Abstract
Lysine methylation of histone and non-histone substrates by the methyltransferase G9a is mostly associated with transcriptional repression. Recent studies, however, have highlighted its role as an activator of gene expression through mechanisms that are independent of its methyltransferase activity. Here we review the growing repertoire of molecular mechanisms and substrates through which G9a regulates gene expression. We also discuss emerging evidence for its wide-ranging functions in development, pluripotency, cellular differentiation and cell cycle regulation that underscore the complexity of its functions. The deregulated expression of G9a in cancers and other human pathologies suggests that it may be a viable therapeutic target in various diseases.
G9a Structure and Activity
In an attempt to identify genes related to autoimmune disorders in the unexplored regions of MHC class III cluster on human chromosome 6, an undefined gene G9a or BAT8 was found using a combination of chromosome walking with overlapping cosmid genomic clones and pulsed field gel electrophoresis.Citation1,Citation2 The human G9a/EHMT2 protein which was initially thought to contain 1001 amino acids was later found to lack NG36 transcripts.Citation3 Further investigations revealed that full length G9a protein (isoform a) is a product of both NG36 (encoded by 4 exons) and G9a (encoded by 24 exons) transcripts ().Citation4 In addition, a shorter isoform has been characterized (isoform b) which lacks exon 10. The mouse homolog, found on chromosome 17, has two alternatively spliced isoforms as well. The long isoform (G9a L) lacks exon 1 and includes all of exon 2, while the short isoform (G9a S) contains exon 1 but splices out part of exon 2 to result in a N-terminal region resembling the human homolog. A closely related paralog GLP/EHMT1Citation5 has been identified which exhibits 45% identity with human G9a and differs primarily in the N-terminus.Citation6
Figure 1. Schematic representation of G9a domain structure. Human and mouse G9a isoforms are shown with their respective NCBI accession ID (left). The domain structure was constructed using DOG 1.0 software. The Cysteine (Cys) rich region, ankyrin repeats (ANK) and the catalytic SET domain with flanking Pre SET and post SET regions are shown. The site for methylation (Me),nuclear localization signal (NLS) and the glutamic acid (E) rich region are denoted. Numbers indicate amino acid residues.
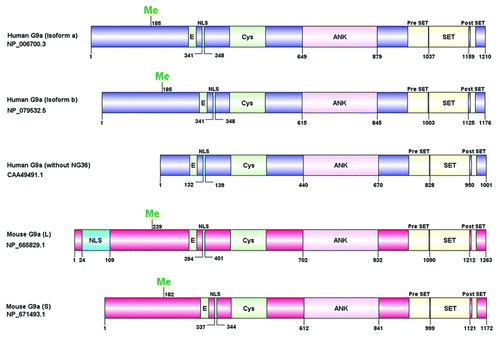
G9a belongs to the SET domain containing Su(var)3–9 family of proteins which include Suv39h1/h2, SETDB1, SETDB2 and DIM-5.Citation7-Citation11 G9a contains an evolutionarily conserved SET domain which contributes to its methyltransferase activity, a domain containing ankyrin repeats which is involved in protein-protein interactionsCitation3,Citation7 and nuclear localization signals that have been identified at the N-terminal region.Citation12 The catalytic SET domain with variable I-SET insert is flanked by pre- and post-SET regions consisting of a pseudo knot. The substrate binding groove is formed by I-SET and post-SET domains.Citation13 When transiently expressed, G9a and GLP form homomeric and heteromeric complexes via their SET domain; however, endogenously they function exclusively as a heteromeric complex in a wide variety of human and mouse cells.Citation14
Biochemical studies have revealed that G9a and GLP possess the same substrate specificities on histones. Both enzymes have histone 3 (H3) lysine 9 (K9) methyltransferase activity in vitro.Citation5,Citation7 G9a transfers methyl groups from S-adenosyl-l-methionine (SAM) to ε-amino group of the target lysine residue, resulting in mono- and di-methylation of K9 (H3K9me1 and H3K9me2 respectively), and to a lesser extent, K27of H3.Citation7 Two tyrosine (Y) residues of G9a are of particular importance for catalytic activity and H3K9 di-methylation specificity. Y1154, participates in catalysis and Y1067 is involved in hydrogen bonding with the nitrogen atom of the ε-amino group of the substrate lysine residue. Loss of Y1154 renders G9a catalytically inactive, whereas replacement of Y1067 to phenylalanine allows it to tri-methylate H3K9 (H3K9me3). The proposed model of G9a action involves interaction of the peptide substrate at the rigid I-SET domain.Citation12 Concurrent binding of SAM at the flexible post-SET domain stimulates domain folding and orients the remaining peptide-binding residues. As such the post-SET domain can “close” onto the substrate to complete the substrate-binding groove and allow catalysis to occur. This concurs with the random order of substrate and co-factor binding as previously suggested.Citation15 Commercially available compounds BIX-01294 and UNC0638 act as competitive inhibitors of G9a activity by binding to the substrate binding groove and render it inaccessible to substrates.Citation16,Citation17
Substrate Recognition and Specificity
G9a interacts with amino acid residues 6–11 of H3, and the minimum substrate recognition criterion in vitro is a seven amino acid heptapeptide with RK/ARK consensus e.g., 6-TARKSTG-12 of H3.Citation18-Citation20 In the H3 peptide, arginine (R) residue at position 8 adjacent to a lysine residue is an absolute requirement for G9a activity; any other amino acid substituted at this position abolishes methylation of the H3 peptide substrate. Other than the RK specificity, G9a exhibits a preference for hydrophilic amino acids at position 6; smaller amino acids such as alanine (A) are preferred at position 7, whereas hydrophilic and hydrophobic amino acids respectively are favored at positions 10 and 11.Citation20 Apart from these positional preferences, modification of H3 proximal amino acids serine (S)10 and threonine (T)11 by phosphorylation impairs methylation by G9a.Citation18,Citation20 In addition to H3, the G9a recognition motif is found in several non-histone proteins. In fact, G9a itself has such a motif at its non-catalytic N-terminus which is automethylated.Citation18,Citation21 Auto-methylation of K239 (mouse) does not result in a loss of its catalytic activity indicating that methylation does not render G9a inactive. The auto-methylated G9a peptide resembles tri-methylated H3K9 (H3K9me3). Similar to H3K9me3 associated HP1 interaction, auto-methylated G9a peptide interacts with HP1 which might stage recruitment of other repressors such as HDAC1 and DNMT1.Citation18 G9a also methylates other methyltransferases such as mAM, an activator protein of SetDB1, and GLP, suggesting a complex internal regulation of these histone methyl transferases.Citation18,Citation21 Several additional non-histone substrates have been identified including WIZ, CDYL1, CSB, ACINUS, HDAC1, DNMT1 and KLF12.Citation20 While the functional relevance of methylation of non-histone proteins has not been established in each case, G9a-mediated methylation of Reptin at K67 under hypoxic conditions results in Reptin-dependent inhibition of hypoxia responsive genes, providing a mechanism to adjust the cellular response to hypoxia.Citation22 Many transcription factors are methylated by G9a which appears to negatively impact their transcriptional activity. For instance, G9a methylates CEBP β at K39 which interferes with activation of myeloid genes.Citation23,Citation24 Similarly, MyoD methylation at K104 blocks its transcriptional activity and expression of muscle differentiation genes,Citation25 and G9a/GLP complex methylates p53 at K373 rendering it transcriptionally inactive.Citation26
Changing Partners and Switching Roles: From Repressor to Activator
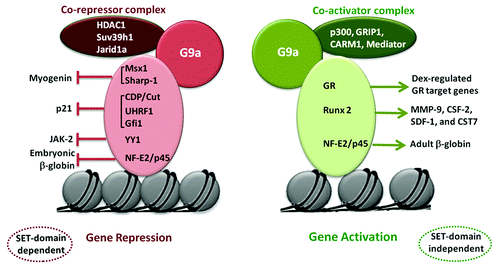
While the vast number of studies have implicated its function in mediating H3K9me2 marks that is associated with transcriptional repression, in erythroid cells, G9a exhibits a more complex role in regulating transcription of the β globin gene.Citation27 G9a is recruited to the β globin locus in a NF-E2/p45-dependent manner. Interestingly, G9a represses embryonic globin Ey expression, whereas it activates the adult β globin βmaj gene. Through pull-down assays followed by mass spectrometry, G9a was found to associate with the H3K4 demethylase Jarid1a at the embryonic Ey globin promoter resulting in silencing of its expression. On the other hand, G9a recruits Mediator only to the βmaj promoter, activating its expression.Citation28 Thus differential interaction with Jarid1a and Mediator in distinct co-repressor and co-activator complexes accounts for its opposing impact at the Ey and βmaj-globin promoters. Similar to these findings, other studies have also reported G9a to act as a co-activator together with GRIP1, CARM1 and p300 for nuclear receptors in a methyltransferase activity-independent manner.Citation29 G9a is recruited by ligand-activated GR bound to GR-binding sites where it functions as a scaffold for recruitment of p300 and CARM1 to positively regulate gene expression. The N-terminal region of G9a is sufficient to bring about recruitment/stabilization of this activator complex in response to dexamethasone treatment.Citation30 G9a is also recruited by Runx2 to MMP-9, CSF-2, SDF-1 and CST7 promoters to activate their expression independent of its methyltransferase activity.Citation31 From these studies, it appears that the role of G9a as an activator does not require its methyltransferase activity; and suggest its function as a repressor or activator depends upon its interacting partners ().
Figure 2. Transcriptional repression and activation by G9a. G9a recruitment by transcription factors and association with distinct co-factor complexes leads to opposing outcomes on gene expression. Transcriptional repression of genes such as myogenin, p21, JAK2 and embryonic β-globin is dependent on G9a methyltransferase activity (SET domain). On the other hand, G9a recruitment by GR, Runx2 and NF-E2/p45 leads to activation of target genes in a SET-domain independent manner. This occurs through association of G9a with co-activators such as p300 and CARM1, as well as the Mediator complex.
Besides regulation of RNA polymerase II transcription, G9a has also been found to regulate RNA polymerase I-mediated transcription. This occurs through interaction of G9a with CSB, resulting in H3K9 methylation at the coding region of rRNAs.Citation32 Moreover, both ATPase activity of CSB and methyltransferase activity of G9a are required for association of RNA polymerase I to rDNA. In view of this, a role for G9a has been proposed in the inactivation of actively-transcribed rRNAs in preparation for transcriptional re-initiation.
Function in Development and Differentiation
G9a is widely expressed in most tissues including fetal liver, bone marrow, peripheral blood leukocytes, thymus, lymph node, spleen and developing skeletal muscles.Citation4,Citation33 Loss of G9a in mice results in embryonic lethality between embryonic (E) days E9.5-E12.5. Even though no abnormalities are seen, G9a−/− embryos at E9.5 morphologically resemble wild type embryos at E8.0-E8.5. This growth retardation is attributed to increased apoptosis rather than growth arrest. Interestingly, G9a−/− embyronic stem (ES) cells do not show overt growth defects in culture conditions, but exhibit severe differentiation defects, suggesting a role for G9a in commitment and differentiation.Citation34 Consistent with this notion, there is substantial evidence that G9a plays a prominent role in early development by repression of Oct-3/4, Nanog and DNMT3L which are required for maintenance of pluripotency in ES cells. Thus Oct-3/4 and Nanog expression is sustained until E7.5 in G9a-null embryos, indicating the importance of G9a in the silencing of pluripotent genes at early differentiation stages.Citation35 Post-implantation repression of these pluripotency genes is achieved through establishment of methylated H3K9 heterochromatinization via the SET domain, as well as through de novo DNA methylation by recruitment of DNMT3a/3b that associate with the ANK repeats of G9a. The establishment of these two separate epigenetic marks prevents reprogramming of ES cells to an undifferentiated state. Consistently, in contrast to wild type cells, G9a−/− ES cells differentiated with retinoic acid revert back to a pluripotent state by expressing Oct-3/4 and Nanog when re-cultured in LIF-containing medium.Citation36,Citation37 In addition to control of ES cell pluripotency, G9a has also been implicated in genomic imprinting. While G9a is not required for X-chromosome inactivation,Citation38 it is recruited by non-coding RNAs Kcnq1ot1 and Air to respective target domains in a lineage-specific manner and stimulate the formation of heterochromatin to cause monoallelic expression of specific genes.Citation39,Citation40
G9a is recruited by LSH, a member of SNF2 family of ATP-dependent chromatin remodelling enzymes, to generate normal DNA methylation patterns and stable gene silencing during lineage commitment and differentiation of distinct cell types.Citation41 Progressive H3K9me2 patterning occurs in a G9a/GLP dependent manner during lineage specification of hematopoietic stem and progenitor cells (HSPCs).Citation42 H3K9me2 marks are deficient in primitive HSCs and are formed de novo during lineage commitment, with highest levels in megakaryocytes and T cells. Treatment of HSPCs with UNC0638 results in preservation of stem cell-like phenotype and function during in vitro expansion. An increase in G9a-mediated H3K9me2 also occurs during retinoic acid-induced differentiation of the leukemic cell line HL-60, concomitant with decreased JAK2 expression. G9a is recruited to the JAK2 promoter to repress its transcription in a YY1-dependent manner.Citation43 G9a is also important in Th2 cell differentiation. CD4+ T cells from conditional knockout mice in which G9a is specifically deleted in T cells fail to differentiate into Th2 cells resulting in impaired cytokine production.Citation44
In contrast to these studies, G9a expression levels and H3K9me2 repression marks decline during differentiation of skeletal muscle precursor cells.Citation25 Overexpression of G9a in myoblasts negatively regulates differentiation, and this is accompanied by elevated H3K9me2 marks on muscle promoters as well as methylation of MyoD, a key regulator of skeletal myogenesis. MyoD methylation inhibits its transcriptional activity and the consequent activation of its downstream targets involved in differentiation.Citation25,Citation45 Recruitment of G9a to muscle promoters is mediated by the bHLH transcription factor Sharp-1, as well as by the homeoprotein Msx1.Citation33,Citation46 Similar to its impact on skeletal myogenesis, G9a represses differentiation of adipocytes, which is dependent upon H3K9me2-mediated inhibition of PPARγ expression. In addition, G9a enhances Wnt10b expression, an inhibitor of adipogenesis, in a SET-domain independent manner.Citation47
G9a/GLP complex functions as master regulator of lineage specific gene expression in the brain. Recruitment of G9a by NRSF is important in the repression of neuronal genes outside of the nervous system.Citation48 Similarly, conditional ablation of GLP/G9a in postnatal neurons of the forebrain using the Camk2a-Cre mice results in expression of early neuronal progenitor genes in various regions of the brain.Citation49 These changes led to brain impairment with reduced exploratory behavior and locomotor activity, which manifests as a mental retardation-like syndrome in adult mice. The phenotype closely resembles human 9q34 mental retardation syndrome that involves the sub-telomeric deletion of human chromosome 9(9q34), including the GLP gene. Recently, G9a/GLP were also found to play a role in memory consolidation.Citation50 Inhibition of GLP/G9a activity with BIX-01294 in the hippocampus disrupted LTM formation, and in the EC, enhanced memory consolidation, by increasing expression of memory permissive genes. This differential regulation of genes in the hippocampus and EC highlights the function of G9a in molecular cross talk between the two brain regions. G9a also plays an important role in cocaine-induced structural and behavioral plasticity.Citation51 Repeated administration of cocaine decreases G9a mRNA levels and global H3K9me2 repressive marks in nucleus accumbens (NAc), resulting in increased gene expression. Repression of G9a and H3K9me2 in the NAc neurons promotes cocaine addiction and conversely, its overexpression has a reciprocal effect. Together these studies highlight the relevance of H3K9me2 in maintaining transcriptional homeostasis in adult neurons, and underscore the function of GLP/G9a in mental retardation, defects in learning, environmental adaptation and motivation in mice and humans.
Role in Cellular Proliferation, Senescence and Cancer
A large body of evidence has indicated a role for G9a in cellular proliferation, quiescence, senescence and replication. Recruitment of G9a by CDP/cut, UHRF1 and Gfi1 to the p21Cip/Waf1 promoter results in repression of its expression.Citation52-Citation54 Consistent with these studies, pharmacological inhibition of G9a activity with BIX-01294 reduces proliferation of fetal pulmonary arterial smooth muscle cells that is accompanied by G1 cell cycle arrest and elevated p21Cip/Waf1 expression. Moreover, migration and contractility was also inhibited with altered global methylation levels.Citation55 In addition to its role in G1 arrest, G9a was reported to be present in a complex with E2F6 and other polycomb group proteins on E2F responsive promoters in G0 cells.Citation5 These studies indicate the involvement of G9a in repression of E2F responsive genes in quiescent cells. Similarly, G9a has also been shown to repress HIV1 gene expression and maintain quiescence of the latent HIV1 provirus by mediating H3K9me2 on the HIV1 long-terminal repeat promoter.Citation56
DNMT1 levels are reduced in senescent cells and knockdown of DNMT1 in human diploid fibroblasts correlates with a decrease in G9a/GLP expression. During Ras-induced senescence, proteosomal degradation of G9a and GLP occurs through APC/Ccdh1, an ubiquitin ligase. Cdc14B and p21 activate APC/Ccdh1, thereby degrading G9a/GLP and causing decreased H3K9 di-methylation globally as well as on IL-6 and IL-8 promoters, which are major components of senescence-associated secretory phenotype. Knockdown of Cdh1 abolishes IL-6 and IL-8 expression in senescent cells, indicating the importance of both G9a/GLP and APC/Ccdh1 in senescence.Citation57
During replication, DNMT1 complexes with G9a at replication foci, coordinating DNA and H3K9 methylation, indicating that both molecules work together to restore heterochromatin at replication forks during the S phase.Citation58 Gene expression analysis performed on G9a conditional knockout mouse ESCs revealed a strong correlation between H3K9me2 and late replication genes including Magea1 and Dub1a. This study indicates that G9a creates facultative heterochromatin by mediating H3K9me2 within late replicating chromatin at the nuclear periphery.Citation59 Consistent with its role during replication, G9a−/− ESCs exhibited significant delay in progression through S phase. Interestingly, G9a mono-methylates H3K56 (H3K56me1), which acts as a docking site for PCNA during G1 phase, and thus facilitates PCNA functionality during the S phase.Citation60
Given its role in cellular proliferation, it is not surprising that G9a expression is high in many cancers compared with normal tissue. Cancer transcriptome analysis has revealed high expression in many tumors including hepatocellular, colon, prostate, lung and invasive transitional cell carcinomas and in B cell chronic lymphocytic leukemia.Citation26 In a number of human bladder and lung carcinoma patients,Citation61 G9a expression is upregulated. Knockdown of G9a in both bladder and lung cancer cell lines caused growth suppression and apoptosis with an increase in sub-G1 population. By microarray and chromatin immunoprecipitation experiments, the tumor suppressor SIAH1Citation62,Citation63 was identified to be a transcriptional target.Citation61 Studies on prostate cancer further corroborate its role in carcinogenesis, where downregulation of G9a causes centrosome disruption, chromosomal instability, inhibition of cell growth and increased cellular senescence in cancer cells.Citation64 G9a is also involved in repression of Runx3 expression which is inactivated in gastric cancer.Citation65 In Runx3-expressing gastric cell lines, hypoxia increases the accumulation of G9a and H3K9me2 marks which leads to Runx3 gene silencing. Overexpression of G9a in gastric cancer cells grown under hypoxic conditions leads to further repression of Runx3 expression. Deletion of the SET domain in G9a results in a failure to repress Runx3 under both normoxic and hypoxic conditions.
In aggressive lung cancer, high levels of G9a correlate with poor prognosis with increased cell migration and invasion in vitro and metastasis in vivo. The metastatic function of G9a in lung cancer is due to repression of Ep-CAM.Citation66 Knockdown of G9a in lung cancer cells leads to de-repression in Ep-CAM expression and decrease in H3K9me2 levels along with reduced DNMT1, HP1 and HDAC1 recruitment at the promoter. Similar studies in CLBC have uncovered the role of G9a in promoting EMT by repression of E-cadherin expression.Citation67 Repression of E-cadherin is brought about by the interaction of Snail with G9a. HP1 and DNA methyltransferases are recruited to the E-cadherin promoter resulting in DNA methylation and H3K9me2. Knockdown of G9a rescued the expression of E-Cadherin, preventing cell migration and invasion in vitro, and decreased tumor growth and metastatic potential in CLBC metastasis models. Thus by targeting and blocking the association of the Snail-G9a-DNMTs complex, novel therapies could be developed to target CLBCs.
Future Perspectives
The abundance of data on G9a and its associated H3K9 methylation activity has led to the view that G9a is a co-repressor that silences genes found in euchromatin. Nevertheless, the fact that G9a has been shown to interact with the Mediator complex and exists in distinct co-activator complexes highlight a new role for G9a in transcriptional activation. It remains to be seen whether, similar to the control of embryonic and adult β-globin expression in differentiating erythroid cells, G9a exerts such dual activities on regulation of other cell fate decisions. As such more work has to be done to determine the significance of this newly discovered function of G9a. Regardless, these studies highlight the importance of elucidating G9a interacting partners and relevant complexes that dictate its activity. In addition, the transcription factors that recruit G9a to target promoters in various cell types need to be identified.
The fact that G9a is overexpressed in many different types of cancers, and has been shown to be responsible for various aspects of tumorigenesis, including cellular differentiation, proliferation and EMT, indicates that G9a may be a feasible target for cancer therapy. This supports the prevailing perception that de-regulation of the epigenome in cells is a contributing, and possibly causative, factor of oncogenesis. Given its myriad roles, it would also be important to investigate the role of G9a in fear-related memory disorders, mental retardation, addictive disorders, metabolic disease, as well as skeletal muscle development and regenerative potential. This will lead to a greater understanding of the pathologies in which G9a is involved, and, as with cancer, the potential for using G9a inhibitors as therapeutic avenues. The efficacy of commercially available G9a inhibitors in cancer and other disease models however, remain to be tested.
| Abbreviations: | ||
| ANK | = | Ankyrin |
| ATRA | = | All Trans retinoic acid |
| βmaj | = | β globin major |
| βminor | = | β globin minor |
| BAT8 | = | HLA-B-Associated Transcript 8 |
| BDNF | = | brain-derived neurotrophic factor |
| CA1 | = | cornu ammonis 1 |
| Camk2a | = | calcium/calmodulin-dependent protein kinase II alpha |
| CARM1 | = | coactivator-associated arginine methyltransferase 1 |
| Cdc 14B | = | CDC14 cell division cycle 14 homolog B |
| Cdk 5 | = | cyclin dependent kinase 5 |
| CDP/cut | = | CCAAT displacement protein/cut homolog |
| CDYL1 | = | chromodomain Y-Like protein |
| CEBP β | = | CCAAT/enhancer binding protein beta |
| CLBC | = | claudin low breast cancer |
| CSB | = | cockayne syndrome group B protein |
| CSF-2 | = | Colony-stimulating factor-2 |
| CST7 | = | cystatin-7 |
| COMT | = | catechol O methyltransferase |
| DIM-5 | = | defective in methylation-5 |
| DNMT1 | = | DNA methyltransferase 1 |
| Ey | = | embryonic β like globin |
| EC | = | entorhinal cortex |
| EMT | = | epithelial to mesenchymal |
| Ep-CAM | = | epithelial cell adhesion molecule |
| FosB | = | FBJ murine osteosarcoma viral oncogene homolog B |
| Gfi1 | = | growth factor independent 1 transcription repressor |
| GLP | = | G9a-like-protein |
| GR | = | glucocorticoid receptor |
| GRIP1 | = | glutamate receptor interacting protein 1 |
| HDAC1 | = | histone deacetylase 1 |
| HIV1 | = | human immunodeficiency virus 1 |
| HP1 | = | heterochromatin protein |
| IL6 | = | interleukin 6 |
| IL8 | = | interleukin 8 |
| JAK2 | = | janus kinase 2 |
| JARID 1a | = | jumonji, AT rich interactive domain 1 |
| KLF-12 | = | kruppel-Like factor-12 |
| LIF | = | leukemia inhibitory factor |
| L-LTP | = | late phase long-term potentiation |
| LSH | = | lymphoid-specific helicase |
| LTM | = | long term memory formation |
| Magea | = | melanoma antigen family A, 1 |
| mAM | = | mouse ATFa-associated modulator |
| MHC | = | major histocompatibility complex |
| MMP-9 | = | matrix metalloproteinsase-9 |
| Msx1 | = | Msh homeobox 1 |
| NAc | = | nucleus accumbens |
| NF-kB | = | nuclear factor kappa light chain enhancer of activated B Cells |
| NLS | = | nuclear localization signals |
| NRSF | = | neuron restrictive silencing factor |
| PCNA | = | proliferating cell nuclear antigen |
| PIC | = | pre initiation Complex |
| PPARγ | = | peroxisome proliferator-activated receptor gamma |
| Runx2 | = | runt-related transcription factor 2 |
| Runx3 | = | runt related transcription factor 3 |
| SAM | = | S-adenosyl-L-methionine |
| SASP | = | senescent associated secretory phenotype |
| SDF-1 | = | stromal differentiating factor-1 |
| SET | = | Su(var)3-9, enhancer of zeste, trithorax |
| SETDB1 | = | SET domain, bifurcated 1 |
| SETDB2 | = | SET domain, bifurcated 2 |
| Sharp-1 | = | enhancer-of-split and hairy-related protein |
| SIAH1 | = | seven in absentiah |
| SNF2 | = | sucrose nonfermentable 2 |
| TA-CA1 | = | Temporo ammonis-cornu ammonis 1 |
| UHRF1 | = | ubiquitin-like with PHD and ring finger domains 1 |
| WIZ | = | widely interspaced zinc finger motifs protein |
| YY1 | = | ying and yang 1 |
Acknowledgments
Work in the authors’ laboratory is supported by the National Medical Research Council, the A*STAR Singapore Stem Cell Consortium. The domain structure in was constructed using DOG 1.0: Illustrator of Protein Domain Structures.Citation68
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Dunham I, Sargent CA, Kendall E, Campbell RD. Characterization of the class III region in different MHC haplotypes by pulsed-field gel electrophoresis. Immunogenetics 1990; 32:175 - 82; http://dx.doi.org/10.1007/BF02114970; PMID: 1977694
- Spies T, Bresnahan M, Strominger JL. Human major histocompatibility complex contains a minimum of 19 genes between the complement cluster and HLA-B. Proc Natl Acad Sci U S A 1989; 86:8955 - 8; http://dx.doi.org/10.1073/pnas.86.22.8955; PMID: 2813433
- Milner CM, Campbell RD. The G9a gene in the human major histocompatibility complex encodes a novel protein containing ankyrin-like repeats. Biochem J 1993; 290:811 - 8; PMID: 8457211
- Brown SE, Campbell RD, Sanderson CM. Novel NG36/G9a gene products encoded within the human and mouse MHC class III regions. Mamm Genome 2001; 12:916 - 24; http://dx.doi.org/10.1007/s00335-001-3029-3; PMID: 11707778
- Ogawa H, Ishiguro K-I, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 2002; 296:1132 - 6; http://dx.doi.org/10.1126/science.1069861; PMID: 12004135
- Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol 2005; 6:227; http://dx.doi.org/10.1186/gb-2005-6-8-227; PMID: 16086857
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem 2001; 276:25309 - 17; http://dx.doi.org/10.1074/jbc.M101914200; PMID: 11316813
- Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 2000; 406:593 - 9; http://dx.doi.org/10.1038/35020506; PMID: 10949293
- O’Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, et al. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Mol Cell Biol 2000; 20:9423 - 33; http://dx.doi.org/10.1128/MCB.20.24.9423-9433.2000; PMID: 11094092
- Tamaru H, Selker EU. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 2001; 414:277 - 83; http://dx.doi.org/10.1038/35104508; PMID: 11713521
- Zhang X, Tamaru H, Khan SI, Horton JR, Keefe LJ, Selker EU, et al. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell 2002; 111:117 - 27; http://dx.doi.org/10.1016/S0092-8674(02)00999-6; PMID: 12372305
- Estève P-O, Patnaik D, Chin HG, Benner J, Teitell MA, Pradhan S. Functional analysis of the N- and C-terminus of mammalian G9a histone H3 methyltransferase. Nucleic Acids Res 2005; 33:3211 - 23; http://dx.doi.org/10.1093/nar/gki635; PMID: 15939934
- Wu H, Min J, Lunin VV, Antoshenko T, Dombrovski L, Zeng H, et al. Structural biology of human H3K9 methyltransferases. PLoS One 2010; 5:e8570; http://dx.doi.org/10.1371/journal.pone.0008570; PMID: 20084102
- Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev 2005; 19:815 - 26; http://dx.doi.org/10.1101/gad.1284005; PMID: 15774718
- Patnaik D, Chin HG, Estève P-O, Benner J, Jacobsen SE, Pradhan S. Substrate specificity and kinetic mechanism of mammalian G9a histone H3 methyltransferase. J Biol Chem 2004; 279:53248 - 58; http://dx.doi.org/10.1074/jbc.M409604200; PMID: 15485804
- Chang Y, Ganesh T, Horton JR, Spannhoff A, Liu J, Sun A, et al. Adding a lysine mimic in the design of potent inhibitors of histone lysine methyltransferases. J Mol Biol 2010; 400:1 - 7; http://dx.doi.org/10.1016/j.jmb.2010.04.048; PMID: 20434463
- Vedadi M, Barsyte-Lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, et al. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat Chem Biol 2011; 7:566 - 74; http://dx.doi.org/10.1038/nchembio.599; PMID: 21743462
- Chin HG, Estève P-O, Pradhan M, Benner J, Patnaik D, Carey MF, et al. Automethylation of G9a and its implication in wider substrate specificity and HP1 binding. Nucleic Acids Res 2007; 35:7313 - 23; http://dx.doi.org/10.1093/nar/gkm726; PMID: 17962312
- Chin HG, Pradhan M, Estève P-O, Patnaik D, Evans TC Jr., Pradhan S. Sequence specificity and role of proximal amino acids of the histone H3 tail on catalysis of murine G9A lysine 9 histone H3 methyltransferase. Biochemistry 2005; 44:12998 - 3006; http://dx.doi.org/10.1021/bi0509907; PMID: 16185068
- Rathert P, Dhayalan A, Murakami M, Zhang X, Tamas R, Jurkowska R, et al. Protein lysine methyltransferase G9a acts on non-histone targets. Nat Chem Biol 2008; 4:344 - 6; http://dx.doi.org/10.1038/nchembio.88; PMID: 18438403
- Sampath SC, Marazzi I, Yap KL, Sampath SC, Krutchinsky AN, Mecklenbräuker I, et al. Methylation of a histone mimic within the histone methyltransferase G9a regulates protein complex assembly. Mol Cell 2007; 27:596 - 608; http://dx.doi.org/10.1016/j.molcel.2007.06.026; PMID: 17707231
- Lee JS, Kim Y, Kim IS, Kim B, Choi HJ, Lee JM, et al. Negative regulation of hypoxic responses via induced Reptin methylation. Mol Cell 2010; 39:71 - 85; http://dx.doi.org/10.1016/j.molcel.2010.06.008; PMID: 20603076
- Leutz A, Pless O, Lappe M, Dittmar G, Kowenz-Leutz E. Crosstalk between phosphorylation and multi-site arginine/lysine methylation in C/EBPs. Transcription 2011; 2:3 - 8; http://dx.doi.org/10.4161/trns.2.1.13510; PMID: 21326902
- Pless O, Kowenz-Leutz E, Knoblich M, Lausen J, Beyermann M, Walsh MJ, et al. G9a-mediated lysine methylation alters the function of CCAAT/enhancer-binding protein-beta. J Biol Chem 2008; 283:26357 - 63; http://dx.doi.org/10.1074/jbc.M802132200; PMID: 18647749
- Ling BMT, Bharathy N, Chung T-K, Kok WK, Li S, Tan YH, et al. Lysine methyltransferase G9a methylates the transcription factor MyoD and regulates skeletal muscle differentiation. Proc Natl Acad Sci U S A 2012; 109:841 - 6; http://dx.doi.org/10.1073/pnas.1111628109; PMID: 22215600
- Huang J, Dorsey J, Chuikov S, Pérez-Burgos L, Zhang X, Jenuwein T, et al. G9a and Glp methylate lysine 373 in the tumor suppressor p53. J Biol Chem 2010; 285:9636 - 41; http://dx.doi.org/10.1074/jbc.M109.062588; PMID: 20118233
- Chaturvedi C-P, Hosey AM, Palii C, Perez-Iratxeta C, Nakatani Y, Ranish JA, et al. Dual role for the methyltransferase G9a in the maintenance of beta-globin gene transcription in adult erythroid cells. Proc Natl Acad Sci U S A 2009; 106:18303 - 8; http://dx.doi.org/10.1073/pnas.0906769106; PMID: 19822740
- Chaturvedi C-P, Somasundaram B, Singh K, Carpenedo RL, Stanford WL, Dilworth FJ, et al. Maintenance of gene silencing by the coordinate action of the H3K9 methyltransferase G9a/KMT1C and the H3K4 demethylase Jarid1a/KDM5A. Proc Natl Acad Sci U S A 2012; 109:18845 - 50; http://dx.doi.org/10.1073/pnas.1213951109; PMID: 23112189
- Lee DY, Northrop JP, Kuo M-H, Stallcup MR. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem 2006; 281:8476 - 85; http://dx.doi.org/10.1074/jbc.M511093200; PMID: 16461774
- Bittencourt D, Wu D-Y, Jeong KW, Gerke DS, Herviou L, Ianculescu I, et al. G9a functions as a molecular scaffold for assembly of transcriptional coactivators on a subset of Glucocorticoid Receptor target genes. Proc Natl Acad Sci U S A 2012; 109:19673 - 8; http://dx.doi.org/10.1073/pnas.1211803109; PMID: 23151507
- Purcell DJ, Khalid O, Ou C-Y, Little GH, Frenkel B, Baniwal SK, et al. Recruitment of coregulator G9a by Runx2 for selective enhancement or suppression of transcription. J Cell Biochem 2012; 113:2406 - 14; http://dx.doi.org/10.1002/jcb.24114; PMID: 22389001
- Yuan X, Feng W, Imhof A, Grummt I, Zhou Y. Activation of RNA polymerase I transcription by cockayne syndrome group B protein and histone methyltransferase G9a. Mol Cell 2007; 27:585 - 95; http://dx.doi.org/10.1016/j.molcel.2007.06.021; PMID: 17707230
- Ling BMT, Gopinadhan S, Kok WK, Shankar SR, Gopal P, Bharathy N, et al. G9a mediates Sharp-1-dependent inhibition of skeletal muscle differentiation. Mol Biol Cell 2012; 23:4778 - 85; http://dx.doi.org/10.1091/mbc.E12-04-0311; PMID: 23087213
- Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 2002; 16:1779 - 91; http://dx.doi.org/10.1101/gad.989402; PMID: 12130538
- Yamamizu K, Fujihara M, Tachibana M, Katayama S, Takahashi A, Hara E, et al. Protein kinase A determines timing of early differentiation through epigenetic regulation with G9a. Cell Stem Cell 2012; 10:759 - 70; http://dx.doi.org/10.1016/j.stem.2012.02.022; PMID: 22704517
- Feldman N, Gerson A, Fang J, Li E, Zhang Y, Shinkai Y, et al. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol 2006; 8:188 - 94; http://dx.doi.org/10.1038/ncb1353; PMID: 16415856
- Epsztejn-Litman S, Feldman N, Abu-Remaileh M, Shufaro Y, Gerson A, Ueda J, et al. De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nat Struct Mol Biol 2008; 15:1176 - 83; http://dx.doi.org/10.1038/nsmb.1476; PMID: 18953337
- Ohhata T, Tachibana M, Tada M, Tada T, Sasaki H, Shinkai Y, et al. X-inactivation is stably maintained in mouse embryos deficient for histone methyl transferase G9a. Genesis 2004; 40:151 - 6; http://dx.doi.org/10.1002/gene.20077; PMID: 15493016
- Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 2008; 32:232 - 46; http://dx.doi.org/10.1016/j.molcel.2008.08.022; PMID: 18951091
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 2008; 322:1717 - 20; http://dx.doi.org/10.1126/science.1163802; PMID: 18988810
- Myant K, Termanis A, Sundaram AYM, Boe T, Li C, Merusi C, et al. LSH and G9a/GLP complex are required for developmentally programmed DNA methylation. Genome Res 2011; 21:83 - 94; http://dx.doi.org/10.1101/gr.108498.110; PMID: 21149390
- Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, et al. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev 2012; 26:2499 - 511; http://dx.doi.org/10.1101/gad.200329.112; PMID: 23105005
- Son H-J, Kim J-Y, Hahn Y, Seo S-B. Negative regulation of JAK2 by H3K9 methyltransferase G9a in leukemia. Mol Cell Biol 2012; 32:3681 - 94; http://dx.doi.org/10.1128/MCB.00673-12; PMID: 22801367
- Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC, et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med 2010; 207:915 - 22; http://dx.doi.org/10.1084/jem.20100363; PMID: 20421388
- Bharathy N, Taneja R. Methylation muscles into transcription factor silencing. Transcr 2012; 3:215 - 20; http://dx.doi.org/10.4161/trns.20914
- Wang J, Abate-Shen C. The MSX1 homeoprotein recruits G9a methyltransferase to repressed target genes in myoblast cells. PLoS One 2012; 7:e37647; http://dx.doi.org/10.1371/journal.pone.0037647; PMID: 22629437
- Wang L, Xu S, Lee J-E, Baldridge A, Grullon S, Peng W, et al. Histone H3K9 methyltransferase G9a represses PPARy expression and adipogenesis. The EMBO Journal 2012; In press http://dx.doi.org/10.1038/emboj.2012.306
- Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell 2004; 14:727 - 38; http://dx.doi.org/10.1016/j.molcel.2004.05.026; PMID: 15200951
- Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, et al. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron 2009; 64:678 - 91; http://dx.doi.org/10.1016/j.neuron.2009.11.019; PMID: 20005824
- Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, et al. G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci 2012; 32:5440 - 53; http://dx.doi.org/10.1523/JNEUROSCI.0147-12.2012; PMID: 22514307
- Maze I, Covington HE 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 2010; 327:213 - 6; http://dx.doi.org/10.1126/science.1179438; PMID: 20056891
- Nishio H, Walsh MJ. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc Natl Acad Sci U S A 2004; 101:11257 - 62; http://dx.doi.org/10.1073/pnas.0401343101; PMID: 15269344
- Duan Z, Zarebski A, Montoya-Durango D, Grimes HL, Horwitz M. Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1. Mol Cell Biol 2005; 25:10338 - 51; http://dx.doi.org/10.1128/MCB.25.23.10338-10351.2005; PMID: 16287849
- Kim JK, Estève P-O, Jacobsen SE, Pradhan S. UHRF1 binds G9a and participates in p21 transcriptional regulation in mammalian cells. Nucleic Acids Res 2009; 37:493 - 505; http://dx.doi.org/10.1093/nar/gkn961; PMID: 19056828
- Yang Q, Lu Z, Singh D, Raj JU. BIX-01294 treatment blocks cell proliferation, migration and contractility in ovine foetal pulmonary arterial smooth muscle cells. Cell Prolif 2012; 45:335 - 44; http://dx.doi.org/10.1111/j.1365-2184.2012.00828.x; PMID: 22691107
- Imai K, Togami H, Okamoto T. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem 2010; 285:16538 - 45; http://dx.doi.org/10.1074/jbc.M110.103531; PMID: 20335163
- Takahashi A, Imai Y, Yamakoshi K, Kuninaka S, Ohtani N, Yoshimoto S, et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol Cell 2012; 45:123 - 31; http://dx.doi.org/10.1016/j.molcel.2011.10.018; PMID: 22178396
- Estève P-O, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, et al. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev 2006; 20:3089 - 103; http://dx.doi.org/10.1101/gad.1463706; PMID: 17085482
- Yokochi T, Poduch K, Ryba T, Lu J, Hiratani I, Tachibana M, et al. G9a selectively represses a class of late-replicating genes at the nuclear periphery. Proc Natl Acad Sci U S A 2009; 106:19363 - 8; http://dx.doi.org/10.1073/pnas.0906142106; PMID: 19889976
- Yu Y, Song C, Zhang Q, DiMaggio PA, Garcia BA, York A, et al. Histone H3 lysine 56 methylation regulates DNA replication through its interaction with PCNA. Mol Cell 2012; 46:7 - 17; http://dx.doi.org/10.1016/j.molcel.2012.01.019; PMID: 22387026
- Cho H-S, Kelly JD, Hayami S, Toyokawa G, Takawa M, Yoshimatsu M, et al. Enhanced expression of EHMT2 is involved in the proliferation of cancer cells through negative regulation of SIAH1. Neoplasia 2011; 13:676 - 84; PMID: 21847359
- Okabe H, Satoh S, Furukawa Y, Kato T, Hasegawa S, Nakajima Y, et al. Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res 2003; 63:3043 - 8; PMID: 12810624
- Medhioub M, Vaury C, Hamelin R, Thomas G. Lack of somatic mutation in the coding sequence of SIAH1 in tumors hemizygous for this candidate tumor suppressor gene. Int J Cancer 2000; 87:794 - 7; http://dx.doi.org/10.1002/1097-0215(20000915)87:6<794::AID-IJC5>3.0.CO;2-B; PMID: 10956387
- Kondo Y, Shen L, Ahmed S, Boumber Y, Sekido Y, Haddad BR, et al. Downregulation of histone H3 lysine 9 methyltransferase G9a induces centrosome disruption and chromosome instability in cancer cells. PLoS One 2008; 3:e2037; http://dx.doi.org/10.1371/journal.pone.0002037; PMID: 18446223
- Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res 2006; 66:9009 - 16; http://dx.doi.org/10.1158/0008-5472.CAN-06-0101; PMID: 16982742
- Chen M-W, Hua K-T, Kao H-J, Chi CC, Wei LH, Johansson G, et al. H3K9 histone methyltransferase G9a promotes lung cancer invasion and metastasis by silencing the cell adhesion molecule Ep-CAM. Cancer Res 2010; 70:7830 - 40; http://dx.doi.org/10.1158/0008-5472.CAN-10-0833; PMID: 20940408
- Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 2012; 122:1469 - 86; http://dx.doi.org/10.1172/JCI57349; PMID: 22406531
- Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. DOG 1.0: illustrator of protein domain structures. Cell Res 2009; 19:271 - 3; http://dx.doi.org/10.1038/cr.2009.6; PMID: 19153597