Abstract
Skin epidermis is an active site of lipid synthesis. The intercellular lipids of human stratum corneum (SC) are unique in composition and quite different from the lipids found in most biological membranes. The three major lipids in the SC are free fatty acids, cholesterol and ceramides. Fatty acids can be synthesized by keratinocytes de novo and, in addition, need to be taken up from the circulation. The latter process has been shown to be protein mediated, and several fatty acid transporters are expressed in skin. Recent studies of transgenic and knockout animal models for fatty acid transporters and the identification of fatty acid transport protein 4 (FATP4 or SLC27A4) mutations as causative for Ichthyosis Prematurity Syndrome highlight the vital roles of fatty acid transport and metabolism in skin homeostasis. This review provides an overview of our current understanding of the role of fatty acids and their transporters in cutaneous biology, including their involvement in epidermal barrier generation and skin inflammation.
Introduction
The skin is a complex and highly specialized organ serving multiple functions in the body, the main of which is to provide a barrier that prevents water loss and protects the body from adverse environmental agents.Citation1 This function is mediated exclusively by the stratum corneum (SC)—the outermost layer of the epidermis, consisting of dead flattened keratinocytes embedded in a lipid matrix, acting together as a “brick and mortar” system that is difficult to penetrate. The lipids that constitute the extracellular lamellar matrix of the stratum corneum have a unique composition and exhibit distinctive properties.Citation2 The major lipids of the human SC are ceramides, cholesterol and fatty acids (FAs), comprising approximately 50, 25 and 15% of the total lipid mass, respectively.Citation3 Interestingly, this mass composition reflects nearly equimolar quantities of ceramides, cholesterol and fatty acids, a ratio that is imperative for normal lamellar membrane organization and epidermal barrier homeostasis.Citation4,Citation5 Both essential and non-essential fatty acids play separate and critical roles in proper skin function. In this review we will focus on the role of fatty acids and their transporters in skin epidermal barrier functions and disease.
Origin of Fatty Acids in the Epidermis
The epidermis is a very active site of lipid synthesis, exceeding even the liver, kidney and gastrointestinal epithelia on a per weight basis.Citation6 Most of the FAs can be synthesized by keratinocytes de novo. FAs with carbon chains of up to C16 are synthesized by cytosolic fatty acid synthase (FAS). In normal human epidermis, FAS is strongly expressed in the stratum granulosum and moderately in the uppermost layer of the stratum spinosum, suggesting that FA synthesis may increase during normal epidermal differentiation.Citation7 Acute disruption of the epidermal barrier stimulates FA synthesis, which is required for full recovery of the barrier.Citation8 A significant amount of the FAs produced by FAS, as well as FAs taken up from diet, are further elongated into very long chain FAs (C ≥18).Citation9 The role of FA elongation in epidermal function will be discussed in detail in the review by Dr. Y. Uchida in this series. We will only mention that during cornification, short-chain FAs are replaced by long-chain, highly saturated species, ranging from 14 to 28 carbons in length. The majority are 20 carbons and longer, with 22–24 carbon lengths being the most abundant.Citation3
However, not all fatty acids can be synthesized by keratinocytes, and not all that can be synthesized are produced in sufficient quantities. Research performed more than 80 years ago show that essential fatty acid (EFA) deficiency leads to abnormalities in skin function, resulting in scaly dermatosis, permeability of skin to water and hair loss.Citation10 The human body can produce all but two of the fatty acids it needs: linoleic acid (C18:2, n6) and alpha-linolenic acid (C18:3, n3). In addition to these, nutritional studies have revealed the existence of so called conditional EFAs, such that the term EFA may be applied to over thirty omega-6 and omega-3 polyunsaturated fatty acids.Citation11 Skin is devoid of delta-5 desaturase and so cannot directly convert gamma-linolenic acid to arachidonic acid. Thus, arachidonic acid (C20:4, n6) in the epidermis is not of keratinocyte origin, but must be synthesized in the liver and transported into keratinocytes therefore being an essential fatty acid for the skin. In addition to linoleic and arachidonic acids, several long chain- and very long chain-fatty acids (LCFA and VLCFA) were identified as EFAs for the skin, and they also have to be translocated across the keratinocyte plasma membrane.
Role of Fatty Acids in the Epidermis
FAs have multiple roles in the epidermis. They are found in bound form in triglycerides, phospholipids, glycosylceramides and ceramides, which are all playing a vital role in formation of the epidermal permeability barrier.Citation12 However, FAs in keratinocytes do not function only as building blocks. In addition to their well known role in energy generation and storage, FAs can be potent signaling molecules, activating the nuclear hormone receptors peroxisome proliferators-activated receptors (Pars).Citation13–Citation15 Moreover, by acylation FAs may modify other signaling molecules, including Sonic hedgehog and Wnt,Citation16 though the role of such modifications in epidermal biology is not well studied. There are a few recent studies concerning the role of protein palmitoylation in skin.Citation17,Citation18
EFAs are integral for normal stratum corneum structure and function. Three acylceramide species unique to epidermis contain linoleic acid esterifies to the terminal omega-hydroxyl group of very long chain fatty acids (C ≥28–34). These ceramides are central to barrier function and essential for mammalian survival.Citation12 Substitution of the linoleic acid with non-essential oleic acid, as in the case of EFA deficiency, will lead to barrier defects accompanied by proliferative epidermal changes.Citation19 Atopic dermatitis and psoriasis, well known inflammatory skin diseases, are characterized by changes in FA composition in keratinocytes, indicating their possible involvement in modulating of inflammatory processes.Citation20–Citation25 Moreover, free FAs are one of the major contributors to the acidic pH at the SC surface regulating permeability and antimicrobial barrier, inflammation and desquamation.Citation26–Citation28 And last, but not least, there is accumulating evidence for the existence of an epidermis-specific hepoxilin pathway for arachidonic acid utilization in keratinocytes, which is important for formation of functional epidermis.Citation29–Citation31 These genes include two lipoxygenases (ALOX12B, ALOXE3), an ATP binding cassette transporter (ABCA12), a potential receptor (ICHTHYIN) and a gene coding for a protein of the cytochrome P450 family (CYP4C22).Citation29,Citation32–Citation34 Mutations in these genes, as well as mutations in transglutaminase 1 (TG1), have been reported as the most common cause of autosomal recessive congenital ichthyosis (ARCI). However, 20–40% of the patients have no mutations in the six known ARCI genes suggesting that more genes are involved in this type of ichthyoses.Citation30,Citation35 The role of lipoxygenases in formation of the epidermal barrier will be reviewed in details by Alan Brash in this series of reviews.
Transport of Fatty Acids to the Epidermis
There are still ongoing debates regarding the mechanisms by which free FAs are transported across cell membranes. On the one hand, due to the lipophilic nature of FAs, it has been proposed that they may be passively transported through the lipid bilayer by “flip-flop” diffusion. This mechanism does not involve any protein mediators.Citation36,Citation37 On the other hand, many studies have provided considerable support for the protein-mediated entry of long chain fatty acids.Citation38 While the exact mechanism by which FAs are preferentially transported into cells is not yet settled, apparently both modes of FA uptake (diffusion or protein-dependant) may coexist in mammalian cells. The preferential mode of transport may depend on: (1) the nature of the membrane (plasma membrane organelle or vesicular membrane); (2) cell type (e.g., adiposities, neurons and keratinocytes may have different systems for FA transport); and (3) functional state of the cell (e.g., resting, activated, proliferative or cancer cells of the same origin may employ different mechanisms). Recently, a unified hypothesis for the role of the lipid bilayer and proteins was proposed for transport of FA into brain cells.Citation39 However, due to space limitations we will not discuss the different models for fatty acid transport in details, but rather refer to several comprehensive reviews which cover this topic.Citation38,Citation40–Citation43
There are several lines of evidence suggesting that keratinocytes must import fatty acids from extracutaneous sites. In addition to EFAs which by definition must be obtained from the diet, eicosapentaenoic acid (C22:6, n3) and docosahexaenoic acid (C20:5, n3) derived from dietary fish oil are incorporated into epidermal lipids.Citation44 Second, plant-derived fatty acids accumulate in the epidermis in certain diseases states, such as in Résumé's disease.Citation45 Third, studies have shown that systemically administered 14C-labelled FAs were delivered to the epidermis.Citation46 Fourth, inhibition of FA synthesis in the epidermis does not completely block barrier recovery after acute disruption, indicating that several sources of fatty acids are available to the keratinocytes.Citation5 And finally, studies have shown the active uptake of FAs by keratinocytes, which is temperature sensitive, has saturable kinetics, and can be reduced by prior treatment with try sin, indicating protein-mediated FA uptake.Citation47 The latter study demonstrated that keratinocytes transport EFAs, i.e., linoleic acid and arachidonic acid, with higher specificity than for non-essential FA, such as oleic acid (C18:1, n9). These transport preferences were not shared with other cell types, such as hepatocytes and dermal fibroblasts, which transport non-essential and essential FA with similar kinetics. However, identity of the protein(s) responsible for FA uptake in keratinocytes was not determined in this study.Citation47
In recent years, a number of proteins have been identified that in some way may facilitate the LCFA transport in mammalian cells. They include fatty acid translocase (CD36/FAT),Citation48 fatty acid transport proteins (FATPs),Citation49 fatty acid binding proteins (FABPs),Citation50,Citation51 long chain fatty acid-CoA synthetics (ACSLs),Citation52 and acyl-CoA binding proteins (ACBPs).Citation53 Despite the fact that these proteins have a different tissue expression pattern and subcellular localization, there are evidences to suggest that each of these transporters can independently increase FA uptake (see references above). In addition, it was proposed that FA transport can occur through the combination of caveolin and CD36/FAT in conjunction with lipid rafts.Citation54,Citation55 Finally, an unknown protein has been reported recently to be involved in uptake of FAs by adipocytes.Citation56
How proteins contribute to FA uptake is again highly controversial. It was proposed that FATPs and CD36/FAT are the only real transporters directly involved in binding and transport of FAs across membranes.Citation57,Citation58 Moreover, they may interact in such a way that FA binds first to CD36, which subsequently deliver the free FA to the FATPs.Citation38 However, whether CD36/FAT and FATPs are directly involved in the translocation process is not clear. Alternatively, these transporters may be indirectly involved in FA uptake by binding and creating high local concentrations of free FAs in close proximity to the membrane, as for CD36/FAT,Citation59 or by vectorial acylation, as for FATPs.Citation43
After translocation to cytosol almost all FAs need to be activated by esterification with coenzyme A (CoA) before they can be utilized, a process which is catalyzed by a family of enzymes called acyl-CoA synthetases.Citation60 FATPs possess acyl-CoA synthetase activity, generally preferring 16–18 carbon FAs, but can also activate FAs as long as 26 carbons.Citation61–Citation64 Activation of FAs into CoA-forms diminish the intracellular pool of free FAs, thus creating a gradient across the membrane and enhancing influx of extracellular FAs, a process called vectorial acylation.Citation43,Citation65,Citation66 The majority of the substrate (FA) and the end product (acyl-CoA) of the enzymatic reaction do not exist free in the cytosol under physiological conditions, but are rather bound to FABPsCitation67 or ACBPs.Citation53 Interestingly, all proteins in this pathway have been reported to mediate FA uptake, indicating the existence of tightly regulated mechanisms controlling concentration of the free FAs and the free acyl-CoA forms in the cytosol. Deficiency in ACSLs or in FABPs will lead to accumulation of LCFAs in the cytosol and indirectly decrease FA uptake. Deficiency in ACBPs will create local high concentration of free acyl-CoA, which may inhibit further esterification and indirectly affect FA transport. In addition, both free LCFAs and free acyl-CoA are potent signaling molecules which may regulate expression of genes involved in lipid metabolism through activation of nuclear hormone receptors. Moreover, if present in high concentrations, free FAs will lead to lipotoxicity and ultimately to cell death.Citation60 It is clear therefore, that the ability to control FA uptake and concentration in the cytosol is vital for cell functions and survival.
Surprisingly, taking into account the high rate of lipid synthesis in keratinocytes, only a few studies have been published reporting differential expression of putative FA transporters in epidermis and skin appendages.Citation68–Citation71 These studies have shown that FATP1, -3, -4 and -6, along with CD36/FAT, are expressed in adult murine epidermis. Interestingly, FATP1 and -3 were expressed predominantly by keratinocytes, whereas FATP4 was strongly expressed by sebaceous glands and FATP6 by hair follicle epithelium.Citation70 As a result of permeability barrier disruption in mice, increase in expression of FATP1 and -6 have been observed, as well as robust increase in CD36 protein and mRNA.Citation69,Citation70 FATPs expression in humans was comparable to that in mice. Experiments with primary human keratinocytes show that the major FATP expressed in culture was FATP4, and induction of differentiation induced by high calcium conditions (1.2 mM Ca2+) results in approximately 50% reduction in the level of FATP4 protein. In marked contrast to human and mouse epidermis, neither FATP1, -3 nor -6 were expressed in either undifferentiated or differentiated human keratinocytes.Citation70 In another study, in addition to FATPs and CD36/FAT, plasma-membrane FABPs and fatty acyl-CoA synthetase have been reported at different levels in undifferentiated and differentiated human keratinocyte cultures, as well as in mouse epidermis.Citation69
The crucial role for proteins in the efficient uptake of LCFAs was highlighted by a number of mouse models with impaired or enhanced FA transport. In the next section we will discuss cutaneous manifestations in these models as well as clinical skin abnormalities that occurs secondary to mutations in FA transporters.
Role of Fatty Acid Transporters in Skin: Mouse Models and Possible Clinical Involvement
Among the number of mouse models with defective FA metabolismCitation72,Citation73 we will in this review discuss only “classical” transporters such as CD36/FAT, FABPs and FATPs.
CD36/FAT.
CD36 is an integral transmembrane glycoprotein with molecular mass of 88 kea in its fully glycosylated form, functioning as a scavenger receptor for oxidized low-density lipoproteins with multiple function in different cell types.Citation74 CD36 was proposed to be a crucial transporter for long-chain fatty acids mediated through interaction with lipid rafts and caveolin.Citation54,Citation55,Citation75 It is expressed in tissues with high FA metabolism, including adipose tissue, heart and skeletal muscles.Citation48,Citation74 CD36 has been also reported to be weakly expressed in normal epidermis, with increased expression after barrier disruption.Citation70 However, CD36 knockout mice do not have any apparent skin phenotype, while defective uptake and utilization of LCFA have been reported in muscles and adipose tissues.Citation76–Citation78 Recent finding indicate that CD36 mediates uptake of LCFAs, VLCFAs and cholesterol in the intestine.Citation79,Citation80 Interestingly, CD36 has four palmitoylation sites on both N- and C-termini, and it was shown that palmitoylation plays a crucial role in targeting CD36 to lipid rafts, where it mediates its function.Citation81 In humans, CD36 deficiency has a prevalence of 0.3–11%, with higher incidences in Asian and African populations,Citation82 while again a skin phenotype was not obvious.Citation83 Apparently, CD36-mediated uptake of FAs in keratinocytes can be easily compensated by other FA transporters.
FABP5.
FABPs can be divided into two main groups: those associated with plasma membrane (FABP-pm) and with intracellular, cytoplasmic proteins (FABPc). So far, nine tissue-specific cytoplasmic FABPs with molecular mass 14–15 kea have been identified, including cytosolic epidermal FABP (FABP5, also termed E-FABP or PA-FABP).Citation67 In contrast, FABP-pm has a higher molecular weight (43 kea) and seems to be identical to mitochondrial aspartame aminotransferase.Citation84 Overexpression of FABP-pm in mammalian tissues can increase uptake of FAs,Citation85 and in human keratinocytes induction of differentiation by high calcium reduced FABP-pm mRNA by 50%.Citation69 However, whether this finding is relevant to the regulation of FA uptake into keratinocytes was not studied. Another study have shown that antibodies directed against FABP-pm did not inhibit FA uptake in human keratinocytes, but did inhibit its uptake in hepatocyte cell line HepG2, indicating that FABP-pm is unlikely to be responsible for the bulk of transport activity in keratinocytes.Citation47
More is known about FABP5 which is predominantly expressed in keratinocytes, where it has been proposed to act as a cellular lipid chaperone in keratinocytes homeostasis.Citation86,Citation87 FABP5 has been detected also in tongue and thymus epithelia, as well as in mammary and adipose tissues, while it seems that FABP5 is the only FABP which is detected in epidermis.Citation87–Citation90 Compared with other tissues active in lipid metabolism, human keratinocytes contain a surprisingly low amount of FABP5, and its specific role in normal skin is not yet fully established. The amount of FABP5 increases with keratinocyte differentiation and immunohistochemical studies have demonstrated that FABP5 expression is strongest in stratum granulosum, where activity of rate limiting enzymes required for barrier formation is the highest.Citation86,Citation91–Citation93 Overexpression of FABP5 is associated with highly hyper-proliferative skin conditions, such as psoriasis, atopic dermatitis and basal and squamous cell carcinomas.Citation94–Citation96 It can be speculated that FABP5 expression is increased in response to increased lipid traffic, which may be related to abnormal proliferation and differentiation of keratinocytes in such conditions.
The skin of FABP5 knockout mice appears normal at the gross and histological level, but the water-barrier function of the epidermis is altered in these mice.Citation89,Citation97 It was shown that FABP5 deletion results in impaired keratinocyte migration, suggesting a connection between FABP5 and cell motility.Citation98 The lack of obvious skin phenotype raises the possibility that other FABP member or even other FA transporters can compensate for FABP5 deficiency during development.
Recent study by Ogawa and colleguesCitation90 helps to clarify the mechanism of FABP5 action in epidermis. It was shown that FABP5 deletion affects keratinocyte differentiation, but not proliferation. Total FA content in FABP5-deficient epidermis was decreased including decreased saturated, monounsaturated and polyunsaturated FAs. Detailed examination revealed that linoleic acid (C18:2, n6) was significantly decreased, while arachidonic and linolenic acid content was unchanged. Furthermore, the authors showed that a linoleic acid derivative, 13-hydroxyoctadecadienoic acid [13(S)-HODE], mediates keratinocyte differentiation by activating the NFκB pathway. Consequently, FABP5 deletion will lead to decreased cellular linoleic acid and 13(S)-HODE contents, resulting in downregulation of NFκB activity and decreased differentiation.Citation90 Given the strong expression of FABP5 in hyperproliferative skin conditions, it can be suggested that FABP5 may have a role in the pathogenesis of such diseases through impaired metabolism of FAs and their derivates. Except for hyperproliferation in keratinocytes, no other clinical conditions caused by either deficiency of overexpression of FABP5 have been reported so far in humans.
FATP4.
Recently, mutations in the FATP4 gene (also known as SLC27A4) were identified as causative for Ichthyosis Prematurity Syndrome (IPS).Citation99 IPS is a rare disorder of cornification belonging to the heterogeneous group of autosomal-recessive congenital ichthyoses (ARCI).Citation100,Citation101 Ultrastructural investigation of the first IPS patients identified in Norway supported the idea that this was a distinct type of congenital ichthyosis, termed ichthyosis congenita type IV.Citation102 IPS has been described almost exclusively in a region in the middle of Norway and Sweden where the estimated heterozygote carrier frequency is 1 in 50.Citation103 Outside of this region, only a few cases have been reported in other European countries, including Germany, Finland, Italy, Denmark and France, as well as in families from North Africa and Middle East.Citation99,Citation104–Citation107 IPS is characterized by the clinical triad of premature birth, scaly erythroderma and neonatal asphyxia in combination with pathognomonic ultrastructural findings, typically showing lipid membrane packages in the granular and horny cells.Citation102,Citation104 The severe skin phenotype at birth significantly improves during the first weeks of life, and recovers into a lifelong non-scaly ichthyosis with dermal atopic dermatitis-like inflammation and severe itching (D. Khnykin et al. unpublished). Interestingly, most of the Norwegian patients have extremely high numbers of eosinophils in peripheral blood and very high serum-Age levels; both are central to etiology of atopic disorders (D. Khnykin et al. unpublished).
FATP4 knockout mice also show a dramatic skin phenotype, together highlighting the importance of this transporter for skin homeostasis.Citation108,Citation109
FATP4 belongs to the family of membrane associated FATPs consisting of six members, designated FATP1-6.Citation49 They are proposed to mediate the translocation of FAs from the extracellular milieu into cells. However, not all members were able to complement FA uptake in yeast studies.Citation110,Citation111 In addition, all six proteins have acyl-CoA synthetase activity and are able to activate LCFAs and VLCFAs to their CoA hoisters for subsequent metabolism.Citation49,Citation112,Citation113 The mechanism by which FATPs mediate FA uptake is not well understood, and it remains to be determined whether they participate in the physical translocation process or facilitate transport by trapping, as CoA derivatives, fatty acids that enter cells by diffusion.Citation38,Citation54,Citation114,Citation115
Several FATPs, including FATP4, are expressed in the epidermis,Citation70 and animal models suggest that FATP4 plays an important role in skin homeostasis. FATP4 knockout mice die either in uteroCitation116 or shortly after birth,Citation108,Citation109 showing disturbed epidermal barrier and hyperkeratosis resulting in very tight, thick skin (wrinkle free phenotype). Given the well-known importance of lipids to the epidermal barrier,Citation2,Citation117 it was reasonable to hypothesize that the absence of FATP4 led to abnormal lipid trafficking and failed production of the lipids required to establish a proper skin barrier. Indeed, FATP4 was shown to be expressed in the epidermis primarily in the granular and upper spinous layers where epidermal barrier lipid synthesis is robust.Citation109,Citation118 Furthermore, analysis of epidermal lipid composition by mass spectrometry in the absence of FATP4 showed an increase in the fraction of ceramides with fatty acid moieties containing 24 or fewer carbon atoms, at the expense of those containing 26 or more.Citation109,Citation118 Ceramides are crucial for barrier formationCitation2,Citation12 so this change in creamed composition likely contributes to the barrier abnormalities. Studies of dermal fibroblast cell lines established from FATP4 knockout mice revealed that FATP4 is in fact a principal VLCFA acyl-CoA synthetase in skin fibroblasts.Citation63 However, mutant fibroblasts also showed reduced uptake of LCFAs and a reduced level of long chain polyunsaturated FAs. Taking into account that FATP4-deficient cells also contain abnormal neutral lipid droplets, this indicates that the metabolic abnormalities in these cells are likely not limited only to VLCFAs.Citation63 Despite the high expression of FATP4 in other tissues, such as intestine and brain,Citation112,Citation119 it was possible to rescue the lethal phenotype of FATP4 knockout mice by keratinocyte-specific transgenic expression of FATP4, resulting in viable and fertile mice with only mild skin and hair abnormalities.Citation118 This suggests that the skin phenotype of FATP4 mutants is indeed due to the absence of FATP4 from epidermis. No structural or functional defects in other tissues have been observed in the transgene-rescued mutants that lack FATP4 expression in non-epidermal tissues.Citation118 Importantly, keratinocytes-specific expression of FATP4 mutated in the acyl-CoA synthetase domain did not rescue the skin phenotype, indicating that the ability of FATP4 to activate fatty acids is a crucial for its function.Citation118
Another strategy was chosen by Herrmann et al.Citation120 who created mice with conditional FATP4 deficiency in the epidermis. This mouse line displays distinct changes in the structure of the epidermis and a compromised barrier function, but the phenotype was not nearly as severe as that seen in mutant neonates.Citation120 Interestingly, IPS patients carrying mutations in FATP4 gene show much more severe phenotype immediately after birth and in neonatal period, and show a mild form of ichthyosis in later life (see below). The explanation for this apparent difference could be that in adult skin FATP4 is compensated by other FATPs. As was discussed above, adult skin expresses several FATPs, including FATP1, -3, -4 and -6,Citation70 and FATP1 and 3 are predominantly expressed by keratinocytes. However, studies of embryonic expression at day 18.5 revealed that FATP1 is not expressed in epidermis, whereas expression of FATP4 was relatively increased compared with expression in adult epidermis.Citation70 It can be speculated therefore, that FATP4 is more critical for the generation of the epidermal barrier and less important for its maintenance, because after birth the other FATPs may partially compensate for the deficiency of FATP4.
To gain insights into why the absence of FATP4 in utero causes the severe skin defects in mice, gene expression profiling was used to search for significant changes at embryonic day 15.5.Citation121 This is the gestational age at which the wrinkle free phenotype first becomes detectable in some but not all Fatp4 mutants; thus, the hope was that any changes observed would be directly related to the absence of FATP4 rather than secondary to the abnormal skin phenotype. The results suggested that there was premature expression of genes relevant to establishing the barrier, and this was validated by the presence of a precocious but incomplete skin barrier at E16.5 that never progressed into a complete barrier.Citation121 Four members of the epidermal growth factor (EGF) family of ligands were also upregulated. This was associated with increased activation of the EGF receptor (EGFR) and of at least one of its downstream effectors, signal transducer and activator of transcription-3 (STAT3). Pharmacological studies in vivo with curcumin and AG1478, inhibitors of EGFR and/or STAT3 signaling, showed that activation of EGFR and STAT3 caused suprabasal keratinocyte hyperproliferation and thickening, as well as precocious barrier formation.Citation121 However, the lack of skin wrinkles was not ameliorated, indicating that other signaling pathways must contribute to that aspect of the phenotype. Despite these insights into the signaling pathways associated with the skin defects, why the absence of FATP4 leads to their activation (i.e., to the overexpression of EGF-like ligands) is an important remaining question.
FATP4 deficient mice models showed a non redundant role for FATP4 in epidermis, comparing with other tissues,Citation122,Citation123 indicating the unique, specific role of FATP4 in generation and maintenance of the skin barrier.
The FATP4 gene (also known as SLC27A4) consists of 1 non-coding and 12 coding exons, encoding a protein of 643 residues with a predicted size of 71 kDa.Citation124 FATP4 is highly conserved among widely divergent species and contains an N-terminal transmembrane (TM) region, an ER localization signal (ERx), as well as two functioning domains: ATP/AMP motif, responsible for ATP binding and FATP/VLACS motif, possessing very long chain acyl-CoA synthetase activity and contributing to fatty acid transport.Citation111 Molecular genetic analysis of DNA from members of 18 Scandinavian IPS patients revealed that they all were either homozygous or compound heterozygous for a nonsense mutation in exon 3 of the FATP4 gene, whereas a North African patient and a patient from the Middle East were homozygous for a splice site mutation in exon 5 and a missense mutation in exon 12, respectively.Citation99 Two new mutation in the FATP4 gene leading to IPS were identified recently: missense mutation c.1120C>T in a highly conserved region of exon 8,Citation107 and a homozygous A>G mutation in exon 2 (c.1a>g) which leads to conversion of the initiation codon ATG to GTG (p.M1V) (Inhoff et al. in press).
All together, nine mutations in FATP4, affecting several domains of FATP4 protein, have been found so far in IPS patients worldwide. The most frequent mutation is located in exon 3.Citation99 If the mRNA for this allele is translated it will produce truncated protein without any FA transport or acyl-CoA synthetase activity.Citation111 Of interest, mutation in mouse Fatp4 leads to generation of a protein containing the first 133 amino acids of FATP4 fused to 50 amino acids encoded by retrotransposon antisense RNA,Citation108 which will also lack acyl-CoA synthetase activity. However, the mice exhibit a much more severe, lethal phenotype, which may be due to a higher surface to volume ratio as compared with humans, leading to excess loss of water through the skin.
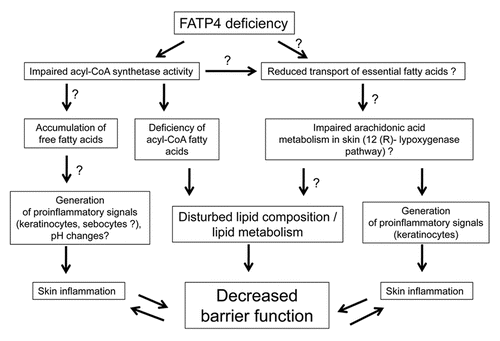
The mechanism by which FATP4 deficiency leads to a defective barrier and skin inflammation is not known and is only beginning to be elucidated. Some of the possible mechanisms are shown in .
It would be tempting to speculate that impaired acyl-CoA synthetase activity is the major trait associated with IPS pathology. FATP4 carrying a mutated acyl-CoA domain is functionally inactive in vivo and not able to rescue the skin phenotype in FATP4 deficient mice, indicating a critical role for FA esterification by FATP4 in skin barrier formation.Citation118 Indeed, tissues from FATP4 deficient mice, as well as both human and mouse FATP4 deficient fibroblasts, show a decreased activation and incorporation of very VLCFAs into complex structural lipids.Citation62,Citation63,Citation99 Analysis of lipid composition from FATP4 deficient epidermis revealed deficiency of VLCFAs (C ≥26) in epidermal ceramides,Citation109,Citation118 which might affect barrier properties of the skin. But barrier function may not be the only aspect of the epidermis affected by the lack of FATP4 activity. The inability of FATP4 to activate VLCFA and LCFA could lead to intracellular accumulation of free FAs in the cell, leading to ER-stress and activation of proinflammatory pathways.Citation60
Mouse FATP4 shows considerable activity in the esterification of arachidonic acid in uptake- and activation-studies in yeast.Citation110,Citation111 Moreover, the level of arachidonic acid was almost 50% lower in FATP4 deficient fibroblasts compared to control cells, while the level of linoleic acid was unchanged.Citation63 Arachidonic acid is a precursor of potent pro- and anti-inflammatory eicosanoids, and it appears that the 12(R)-lipoxygenase pathway of arachidonic acid utilization plays a critical role in epidermal barrier formation.Citation30,Citation31 Therefore, it cannot be excluded that altered arachidonic acid metabolism are directly involved in the skin pathology observed in humans and mice with FATP4 deficiency.
Given the highly purity nature of IPS, another possible factor affecting the phenotype could be related to regulation of skin pH. The changes in stratum corneum pH may be due to accumulation of free FAs, generated both in epidermis and sebaceous glands. Local changes in pH can activate proteases and their receptors in inappropriate temporal and spatial patterns, leading to inflammation and itching.Citation125–Citation127 One more family of proteins which are expressed in epidermis and are pH-sensitive is the transient receptor potential (TRP) channel family. Members of this family has been shown to be involved in the pathogenesis of itchCitation128–Citation130 and in skin barrier formation, where it regulates EGFR signaling.Citation131
Finally, proinflammatory signals may be generated not only in epidermis but also in skin appendages. FATP4 is highly expressed in sebaceous glands,Citation70 and absence of its acyl-CoA synthetase activity may lead to accumulation of free fatty acids in sebocytes, as well as in keratinocytes. Sebum free FAs may enhance production and secretion of antimicrobial peptides, which in turn may stimulate production of proinflammatory cytokines and chemokines by keratinocytes.Citation132,Citation133
Concluding Remarks
Although the exact mechanism by which FAs are transported into keratinocytes remains unclear, studies of different FA transporters demonstrate their unique and important roles in the epidermis for maintaining skin barrier and keratinocyte homeostasis. Animal models of FATP4 deficiency, as well as human phenotype in IPS, have demonstrated a vital role for FATP4 in mammalian skin development and formation of epithelial barrier. Pathomechanism of FATP4 deficiency in skin is still poorly understood and clarifying of specific pathways affected by such deficiency may yield new insights for the role of fatty acid metabolism in epidermal biology.
Abbreviations
| FA | = | fatty acids |
| LCFA | = | very long chain fatty acids |
| VLCFA | = | very long chain fatty acids |
| SC | = | stratum corneum |
| FAS | = | fatty acid synthase |
| CD36/FAT | = | fatty acid translocase |
| FATP | = | fatty acid transport proteins |
| FABP | = | fatty acid binding proteins |
| ACSL | = | long chain fatty acid-CoA synthetics |
| ACBP | = | acyl-CoA binding proteins |
| ARCI | = | autosomal recessive congenital ichthyosis |
| IPS | = | ichthyosis prematurity syndrome |
References
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol 2008; 17:1063 - 1072
- Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 2007; 48:2531 - 2546
- Wertz P. PM E, Feingold K. Biochemistry of Human Statum Corneum Lipids, in Skin Barrier 2006; New York Taylor & Francis 33 - 42
- Man MQ, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol 1996; 106:1096 - 1101
- Man MQ, Feingold KR, Elias PM. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Arch Dermatol 1993; 129:728 - 738
- Feingold KR. The regulation and role of epidermal lipid synthesis. Adv Lipid Res 1991; 24:57 - 82
- Uchiyama N, Yamamoto A, Kameda K, Yamaguchi H, Ito M. The activity of fatty acid synthase of epidermal keratinocytes is regulated in the lower stratum spinousum and the stratum basale by local inflammation rather than by circulating hormones. J Dermatol Sci 2000; 24:134 - 141
- Man M, Elias PM, Feingold KR. Fatty acids are required for epidermal permeability barrier function. J Clin Invest 1993; 92:791 - 798
- Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 2006; 45:237 - 249
- Burr GOBM. On the nature and role of fatty acids essential in nutrition. J Biol Chem 1930; 86:587 - 621
- Cunnane SC. Problems with essential fatty acids: time for a new paradigm?. Prog Lipid Res 2003; 42:544 - 568
- Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci 2008; 51:77 - 87
- Schmuth M, Watson RE, Deplewski D, Dubrac S, Zouboulis CC, Griffiths CE. Nuclear hormone receptors in human skin. Horm Metab Res 2007; 39:96 - 105
- Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic Review Series: Skin Lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res 2008; 49:499 - 509
- Sertznig P, Seifert M, Tilgen W, Reichrath J. Peroxisome proliferator-activated receptors (PPARs) and the human skin: importance of PPARs in skin physiology and dermatologic diseases. Am J Clin Dermatol 2008; 9:15 - 31
- Steinhauer J, Treisman JE. Lipid-modified morphogens: functions of fats. Curr Opin Genet Dev 2009; 19:308 - 314
- Saleem AN, Chen YH, Baek HJ, Hsiao YW, Huang HW, Kao HJ, et al. Mice with alopecia, osteoporosis and systemic amyloidosis due to mutation in Zdhhc13, a gene coding for palmitoyl acyltransferase. PLoS Genet 2010; 6:1000985
- Mill P, Lee AW, Fukata Y, Tsutsumi R, Fukata M, Keighren M, et al. Palmitoylation regulates epidermal homeostasis and hair follicle differentiation. PLoS Genet 2009; 5:1000748
- Wertz PW, Cho ES, Downing DT. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rat. Biochim Biophys Acta 1983; 753:350 - 355
- Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr 2000; 71:361 - 366
- Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr 2000; 71:367 - 372
- McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol 2010; 28:440 - 451
- Sala-Vila A, Miles EA, Calder PC. Fatty acid composition abnormalities in atopic disease: evidence explored and role in the disease process examined. Clin Exp Allergy 2008; 38:1432 - 1450
- Ziboh VA, Miller CC, Cho Y. Significance of lipoxygenase-derived monohydroxy fatty acids in cutaneous biology. Prostaglandins Other Lipid Mediat 2000; 63:3 - 13
- Ziboh VA, Cho Y, Mani I, Xi S. Biological significance of essential fatty acids/prostanoids/lipoxygenase-derived monohydroxy fatty acids in the skin. Arch Pharm Res 2002; 25:747 - 758
- Bouwsta JA, Gooris GS, Dubbelaar FE, Ponec M. Phase behaviour of skin barrier model membranes at pH 7.4. Cell Mol Biol (Noisy-le-grand) 2000; 46:979 - 992
- Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol 2001; 117:44 - 51
- Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis and stratum corneum integrity/cohesion. J Invest Dermatol 2003; 121:345 - 353
- Lefevre C, Bouadjar B, Ferrand V, Tadini G, Megarbane A, Lathrop M, et al. Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet 2006; 15:767 - 776
- Fischer J. Autosomal recessive congenital ichthyosis. J Invest Dermatol 2009; 129:1319 - 1321
- Brash AR, Yu Z, Boeglin WE, Schneider C. The hepoxilin connection in the epidermis. Febs J 2007; 274:3494 - 3502
- Jobard F, Lefevre C, Karaduman A, Blanchet-Bardon C, Emre S, Weissenbach J, et al. Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet 2002; 11:107 - 113
- Lefevre C, Bouadjar B, Karaduman A, Jobard F, Saker S, Ozguc M, et al. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet 2004; 13:2473 - 2482
- Lefevre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, Boughdene-Stambouli O, et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet 2003; 12:2369 - 2378
- Eckl KM, de Juanes S, Kurtenbach J, Natebus M, Lugassy J, et al. Molecular analysis of 250 patients with autosomal recessive congenital ichthyosis: evidence for mutation hotspots in ALOXE3 and allelic heterogeneity in ALOX12B. J Invest Dermatol 2009; 129:1421 - 1428
- Pownall HJ, Hamilton JA. Energy translocation across cell membranes and membrane models. Acta Physiol Scand 2003; 178:357 - 365
- Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids 2006; 75:149 - 159
- Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology 2006; 21:259 - 268
- Hamilton JA, Brunaldi K. A model for fatty acid transport into the brain. J Mol Neurosci 2007; 33:12 - 17
- Hamilton JA. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot Essent Fatty Acids 2007; 77:355 - 361
- Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010; 90:367 - 417
- Bonen A, Chabowski A, Luiken JJ, Glatz JF. Is membrane transport of FFA mediated by lipid, protein or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical and physiological evidence. Physiology 2007; 22:15 - 29
- Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes and vectorial esterification. Microbiol Mol Biol Rev 2003; 67:454 - 472
- Ziboh VA, Cohen KA, Ellis CN, Miller C, Hamilton TA, Kragballe K, et al. Effects of dietary supplementation of fish oil on neutrophil and epidermal fatty acids. Modulation of clinical course of psoriatic subjects. Arch Dermatol 1986; 122:1277 - 1282
- Reynolds DJ, Marks R, Davies MG, Dykes PJ. The fatty acid composition of skin and plasma lipids in Refsum's disease. Clin Chim Acta 1978; 90:171 - 177
- Grubauer G, Feingold KR, Elias PM. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res 1987; 28:746 - 752
- Schurer NY, Stremmel W, Grundmann JU, Schliep V, Kleinert H, Bass NM, Williams ML. Evidence for a novel keratinocyte fatty acid uptake mechanism with preference for linoleic acid: comparison of oleic and linoleic acid uptake by cultured human keratinocytes, fibroblasts and a human hepatoma cell line. Biochim Biophys Acta 1994; 1211:51 - 60
- Coburn CT, Hajri T, Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J Mol Neurosci 2001; 16:117 - 121
- Gimeno RE. Fatty acid transport proteins. Curr Opin Lipidol 2007; 18:271 - 276
- Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta 2000; 1486:28 - 44
- Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 2010; 82:149 - 154
- Digel M, Ehehalt R, Stremmel W, Fullekrug J. Acyl-CoA synthetases: fatty acid uptake and metabolic channeling. Mol Cell Biochem 2009; 326:23 - 28
- Knudsen J, Neergaard TB, Gaigg B, Jensen MV, Hansen JK. Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J Nutr 2000; 130:294 - 298
- Pohl J, Ring A, Ehehalt R, Herrmann T, Stremmel W. New concepts of cellular fatty acid uptake: role of fatty acid transport proteins and of caveolae. Proc Nutr Soc 2004; 63:259 - 262
- Ehehalt R, Sparla R, Kulaksiz H, Herrmann T, Fullekrug J, Stremmel W. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biol 2008; 9:45
- Kampf JP, Parmley D, Kleinfeld AM. Free fatty acid transport across adipocytes is mediated by an unknown membrane protein pump. Am J Physiol Endocrinol Metab 2007; 293:1207 - 1214
- Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 2002; 239:181 - 192
- Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab 2001; 12:266 - 273
- Stremmel W, Pohl L, Ring A, Herrmann T. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids 2001; 36:981 - 989
- Li LO, Klett EL, Coleman RA. Acyl-CoA synthesis, lipid metabolism and lipotoxicity. Biochim Biophys Acta 2009; 1801:246 - 251
- Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem 1999; 274:36300 - 36304
- Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem 2005; 280:11948 - 11954
- Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J Biol Chem 2007; 282:20573 - 20583
- Mihalik SJ, Steinberg SJ, Pei Z, Park J, Kim DG, Heinzer AK, et al. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J Biol Chem 2002; 277:24771 - 24779
- Tong F, Black PN, Coleman RA, DiRusso CC. Fatty acid transport by vectorial acylation in mammals: roles played by different isoforms of rat long-chain acyl-CoA synthetases. Arch Biochem Biophys 2006; 447:46 - 52
- Black PN, DiRusso CC. Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found Symp 2007; 286:127 - 138
- Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 2006; 47:39 - 48
- Juhlin L. Expression of CD36 (OKM5) antigen on epidermal cells in normal and diseased skin. Acta Derm Venereol 1989; 69:403 - 406
- Harris IR, Farrell AM, Memon RA, Grunfeld C, Elias PM, Feingold KR. Expression and regulation of mRNA for putative fatty acid transport related proteins and fatty acyl CoA synthase in murine epidermis and cultured human keratinocytes. J Invest Dermatol 1998; 111:722 - 726
- Schmuth M, Ortegon AM, Mao-Qiang M, Elias PM, Feingold KR, Stahl A. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J Invest Dermatol 2005; 125:1174 - 1181
- Lisby S, Ralfkiaer E, Hansen ER, Vejlsgaard GL. Keratinocyte and epidermal leukocyte expression of CD36 (OKM5) in benign and malignant skin diseases. Acta Derm Venereol 1990; 70:18 - 22
- Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 2004; 279:11767 - 11776
- Lee L, DeBono CA, Campagna DR, Young DC, Moody DB, Fleming MD. Loss of the acyl-CoA binding protein (Acbp) results in fatty acid metabolism abnormalities in mouse hair and skin. J Invest Dermatol 2007; 127:16 - 23
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis and behavior. Sci Signal 2009; 2:3
- Ehehalt R, Fullekrug J, Pohl J, Ring A, Herrmann T, Stremmel W. Translocation of long chain fatty acids across the plasma membrane—lipid rafts and fatty acid transport proteins. Mol Cell Biochem 2006; 284:135 - 140
- Febbraio M, Guy E, Coburn C, Knapp F Jr, Beets AL, Abumrad NA, et al. The impact of overexpression and deficiency of fatty acid translocase (FAT)/CD36. Mol Cell Biochem 2002; 239:193 - 197
- Coburn CT, Knapp F Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 2000; 275:32523 - 32529
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274:19055 - 19062
- Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 2007; 282:19493 - 19501
- Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, et al. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 2008; 283:13108 - 13115
- Thorne RF, Ralston KJ, de Bock CE, Mhaidat NM, Zhang XD, Boyd AW, Burns GF. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta 2010; 1803:1298 - 1307
- Lee K, Godeau B, Fromont P, Plonquet A, Debili N, Bachir D, et al. CD36 deficiency is frequent and can cause platelet immunization in Africans. Transfusion 1999; 39:873 - 879
- Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med 2003; 13:136 - 141
- Stump DD, Zhou SL, Berk PD. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol 1993; 265:894 - 902
- Clarke DC, Miskovic D, Han XX, Calles-Escandon J, Glatz JF, Luiken JJ, et al. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism. Physiol Genomics 2004; 17:31 - 37
- Siegenthaler G, Hotz R, Chatellard-Gruaz D, Jaconi S, Saurat JH. Characterization and expression of a novel human fatty acid-binding protein: the epidermal type (E-FABP). Biochem Biophys Res Commun 1993; 190:482 - 487
- Krieg P, Feil S, Furstenberger G, Bowden GT. Tumor-specific overexpression of a novel keratinocyte lipid-binding protein. Identification and characterization of a cloned sequence activated during multistage carcinogenesis in mouse skin. J Biol Chem 1993; 268:17362 - 17369
- Owada Y, Suzuki R, Iwasa H, Spener F, Kondo H. Localization of epidermal-type fatty acid binding protein in the thymic epithelial cells of mice. Histochem Cell Biol 2002; 117:55 - 60
- Owada Y, Suzuki I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol Cell Biochem 2002; 239:83 - 86
- Ogawa E, Owada Y, Ikawa S, Adachi Y, Egawa T, Nemoto K, et al. Epidermal FABP (FABP5) Regulates Keratinocyte Differentiation by 13(S)-HODE-Mediated Activation of the NFkappaB Signaling Pathway. J Invest Dermatol 2011; 131:604 - 612
- Siegenthaler G, Hotz R, Chatellard-Gruaz D, Didierjean L, Hellman U, Saurat JH. Purification and characterization of the human epidermal fatty acid-binding protein: localization during epidermal cell differentiation in vivo and in vitro. Biochem J 1994; 302:363 - 371
- Watanabe R, Fujii H, Yamamoto A, Hashimoto T, Kameda K, Ito M, Ono T. Immunohistochemical distribution of cutaneous fatty acid-binding protein in human skin. J Dermatol Sci 1997; 16:17 - 22
- Watanabe R, Fujii H, Yamamoto A, Yamaguchi H, Takenouchi T, Kameda K, et al. Expression of cutaneous fatty acid-binding protein and its mRNA in rat skin. Arch Dermatol Res 1996; 288:481 - 483
- Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly upregulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol 1992; 99:299 - 305
- Masouye I, Saurat JH, Siegenthaler G. Epidermal fatty-acid-binding protein in psoriasis, basal and squamous cell carcinomas: an immunohistological study. Dermatology 1996; 192:208 - 213
- Yamane Y, Moriyama K, Yasuda C, Miyata S, Aihara M, Ikezawa Z, Miyazaki K. New horny layer marker proteins for evaluating skin condition in atopic dermatitis. Int Arch Allergy Immunol 2009; 150:89 - 101
- Owada Y, Takano H, Yamanaka H, Kobayashi H, Sugitani Y, Tomioka Y, et al. Altered water barrier function in epidermal-type fatty acid binding protein-deficient mice. J Invest Dermatol 2002; 118:430 - 435
- Kusakari Y, Ogawa E, Owada Y, Kitanaka N, Watanabe H, Kimura M, et al. Decreased keratinocyte motility in skin wound on mice lacking the epidermal fatty acid binding protein gene. Mol Cell Biochem 2006; 284:183 - 188
- Klar J, Schweiger M, Zimmerman R, Zechner R, Li H, Torma H, et al. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am J Hum Genet 2009; 85:248 - 253
- Akiyama M, Shimizu H. An update on molecular aspects of the non-syndromic ichthyoses. Exp Dermatol 2008; 17:373 - 382
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol 2010; 63:607 - 641
- Anton-Lamprecht I, Papadimitrou HDWSDV. Jm, The Skin, in Diagnostic ultastructure of non-neoplastic diseases 1992; Edinburgh Churchill Livingstone 459 - 550
- Melin M, Klar J, Gedde-Dahl T Jr, Fredriksson R, Hausser I, Brandrup F, et al. A founder mutation for ichthyosis prematurity syndrome restricted to 76 kb by haplotype association. J Hum Genet 2006; 51:864 - 871
- Niemi KM, Kuokkanen K, Kanerva L, Ignatius J. Recessive ichthyosis congenita type IV. Am J Dermatopathol 1993; 15:224 - 228
- Brusasco A, Gelmetti C, Tadini G, Caputo R. Ichthyosis congenita type IV: a new case resembling diffuse cutaneous mastocytosis. Br J Dermatol 1997; 136:377 - 379
- Bygum A, Westermark P, Brandrup F. Ichthyosis prematurity syndrome: a well-defined congenital ichthyosis subtype. J Am Acad Dermatol 2008; 59:71 - 74
- Morice-Picard F, Leaute-Labreze C, Decor A, Boralevi F, Lacombe D, Taieb A, Fischer J. A novel mutation in the fatty acid transport protein 4 gene in a patient initially described as affected by self-healing congenital verruciform hyperkeratosis. Am J Med Genet A 2010; 152:2664 - 2665
- Moulson CL, Martin DR, Lugus JJ, Schaffer JE, Lind AC, Miner JH. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci USA 2003; 100:5274 - 5279
- Herrmann T, van der HF, Grone HJ, Stewart AF, Langbein L, Kaiser I, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol 2003; 161:1105 - 1115
- DiRusso CC, Li H, Darwis D, Watkins PA, Berger J, Black PN. Comparative biochemical studies of the murine fatty acid transport proteins (FATP) expressed in yeast. J Biol Chem 2005; 280:16829 - 16837
- DiRusso CC, Darwis D, Obermeyer T, Black PN. Functional domains of the fatty acid transport proteins: studies using protein chimeras. Biochim Biophys Acta 2008; 1781:135 - 143
- Herrmann T, Buchkremer F, Gosch I, Hall AM, Bernlohr DA, Stremmel W. Mouse fatty acid transport protein 4 (FATP4): characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene 2001; 270:31 - 40
- Watkins PA. Very-long-chain acyl-CoA synthetases. J Biol Chem 2008; 283:1773 - 1777
- Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, et al. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci 2006; 119:4678 - 4688
- Jia Z, Pei Z, Maiguel D, Toomer CJ, Watkins PA. The fatty acid transport protein (FATP) family: very long chain acyl-CoA synthetases or solute carriers?. J Mol Neurosci 2007; 33:25 - 31
- Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, et al. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem 2003; 278:49512 - 49516
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res 2009; 50:417 - 422
- Moulson CL, Lin MH, White JM, Newberry EP, Davidson NO, Miner JH. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J Biol Chem 2007; 282:15912 - 15920
- Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, et al. Identification of the major intestinal fatty acid transport protein. Mol Cell 1999; 4:299 - 308
- Herrmann T, Grone HJ, Langbein L, Kaiser I, Gosch I, Bennemann U, et al. Disturbed epidermal structure in mice with temporally controlled fatp4 deficiency. J Invest Dermatol 2005; 125:1228 - 1235
- Lin MH, Chang KW, Lin SC, Miner JH. Epidermal hyperproliferation in mice lacking fatty acid transport protein 4 (FATP4) involves ectopic EGF receptor and STAT3 signaling. Dev Biol 2010; 344:707 - 719
- Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, et al. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res 2009; 50:491 - 500
- Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res 2007; 48:609 - 620
- Gertow K, Bellanda M, Eriksson P, Boquist S, Hamsten A, Sunnerhagen M, Fisher RM. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J Clin Endocrinol Metab 2004; 89:392 - 399
- Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci 2009; 34:453 - 463
- Meyer-Hoffert U. Reddish, scaly and itchy: how proteases and their inhibitors contribute to inflammatory skin diseases. Arch Immunol Ther Exp (Warsz) 2009; 57:345 - 354
- Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J 2010; 51:808 - 822
- Biro T, Toth BI, Marincsak R, Dobrosi N, Geczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta 2007; 1772:1004 - 1021
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Arimura, Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol 2009; 129:714 - 722
- Steinhoff M, Biro T. A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch. J Invest Dermatol 2009; 129:531 - 535
- Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010; 141:331 - 343
- Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol 2010; 130:985 - 994
- Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 2007; 127:594 - 604