory for skin oncologic prevention, and their age ranged between 0 and 18 years. For every woman the following personal informations were acquired: smoking habit, number of cigarettes smoked, juvenile acne, possible hormonal pathologies. Ex-smokers, who stopped smoking less than one year before the interview, were excluded from the study.
The participants were evaluated by a group of dermatologists to verify the presence of acne, the number and type of lesions and possible signs of hyperandrogenism (hirsutism, alopecia).
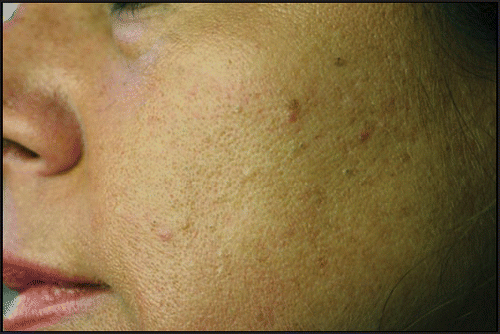
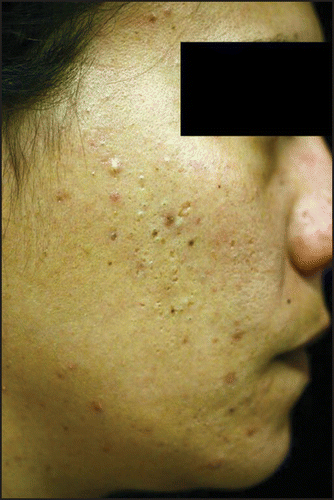
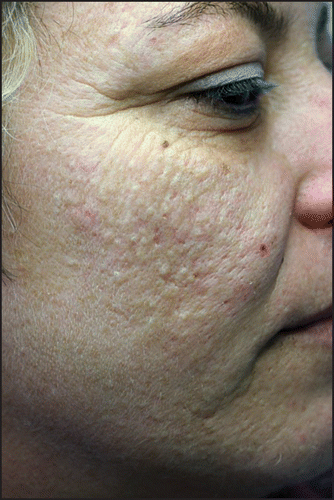
Patients affected by acne were divided, according to the type, the number and layout of the lesions (), into three groups: classical post-adolescent acne (CPAA), mild to moderate atypical post-adolescent acne (mAPAA) ( and ), severe atypical post-adolescent acne (sAPAA) ().
In addition patients with acne who were clinically suspicious for hormonal alterations (hyrsutism, menstrual alterations, obesity), underwent the following series of examinations: sexual hormonebinding globulin (SHBG), testosterone, dihydrotestosterone, dehydroepiandrosterone (DHEA), δ-4 androstenedione and an ovarian ecography. Patients affected by hormonal, either laboratory or ecographical, alterations were excluded from the study.
To analyze sebum peroxidation and vitamin E production, total of 60 female subjects aged between 25 and 50 years were included in the study. Subjects were divided in the following groups: 20 smokers affected by acne (acne-smoking: AS); 20 healthy non-smokers (CTR); 20 healthy smokers (CTR-S). Patients from AS and CTR-S groups smoked between 8 and 14 cigarettes/day for more than 5 years. From each subject sebum was collected.
Chemicals
All chemicals and solvents were of the highest analytical or high performance liquid chromatography (HPLC) grade, unless otherwise specified. Solvents were from Merck, Italy; α and γ-tocopherol, squalene, squalane were from Sigma-Aldrich, Italy.
Sebum Collection
Samples of sebum were collected from the forehead of the volunteers using Sebutapes® (Cuderm, Dallas, TX) as previously described.Citation7 Briefly, forehead skin was cleaned by means of sterile gauze soaked with 70% ethanol solution. Three tapes were applied for each volunteer for 30 minutes. Each tape was weighted before and after sebum collection. Sebum enriched tapes were stored at −80°C until extraction.
Sebum Extraction
Sebutapes® were extracted by HPLC grade ethanol by vortexing each tape in 1.5 ml Eppendorf tube. One milliliter of ethanol containing butylated hydroxy toluene (250 µg/ml) was used as antioxidant for every 1 mg of sebum collected. The tapes were then removed and the solution was centrifuged at 2920 × g at 4°C for 10 minutes. Supernatants were collected and analyzed.Citation8
Alpha-Tocopherol Analysis
One-hundred twenty microliters of sebum extract was added with γ-tocopherol (37.5 ng) as internal standard, dried under N2 flow and then directly silylated with 30 µl of N, O-bis-(trimethylsilyl)- trifluoroacetamide containing 1% trimethyl-chlorosilane as catalyst. The samples were analyzed by gas chromatographymass spectrometry, after 30 min at 60°C (Trace GC Ultra, Trace DSQ, Thermofinnigan). The separation was performed by capillary column RTX-5MS (30 m × 0.25 mm × 0.25 µm, Restek Corporation, Bellefonte, PA) using helium as gas carrier. An oven temperature gradient from 110 to 280°C at 25°C/min was used. Mass spectra were recorded in Electronic Impact and in SIM modality. Selected ions were 237, 502 for α-tocopherol; 223, 488 for γ-tocopherol.
Squalene Analysis
One-hundred twenty microliters of sebum extract was added with squalene (6 µg) as internal standard, dried under N2 flow and resuspended in 30 µl of hexane. Samples were analyzed by gas chromatography-mass spectrometry (Trace GC Ultra, Trace DSQ, Thermofinnigan). Separation was performed by capillary column RTX-5MS (30 m × 0.25 mm × 0.25 µm, Restek Corporation, Bellefonte, PA) using helium as a gas carrier. An oven temperature gradient from 100 to 280°C at 10°C/min was used. Mass spectra were recorded in Electronic Impact and in SIM modality. Selected ions were 69, 410 for squalene; 71, 239, 407 for squalene.
Squalene Monohydroperoxide Analysis
Squalene monohydroperoxide was analyzed by means of liquid chromatography-mass spectrometry (Hewlett Packard Series 1100 HPLC-MSD) as previously described.Citation7 Briefly, the samples were analyzed using Ultra C18 column (250 × 4.6 mm, Restek Corporation, Bellefonte, PA) with HPLC grade ethanol: methanol (1:1, v/v) as mobile phase at a flow rate of 1.2 ml/min and detection at 210 nm. Squalene peroxide was identified by the mass spectra obtained on a single quadrupole mass spectrometer equipped with Atmospheric Pressure Chemical Ionization (APCI) interface. Mass spectra were recorded in SIM modality. Selected ions were 409, 425.
Statistical Analysis
Statistical analysis was performed with GraphPad PRISM4 version software. The Chi-square test was used to evaluate contingency tables. For laboratory investigations numbers represent mean value ± standard deviation. Statistical differences were evaluated using the Student’s test. Results were considered significant at p < 0.05.
Results
Clinical evidences. From 1046 randomly selected women, 42 patients who stopped smoking less than 1 year before the study and 4 patients with hormonal alterations were excluded from the study. Among 1000 patients, 277 (27.7%) were smokers and 723 (72.3%) non-smokers. 185 (18.5%) patients were affected by acne. Mean age in the whole sample was 34.6 (median 36). No significative difference in mean age or age-distribution were found between smokers and non-smokers, acne and healthy patients, CPAA and APAA. A statistically significant difference was noticed for the prevalence of acne among the smokers [115 out of 277 (41.5%)] and the non-smokers [(70 out of 723 (9.7%)] (p < 0.0001) (). Among the patients affected by acne, APAA was the most frequent form, affecting 138 (74.6%) patients, 105 (76.1%) of whom were smokers and 33 (23.9%) were non-smokers, while CPAA was present in only 47 patients (25.4%). Among smokers 91.3% had APAA, while CPAA was the most frequent among non-smokers (37 out of 70, 52.8%) (Fig. 4B) (p = 0.0003).
The characteristics of the sample are summarized in . Among the non-smokers affected by APAA, predisposing factors were identified in 16 cases ().
Among patients with APAA, 127 (92.0%) were classified as mild-moderate and 11 (8.0%) as severe. Among them, 9 (81.8%) were smokers and only 2 (18.2%) non-smokers (). Due to classification limits it was not possible to found a linear correlation between acne severity and number of cigarettes, but all the patients with serious acne were heavy smokers (>15 cigarettes/day).
Regarding the sequential correlation between acne and smoking, 35 patients referred that initiation of smoking definitely preceded the appearance of acne (with an average latency period of 1–2 years), 11 reported with certainty that they commenced smoking after appearance of the disease. Sixty-nine patients began smoking during adolescence (in temporal coincidence with acne onset). The numbers of patients reported here were not analyzed for statistical correlations due to uncertainty of the history.
Taking into account the patients with a positive history for adolescent acne (42% of the sample), 47% among the smokers were affected by acne at the moment of the clinical evaluation, opposed by 18% of the non-smokers () (p = 0.0042; OR: 4.05; CI 95%: 2.6–6.3). It can be stated with a 95% confidence that among patients with adolescent acne, the probability to be affected by current acne in smokers was between 2.6–6.3 times higher than in non-smokers.
We found no statistical differences between smokers with and without acne regarding the cumulative smoking dose. The cumulative dose was calculated by multiplying the number of cigarettes smoked per day × the number of days per year (365) × the number of years. Based on our results the average cumulative dose for smokers with acne was 70875.7 (range 4380–233600, median value 63802) and for smokers without acne 65364.2 (range 3285–219000, median value 59422).
Histological evaluation.
In order to verify if acne was always associated with elastotic alterations, we carried out six skin biopsies on smokers affected by acne (mean age 35.6 range 29–38 years). The histological examinations () revealed open and closed comedones and a normal sebaceous gland, while elastosis was present only in two women (>35 years, >20 cigarettes/day). Numbers were too small for a statistical analysis.
Sebum composition and Vitamin E levels.
The analysis highlighted significant differences in the sebum composition between smokers and non-smokers, independently if they were affected by acne or not. In particular, in the smokers the levels of sebaceous vitamin E was halved compared to non-smokers (). The decrease of vitamin E levels was also associated with an increase in the grade of lipid peroxidation which was evaluated considering the amount of squalene, a characteristic lipid of human sebum, and its peroxide (P-Sq). The smokers presented a decrease in squalene, equivalent to 50% compared to non-smokers in parallel with an increase in the peroxidation product (). The sebum excretion, expressed as mg/min, is the only parameter evaluated that differentiates smokers affected by acne from both healthy smokers and non-smokers. In particular, smokers affected by acne show a sebum excretion that is about three times the one of healthy controls ().
Discussion
Our study detected a prevalence of post-adolescent acne of 18.5% (), slightly higher than that reported by other authors (12–14%),Citation2,Citation3 and particularly frequent among smokers ( and ). Post adolescent acne is commonly described as a mild to moderate acne, mainly inflammatory and localized on the chin, on the lower jaw and the neck.Citation2,Citation3 However, we found that APAA was clearly the most frequent form (74.6%), and that it was particularly frequent among smokers (p = 0.0003). Among smokers, APAA seems to represent a peculiar form, which in the most serious cases could be considered a true “smoker’s acne,” analogous to the “smoker’s face” described by other authors.Citation5 In fact, in our study 81.8% of subjects with severe APAA were smokers. The only two non-smokers with serious APAA were patients who lived in environmental predisposing conditions: a 32-year-old woman strongly exposed to passive smoking (both parents were heavy smokers) (), and a 36-year-old cook. Among smokers, CPAA was scarcely represented (), while it prevailed among the non-smokers (61.6%), and was always mild-moderate in severity ().
Data on the correlation between acne and smoking are still controversial. The absence of published data comparable to our data could probably be explained by a different approach to the problem. In fact published statistics were mainly carried out on adolescent acne or on a non-homogenous population. A study by Schäfer et al. on a general population showed a higher prevalence of acne among smokers compared to non-smokers and a linear correlation between the severity of acne and the number of cigarettes smoked.Citation9 These results seem to be confirmed by a more recent study by Chuh et al.Citation10 In the meanwhile, Mills et al. reported an inverse correlation between the incidence of acne and smoking,Citation11 but authors considered a highly selected population, consisting of hospitalized male patients affected by serious inflammatory acne. A recent study by Klaz et al.Citation12 reported an inverse correlation between the severity of acne and the number of cigarettes smoked. Also in this case, data are not comparable to ours because the cohort consisted exclusively of young male adults.
In a study by Cunliffe et al. on post-adolescent acne, among possible pathogenetic factors (hormonal alteration, stress, cosmetic use, occupational), smoking has inexplicably not been taken into consideration.Citation3
Williams and Layton claimed that data on the correlation between acne and smoking could be confounded by the fact that they do not clearly indicate a temporal correlation between smoking and acne.Citation2 Indeed, in our sample, only approximately 1/3 of the APAA group referred the appearance of acne 1–2 years after starting smoking, moreover this finding was not considered completely reliable due to the difficulty encountered in the collection of history data. Despite this, the strong correlation found made the temporal correlation between smoking habits and acne of secondary importance. Moreover, among patient who had experienced adolescent acne, smokers were more frequently affected by active acne (). This finding suggest that smoking could be a major contributing factor for post-adolescent acne in predisposed patients.
Several factors (hormonal alterations, stress, environmental pollution, light exposure), other than smoking, can contribute to the pathogenesis of APAA.Citation13 In our study, 33 non-smokers were affected by APAA (2 with a severe form). In 16 of these patients predisposing factors were identified (). In particular, light exposure is a predisposing condition to the onset of retentional lesions, as described in Favre-Rochochout syndrome.Citation14 In our geographical area light exposure is widespread and this could have influenced our statistics, even if, according to our biopsies, the presence of severe acne in young women in the absence of elastosis, suggested that the influence of light exposure on APAA was only marginal. Experimental evidence supports the hypothesis of a pathogenetic role of smoking on APAA. Keratinocytes have nicotine acetylcholine receptors that increase their adhesion, differentiation, apoptosis and inhibit migration.Citation7,Citation15 At higher concentrations of 100 µg/ml, nicotine induces cutaneous hyperkeratinisation. Citation16
Nicotine and other components of cigarette smoke induce microcirculation alterations with consequent vasoconstriction and hypoxyemiaCitation17,Citation18 and exhibit an inhibitory effect on the chemotaxis of neutrophils and lymphocytes.Citation19 Among alterations caused on the skin by smoking an important role could be played by alterations in sebum composition; in fact smoking seems to cause an increase in oxidative stress and reduces the levels of α-tocopherol in plasma.Citation20 Our data show that the oxidative damage of smoke is also affecting sebum production: sebum of smokers presented significant lower concentrations of α-tocopherol, which is the principal antioxidant transported by sebum on the skin surface,Citation21 probably in order to maintain low levels of peroxidated products of sebaceous lipids.Citation3,Citation21,Citation22 These products seem to be involved in the pathogenesis of acne; previous studies demonstrated that patients affected by acne had a higher grade of lipid peroxidation.Citation24
Among peroxidated lipids, squalene is particularly important because it is a characteristic lipid of human sebum and because its peroxides have a hyperproliferative effect on keratinocytes and are, therefore, comedogenic.Citation25,Citation26 Our data show that along with a decrease in α-tocopherol there is also an increase in squalene peroxide in the sebum of smokers. Thus, cigarette smoke produces an alteration in sebum composition, through the production of reactive oxygen species (ROS), which is similar to that found in acne. Although we detected no difference between the sebum of healthy smokers and smokers with acne, it could be hypothesized that smoking is associated with a higher susceptibility to acne and in particular to APAA. Taking into consideration the role of lipid peroxidation in acne, and the incidence of acne subjects in our sample, there could be a possible association between smoking habits and this particular type of acne. The ability of cigarette smoke to induce peroxidation on sebaceous lipids and the relative deficiency of antioxidants could be considered as an element capable of contributing to the onset and/or exacerbation of this pathology in subjects already predisposed to this disease, such as individulas that present an excess of sebum production considered one of the principal factors involved in the pathogenesis of acne.
The lack of significant statistical difference between smokers with and without acne regarding the cumulative smoking dose (considering that the calculation does not take numerous variables into account such as type of cigarettes smoked, manner of smoking, passive smoking, possible suspension periods, inaccurate number of cigarettes referred, possible variations in the number of cigarette smoked) could suggest that the clinical expression of acne in these patients could be related to genetic predisposition. Several studies demonstrated that smoking is an independent risk factor for the early onset of facial wrinklesCitation27 and that there is an association between premature facial wrinkling and pulmonary function impairment. Kadunce et al. have suggested a common pathogenesis for the early development of facial wrinkles and chronic-obstructive pulmonary disease (COPD).Citation28 In agreement with this hypothesis, Lange et al. found a significant association of both conditions in smokers.Citation29 A recent work by Patel et al. demonstrated a correlation between cutaneous aging in smokers and COPD.Citation30 Future studies would verify, if the presence of APAA could be a marker for predisposition to systemic damage by smoke as for early cutaneous aging.
The percentage of adult women affected by acne is increasing.Citation31 External factors such as cosmetics and active occupation do not seem to play a significant pathogenetic role.Citation32 However, stress-induced alterations of the curaneous androgen metabolism remain a hypothesis which has still to be verified.Citation33,Citation34 Our data confirm that APAA affects a high percentage of adult women. Among these patients the number of smokers is so high, which might partially explain the noticeable increase in this pathology.
Recognizing this form is fundamental for proper information on the effects of tobacco on the skin and can contribute to the anti-smoking information programs, especially among adolescents where aesthetic motivation plays an important role.
In some patients the severity of acne, the clinical variation, the strong correlation with smoking and the biochemical data could lead to consider APAA as a new entity among smoking-related cutaneous diseases (“smoker’s acne face”).
Figures and Tables
Figure 4 (A) Acne is more frequent among smokers. (B) APAA is more frequent among smokers, CPAA among non smokers. (C) among patients who had post-adolescent acne, smokers have a higher probability to be affected by current acne (p = 0.0042; OR: 4.05; CI 95%: 2.6–6.3).
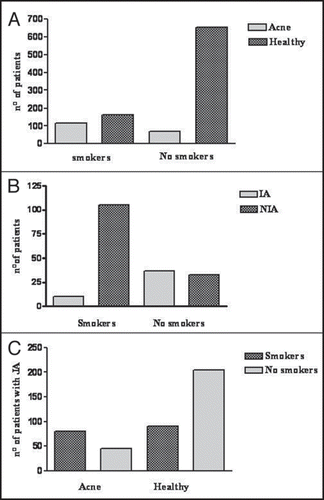
Figure 5 Amounts of vitamin E in the sebum of smokers is significantly lower than in healthy controls (p = 0.02). Ctr, healthy non smokers; AS, acne-smokers; Ctr-S, healthy smokers.
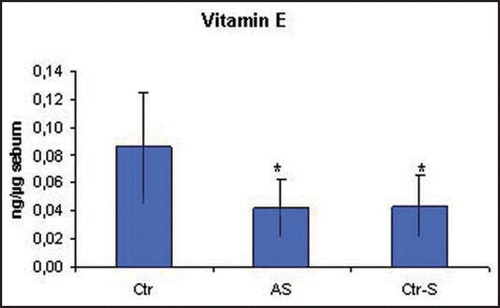
Figure 6 Amounts of squalene in the sebum of smokers (AS, Ctr-S) is halved compared to that present in the sebum of non smokers (Ctr) (A). In parallel with this reduction a relative increase of squalene peroxide is seen (B) (p < 0.05). Ctr, healthy non smokers; AS, acne-smokers; Ctr-S, healthy smokers.
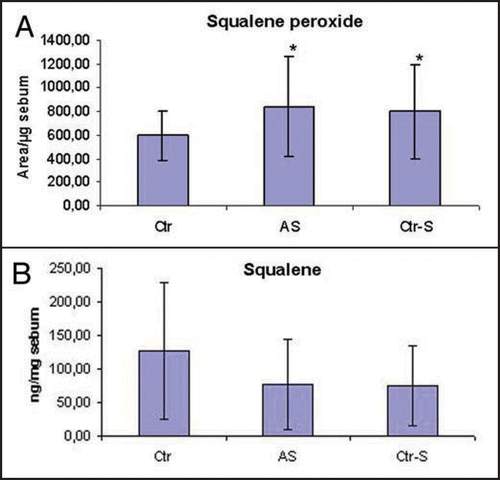
Figure 7 Smokers affected by acne show a significant increase in sebum excretion in comparison with non smokers, affected or not affected by acne (p = 0.04). Ctr, healthy non smokers; AS, acne-smokers; Ctr-S, healthy smokers.
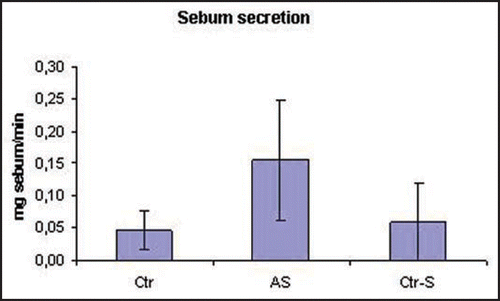
Figure 8 Hematoxylin-eosin 4x; (A) patient A, 30-year-old: open comedone; presence of inflammatory infiltrate and absence of elastosis; (B) patient B, 36-year-old: closed comedone, scarce inflammatory infiltrate and considerable elastosis.
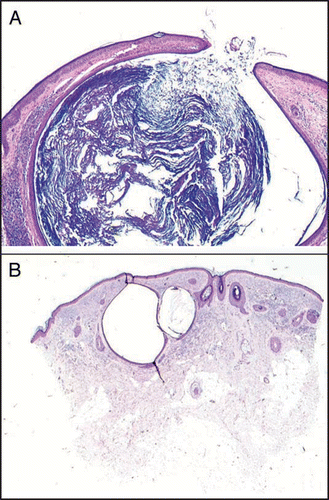
Table 1 Classification criteria
Table 2 Post-adolescent acne and smoking
Table 3 Additional factors possibly associated with post-adolescent acne
References
- Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity and severity risk factors of acne in high school pupils: A community-based study. J Invest Dermatol 2009; In press
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol 2006; 7:281 - 290
- Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol 1997; 136:66 - 70
- Misery L. Nicotine effects on the skin: are they positive or negative?. Exp Dermatol 2004; 13:665 - 670
- Ko JS, Kang H, Woo Choi S, Kim HO. Cigarette smoking associated with premature facial wrinkling: image analysis of facial skin replicas. Int J Derm 2002; 41:21 - 27
- Pelle E, Miranda EP, Fthenakis C, Mammone T, Marenus K, Maes D. Cigarette smoke-induced lipid peroxidation in human skin and its inhibition by topically applied antioxidants. Skin Pharmacol Appl Skin Physiol 2002; 15:63 - 68
- Thiele JJ, Packer L. Non-invasive measurement of alpha-tocopherol gradients in human stratum corneum by HPLC analysis of sequential tape strippings. Methods Enzymol 1999; 300:413 - 419
- Ekanayake Mudiyanselage S, Hamburger M, Elsner P, Thiele JJ. Ultraviolet A induces Generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J Invest Dermatol 2003; 120:915 - 922
- Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in general population: the risk of smoking. Br J Dermatol 2001; 145:100 - 104
- Chuh AA, Zawar V, Wong WC, Lee A. The association of smoking and acne in men in Hong Kong and in India: a retrospective case-control study in primary care settings. Clin Exp Dermatol 2004; 29:597 - 599
- Mills CM, Peters TJ, Finlay AY. Does smoking influence acne?. Clin Exp Dermatol 1993; 18:100 - 101
- Klaz I, Kochba I, Shohat T, Zarka S, Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol 2006; 126:1749 - 1752
- Cunliffe WJ, Holland DB, Jeremy A. Comedone formation: etiology, clinical presentaton and treatment. Clin Dermatol 2004; 22:367 - 374
- Patterson WM, Fox MD, Schwartz RA. Favre-Racouchot disease. Int J Dermatol 2004; 43:167 - 169
- Grando SA, Horton RM, Mauro TM, Kist DA, Lee TX, Dahl MV. Activation of Keratinocyte nicotinic cholinergic receptors stimulates calcium influx and enhances cell differentiation. J Invest Dermatol 1996; 107:412 - 418
- Theilig C, Bernd A, Ramirez-Bosca A, Görmar FF, Bereiter-Hahn J, Keller-Stanislawski B, et al. Reactions of human keratinocytes in vitro after application of nicotine. Skin Pharmacol 1994; 7:7 - 315
- DI Carlo A, Ippolito F. Early effects of sigarette smoking in hypertensive and normotensive subjects. An ambulatori blood pressare and thermographic study. Minerva Cardioangiol 2003; 51:387 - 393
- Monfrecola G, Riccio G, Bavarese C, et al. The acute effect of smoking on cuteneous microcirculation blood flow in habitual smokers and non smokers. Dermatology 1998; 197:115 - 118
- Sopori ML, Kozak W, Savage SM, Geng Y, Kluger MJ. Nicotine-induced modulation of T cell function. Implication for inflammation and infection. Adv Exp Med Biol 1998; 437:279 - 289
- Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids and retinol in human plasma by cigarette smoke. Am J Clin Nutr 1996; 63:559 - 565
- Passi S, De Pità O, Puddu P, Littarru GP. Lipophilic antioxidant in human sebum and aging. Free Radic Re 2002; 36:471 - 477
- Ekanayake-Mudiyanselage S, Thiele JJ. Sebaceous glands are transporters of vitamin E. Hautarzt 2006; 57:291 - 296
- Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is the major physiologic route of vitamin E delivery to skin. J Invest Dermatol 1999; 113:1006 - 1010
- Zouboulis CC, Nestoris S, Adler YD, Orth M, Orfanos CE, Picardo M, Camera E, et al. A new concept for acne theraphy: a pilot study with Zileuton, an oral 5-lipoxygenase inhibitor. Arch Dermatol 2003; 139:668 - 670
- Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes. A possible role in acne vulgaris. J Invest Dermatol 2006; 126:2430 - 2437
- Chiba K, Yoshizawa K, Makino I, Kawakami K, Onoue M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J Toxicol Sci 2000; 25:77 - 83
- Just M, Monsó E, Ribera M, Lorenzo JC, Morera J, Ferrandiz C. Relationships between lung function, smoking and morphology of dermal elastic fibres. Exp Dermatol 2005; 14:744 - 751
- Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ. Cigarette smoking: risk factor for premature facial wrinkling. Ann Int Med 1991; 114:840 - 844
- Lange P, Schnohr P. The relationship between facial wrinkling and airflow obstruction. Int J Dermatol 1994; 33:123 - 126
- Patel BD, Loo WJ, Tasker AD, Screaton NJ, Burrows NP, Silverman EK, et al. Smoking related COPD and facial wrinkling: is there a common susceptibility?. Thorax 2006; 61:568 - 671
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999; 41:577 - 580
- Kligman AM. A critical look at acne cosmetica. J Cutan Ageing Cosmet Dermatol 1988; 109:109 - 112
- Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, et al. Corticotropin releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci USA 2002; 99:7148 - 7153
- Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin- releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol 2009; 160:345 - 352