Abstract
DNA cytosine methylation is a reversible epigenetic mark regulating gene expression. Aberrant methylation profiles are concomitant with developmental defects and cancer. Numerous studies in the past decade have identified enzymes and pathways responsible for active DNA demethylation both on a genome-wide as well as gene-specific scale. Recent findings have strengthened the idea that 5-methylcytosine oxidation catalyzed by members of the ten-eleven translocation (Tet1–3) oxygenases in conjunction with replication-coupled dilution of the conversion products causes the majority of genome-wide erasure of methylation marks during early development. In contrast, short and long patch DNA excision repair seems to be implicated mainly in gene-specific demethylation. Growth arrest and DNA damage-inducible protein 45 a (Gadd45a) regulates gene-specific demethylation within regulatory sequences of limited lengths raising the question of how such site specificity is achieved. A new study identified the protein inhibitor of growth 1 (Ing1) as a reader of the active chromatin mark histone H3 lysine 4 trimethylation (H3K4me3). Ing1 binds and directs Gadd45a to target sites, thus linking the histone code with DNA demethylation.
Introduction
In mammalian DNA, cytosines within a CpG dinucleotide context are commonly marked by a methyl group at carbon 5 of the pyrimidine ring. By influencing, typically silencing, gene expression, the resulting modification, 5-methylcytosine (5mC), has been implicated to bear pivotal roles during embryonic development, imprinting, X-chromosome inactivation and cancer.Citation1-Citation3 DNA cytosine methylation permits organisms to gain an additional layer of genetic information on top of the primary DNA sequence and, hence, is classified as an epigenetic mark.
Methylation marks are commonly maintained during DNA replication by the action of the DNA methyltransferase DNMT1.Citation4 Consequently, once set, DNA methylation has been thought to be stable even through cell divisions. Additionally, loss of methylation marks observed in dividing cells was referred to, by default, as passive DNA demethylation after several rounds of replication in the absence of DNMT1. However, research in the last decade uncovered scenarios in which methylated DNA is demethylated in a replication-independent, active manner, both at the genome-wide level as well as at specific genomic loci.
After fertilization, DNA methylation marks of the paternal pronucleus in the mouse zygote are globally erased prior to the first cell cycle.Citation5,Citation6 Similarly, in primordial germ cells (PGCs), the progenitor cells of gametes, methylation is lost genome-wide during their migration to the genital ridge between embryonic days E8.5–E11.5.Citation7
A remarkable example of loci-specific active DNA demethylation in human cells was described at the estrogen receptor target gene pS2. After estrogen stimulation the pS2-promoter undergoes cycles of methylation and demethylation in less than 100 min indicating DNA methylation to be not only reversible but also highly dynamic.Citation8,Citation9
DNA demethylation is of particular importance for the generation of induced pluripotent stem cells (iPSCs), the artificial reprogramming of somatic cells to their pluripotent ground state. Genes for key transcription factors like Oct4 and Nanog are fully demethylated during reprogramming and this demethylation, in turn, drives their expression.Citation10,Citation11
Enzymes of Active DNA Demethylation
While the enzymes responsible for cytosine methylation are confined and well characterized,Citation12 the enzymes responsible for mammalian active DNA demethylation remain rather numerous and controversial ().Citation13 A single enzymatic reaction that releases the methyl group from 5mC but keeps the DNA backbone untouched (a DNA “demethylase” reaction) seems difficult to conceive. Thermodynamically it is challenging to break unpolar carbon-carbon bonds. Nevertheless, the Fe-S radical S-adenosylmethionine (SAM) domain of elongator complex protein 3 (Elp3) was shown to be involved in paternal DNA demethylation in the mouse zygote leading to the hypothesis of a direct radical SAM-mediated demethylation mechanism.Citation14
Figure 1. The multiple faces of mammalian DNA demethylation. Schematic representation of the enzymology implicated in 5-methylcytosine (5mC) demethylation. The exocyclic group at carbon 5 of each cytosine derivative is highlighted in red. C, cytosine; T, thymine; 5hmC, 5-hydroxymethylcytosine; 5fC, 5-formylcytosine; 5caC, 5-carboxylcytosine; BER: base excision repair; NER, nucleotide excision repair. Note: Direct base excision repair of 5mC and potential deamination of 5hmC to 5-hydroxymethyluracil has not been considered due to lack of experimental confirmation. For details see main text.
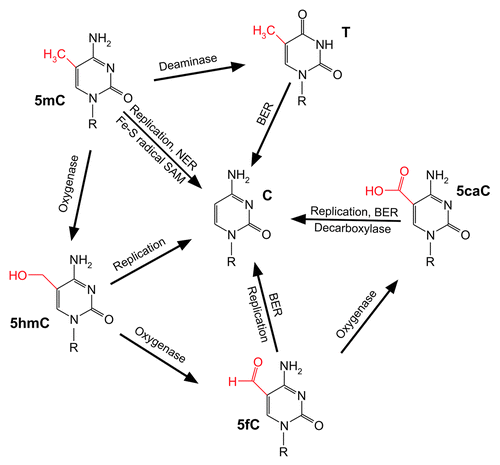
On the other hand, DNA repair mechanisms that exchange complete nucleotides by canonical (unmethylated) DNA building blocks attracted attention in regard to active DNA demethylation. In fact, DNA glycosylases from the Demeter/ROS1 family initiate base excision repair (BER) of methylated cytosines in plants leading to DNA demethylation.Citation15-Citation17 In mammals, however, a 5mC-specific DNA glycosylase could not be confirmed.Citation18-Citation21 An elegant loophole was provided by the idea that 5mC is first deaminated to thymine resulting in a T/G mismatch which, in turn, is processed by thymine-specific DNA glycosylases such as TDG. PGCs deficient in activation-induced (DNA-cytosine) deaminase (AID) show a significant albeit far from complete impairment of global demethylation.Citation22 In line with this, using an interspecies heterokaryon technology for reprogramming it was shown that AID is required to demethylate the critical genes OCT4 and NANOG in human fibroblasts.Citation23 Moreover, reprogramming of mouse embryonic fibroblasts (MEFs) to iPSCs by the four Yamanaka factors Oct4, Sox2, c-Myc and Klf4Citation11 was demonstrated to depend on a catalytically active AID.Citation24 Interestingly, in absence of the cofactor SAM the de novo DNA methyltransferases Dnmt3a and Dnmt3b are able to deaminate 5mC, thereby, potentially enabling dynamical methylation/demethylation cylces in short periods of time on specific genes.Citation9
The paternal pronucleus in the mouse zygote exhibits high amounts of DNA strand breaks within the critical time frame of global demethylation indicative for an involvement of DNA repair in this process.Citation25 Strikingly, DNA strand breaks in direct vicinity of 5mCs were found in an enhancer region of the tyrosine aminotransferase gene that is demethylated after hormone stimulation.Citation26 The result supports short patch base excision repair as being part of the demethylation machinery.
Long patch excision repair has also been attributed to DNA demethylation. Notably, in Xenopus laevis oocytes demethylation of an oct4-reporter was shown to critically depend on the xeroderma pigmentosum complementation group G protein (XPG), the 3′-endonuclease of nucleotide excision repair (NER).Citation27 Demethylation of the same reporter was accompanied by BrdU incorporation, indicating long patch repair synthesis instead of a one nucleotide exchange.Citation27 Demethylation of the human rDNA promoter requires the endonuclease activity of XPGCitation28 and the RARβ2 promoter is occupied by NER factors and exhibits DNA strand breaks, triggering DNA demethylation upon retinoic acid stimulation.Citation29,Citation30
Research in the field was revolutionized by two breakthrough papers in 2009 describing the (re)discovery of 5-hydroxymethylcytosine (5hmC) in human cells.Citation31,Citation32 5hmC is the oxidation product of 5mC catalyzed by the ten-eleven translocation (Tet1–3) family of enzymes.Citation32,Citation33 Tet enzymes can oxidize 5hmC further to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC).Citation34,Citation35 All three oxidation products have been detected in genomic DNACitation35-Citation37 and their role as potential DNA demethylation intermediates has been extensively analyzed since then. Indeed, 5fC and 5caC are substrates for TDGCitation34,Citation38 and the embryonic lethal phenotype of Tdg null mice was explained in part by aberrant methylation during early development.Citation39
Recently, the distribution of 5fC and 5caC in the genome of mouse ES cells (mESCs) has been achieved by high-throughput sequencing of (1) immunoprecipitated DNA using modification-specific antibodies and (2) streptavidin-captured DNA after chemical reduction and specific biotin-labeling of 5fC.Citation40,Citation41 Both studies demonstrated a significant accumulation of 5fC (and 5caC) residues at distal regulatory regions (enhancers) of genes across the genome in TDG-deficient mESCs, as compared with wild-type cells. Hence, 5fC and 5caC serve as intermediates of active DNA demethylation triggered by the Tet-family and TDG/BER at least at distinct genomic loci.
5hmC was postulated to be deaminated by members of the AID/APOBEC family of deaminases with the resulting 5-hydroxymethyluracil (5hmU) being a substrate for 5hmU-glycosylases such as SMUG1.Citation42 However, neither AID nor APOBEC enzymes are able to deaminate 5hmC in vitroCitation43,Citation44 and 5hmU could not be detected in genomic DNA.Citation43
The importance of Tet-mediated DNA demethylation was demonstrated in a recent study describing a modified protocol for the generation of iPSCs. One of the Yamanaka factors, Oct4, could be replaced by Tet1, highlighting the significance of the conversion of 5mC to 5hmC during reprogramming.Citation45
Genomic stability is challenged by strand breaks, base-free sites or nucleotide gaps that necessarily occur during base or nucleotide excision repair. If coping with such toxic intermediates during demethylation at single loci might be a solvable task for cells, the same problem, likely, is of a different nature during global DNA demethylation in a limited time frame. As a matter of fact, in the paternal pronucleus of the mouse zygote, 5hmC signals appear concomitant with the loss of 5mC signals.Citation46-Citation48 A major fraction of 5mCs was, obviously, oxidized, and this was dependent on Tet3. Later, it was also shown that 5fC and 5caC signals occur during late pronuclear stages.Citation36 Strikingly, the signals for 5hmC, 5fC and 5caC diminished subsequently after each round of replication by a factor of two.Citation36,Citation49 The majority of genome-wide DNA demethylation in the paternal pronucleus is therefore a mixture of active enzymatic conversion of 5mCs and passive, replication-coupled dilution of the oxidation products, avoiding any threat for genomic instability.Citation50 Moreover, a similar strategy for global DNA demethylation holds true for mouse PGC reprogramming. In contrast to what happens in the zygote, Tet1 and Tet2 are responsible for the conversion of a majority of 5mCs to 5hmCs in PGCs.Citation51
The three-step oxidation of a methylated pyrimidine, as it is the case for Tet-mediated conversion of 5mC to 5caC, has a precedent. In the thymidine salvage pathway, thymine is converted to uracil, in other words, 5-methyluracil is demethylated. Beside a consecutive three-step oxidation to iso-orotate (5-carboxyluracil), the pathway includes a final decarboxylation to gain uracil.Citation52 In search for a parallel pathway for 5mC demethylation, a decarboxylation activity toward 5caC was indeed demonstrated in a cell-free system using nuclear extracts of mESCs.Citation53 The result opens up a new avenue for non-toxic DNA demethylation.
In conclusion, the mechanisms of active DNA demethylation in mammals not only tend to be highly multifaceted but also offer the promise of surprising us in future research.
Gadd45 Proteins are Regulators of DNA Demethylation
Knowledge about the core enzymes acting on 5mC and its derivatives is just one side of the coin. Non-enzymatic mediators of DNA demethylation shed light on the regulation of the process upstream. Growth arrest and DNA damage-inducible protein 45 a (Gadd45a) were shown to promote DNA demethylation by DNA repair.Citation27,Citation54,Citation55 Gadd45a is an 18 kDa acidic protein without obvious enzymatic activity. Gadd45a is located in the nucleus and associated with ribonucleoprotein speckles.Citation56 Together with Gadd45b and Gadd45g, it is part of a family of histone fold stress-response proteins that modulate diverse cellular processes, one of them being DNA repair.Citation57,Citation58 Epigenetically, Gadd45 proteins act as scaffold proteins for downstream components of the repair machinery, thus directly regulating DNA demethylation. Gadd45 seems to promote gene-specific demethylation only.Citation59-Citation61 Typically, specific external stimuli lead to the upregulation of Gadd45 proteins, which, in turn, drive the demethylation and transcriptional activation of certain target genes. For instance, after electroconvulsive treatment of adult mice neurons Gadd45b is strongly upregulated leading to demethylation and expression of key genes for adult neurogenesis.Citation62
Together with components of the NER machinery (see above) Gadd45a has been demonstrated to be required to demethylate reporter plasmids in Xenopus oocytes as well as the human rDNA and RARβ2 promoter.Citation27,Citation28,Citation30 Gadd45 proteins also promote BER-mediated DNA demethylation, as has been first demonstrated for foreign methylated DNA and endogenous target genes in zebrafish embryos.Citation63 A T/G mismatch intermediate accompanied the demethylation, suggesting deamination of 5mC prior to BER.Citation63 GADD45a was also found to physically interact with AID and TDG in human cells.Citation64 The protein, thus, might regulate pivotal processes during development. However, in contrast to Tdg−/− mutants, mice lacking Gadd45a (but not simultaneously Gadd45b and Gadd45g) are viable and do not show significant alterations in global DNA methylation.Citation59 A potential involvement of Gadd45 in Tet-mediated DNA demethylation remains to be investigated (see below).
Ing1 Directs DNA Demethylation to H3K4me3
Gene-specific DNA demethylation primarily affects methylated CpGs within regulatory regions of limited lengths, whereas 5mCs within the gene body or in intergenic regions are often left untouched. This site specificity suggests a targeting mechanism that guides general demethylation factors to certain CpGs.
Mammalian DNA is wrapped around histone proteins organized in nucleosomes that consist of two H2A-H2B dimers and a H3-H4 tetramer each. Histone proteins are posttranslationally covalently modified by, e.g., methylation, acetylation or phosphorylation of distinct amino acids at the N-terminal tails, constituting the so-called histone code.Citation65 The histone code is an important epigenetic feature regulating gene expression and DNA repair, among other processes. Trimethylation of H3 lysine 4 (H3K4me3) is typically found at promoter regions of genes that are transcriptionally active.Citation66 Thus, the occurrence of H3K4me3 strikingly resembles hypomethylation of promoter regions after gene-specific DNA demethylation and, hence, gene activation. Are both epigenetic marks linked to each other to determine the transcriptional status of the corresponding gene? And, if yes, what is the cause and what is the effect?
The answers to those questions were provided recently by the identification of an H3K4me3 reader that directs Gadd45a and the demethylation machinery to their target 5mCs.Citation67 The protein inhibitor of growth 1 (human ING1b, mouse Ing1) is a known interactor of Gadd45a and exhibits similar properties as both are stress-response proteins influencing the cell cycle and promoting DNA repair.Citation68 Schäfer et al.Citation67 at first confirmed the physical interaction of GADD45a and ING1b in the human cell line HEK293T. They identified that ING1b cooperates with and is required for GADD45a to demethylate and reactivate methylation-silenced reporter plasmids. ING1b consists of an N-terminal PCNA interacting protein domain, a partial bromodomain and a C-terminal plant homeodomain (PHD finger), enabling binding to H3K4me3.Citation69 Importantly, the PHD finger domain was essential for GADD45a-mediated demethylation suggesting a link between the histone mark H3K4me3 and 5mC demethylation. Indeed, in RKO cells, the MAGEB2 promoter, an endogenous target of GADD45a demethylation, was occupied with H3K4me3 marks; GADD45a and ING1b were bound to the same region simultaneously. MEFs lacking Ing1 or Gadd45a had a reduced, double knockouts an abolished potential to induce expression of the Mageb1–3 genes and demethylation of the Mageb2 promoter upon UV-stimulation. Strikingly, knockdown of Wdr5, an essential subunit of the H3K4 methyltransferase complex MLL,Citation70 prevented Mageb1–3 expression and Mageb2 demethylation induced by either UV-irradiation or GADD45a and ING1b overexpression in wild-type MEFs. Moreover, loss of H3K4me3 marks impaired GADD45a and ING1b binding to the Mageb2 locus. Global gene expression profiling of HEK293T cells overexpressing GADD45a, ING1b or both proteins as well as MEFs lacking Gadd45a, Ing1 or both genes revealed roughly a hundred additional endogenous target genes in the human and mouse genome, respectively, potentially regulated by the same mechanism, as exemplified for the Mage genes above. Of note, this does not apply for all genes affected in their gene expression by Gadd45a and Ing1, since a comparable number of genes was, against expectations, also upregulated in double knockout MEFs. However, it should not be neglected that DNA demethylation is just one of many cellular processes influenced by Gadd45a and Ing1.
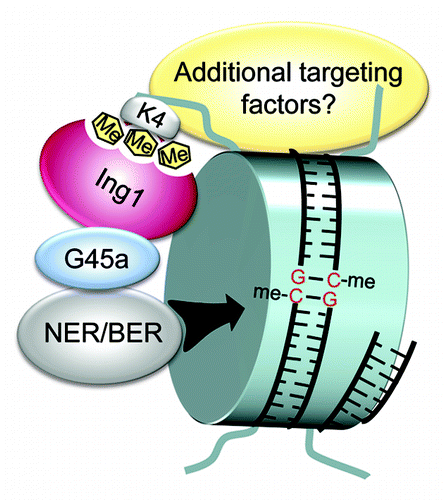
The study uncovered an unknown player in mammalian gene-specific DNA demethylation upstream of the enzymatic reactions on 5mC and links the histone code with DNA demethylation by two factors: Ing1, an H3K4me3 reader, and Gadd45a, a DNA demethylation regulator. The data favors a model by which H3K4me3 serves as a determinant for DNA demethylation via Ing1 and Gadd45a (). Interestingly, in a pull-down assay of mESC nuclear extracts using modified oligonucleotides as bait, Ing1 was found to bind 5fC residues in vitro.Citation71 The result hints at an involvement of Ing1 in Tet-mediated DNA demethylation. In line with this, and as already stated, Gadd45a was shown to directly interact with TDG.Citation64 Thus, Ing1 might target Gadd45a/TDG to oxidized derivatives of 5mC. This assumption has to be clarified in upcoming studies. Additionally, future work has now to decipher the physiological relevance of the targeting process in development and disease, as well as to identify additional cofactors required for regulating demethylation. Finally, the chromatin context should be considered when analyzing the modus operandi of known or yet-to-be-identified demethylation factors.
Figure 2. Ing1 directs Gadd45a and the demethylation machinery to H3K4me3. Proposed model for site-specific demethylation by Gadd45a. At a given gene promoter histone H3 trimethylated at lysine 4 (H3K4me3) is specifically recognized by Ing1. Gadd45a and the repair machinery are recruited through Ing1 binding. Subsequently, 5mCs are excised by DNA repair leading to DNA demethylation. Additional targeting factors may be required for the process. G45a, Gadd45a; NER, nucleotide excision repair; BER, base excision repair. Figure is from ref. Citation67 with permission of the authors.
Summary
The complex world of mammalian DNA demethylation emerges from studies identifying numerous enzymes acting on 5mC and its derivatives. Unequivocally, DNA repair mechanisms are involved in gene-specific DNA demethylation whereas cells presumably avoid hazardous intermediates of DNA repair in genome-wide erasure of methylation marks during development. Gadd45 proteins are regulators of gene-specific and repair-mediated demethylation in different contexts. Site-specific demethylation by Gadd45a is ensured by a targeting mechanism involving the histone mark H3K4me3 as determinant and the histone code reader Ing1 as transducer. The mechanism seems to be valid for the epigenetic regulation of a substantial amount of genes in humans and mice.
Acknowledgment
I thank Andrea Schäfer, IMB Mainz, for providing and apologize to those colleagues whose work was not cited due to space limitations. This work was supported by an ERC senior investigator grant (“DNA Demethylase”) to Christof Niehrs, IMB Mainz.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Costello JF, Plass C. Methylation matters. J Med Genet 2001; 38:285 - 303; http://dx.doi.org/10.1136/jmg.38.5.285; PMID: 11333864
- Ehrlich M. The controversial denouement of vertebrate DNA methylation research. Biochemistry (Mosc) 2005; 70:568 - 75; http://dx.doi.org/10.1007/s10541-005-0150-z; PMID: 15948710
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science 2001; 293:1068 - 70; http://dx.doi.org/10.1126/science.1063852; PMID: 11498573
- Hermann A, Goyal R, Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem 2004; 279:48350 - 9; http://dx.doi.org/10.1074/jbc.M403427200; PMID: 15339928
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature 2000; 403:501 - 2; http://dx.doi.org/10.1038/35000656; PMID: 10676950
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol 2000; 10:475 - 8; http://dx.doi.org/10.1016/S0960-9822(00)00448-6; PMID: 10801417
- Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 2002; 117:15 - 23; http://dx.doi.org/10.1016/S0925-4773(02)00181-8; PMID: 12204247
- Kangaspeska S, Stride B, Métivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, et al. Transient cyclical methylation of promoter DNA. Nature 2008; 452:112 - 5; http://dx.doi.org/10.1038/nature06640; PMID: 18322535
- Métivier R, Gallais R, Tiffoche C, Le Péron C, Jurkowska RZ, Carmouche RP, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 2008; 452:45 - 50; http://dx.doi.org/10.1038/nature06544; PMID: 18322525
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861 - 72; http://dx.doi.org/10.1016/j.cell.2007.11.019; PMID: 18035408
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 2010; 11:204 - 20; http://dx.doi.org/10.1038/nrg2719; PMID: 20142834
- Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 2010; 11:607 - 20; http://dx.doi.org/10.1038/nrm2950; PMID: 20683471
- Okada Y, Yamagata K, Hong K, Wakayama T, Zhang Y. A role for the elongator complex in zygotic paternal genome demethylation. Nature 2010; 463:554 - 8; http://dx.doi.org/10.1038/nature08732; PMID: 20054296
- Agius F, Kapoor A, Zhu JK. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc Natl Acad Sci U S A 2006; 103:11796 - 801; http://dx.doi.org/10.1073/pnas.0603563103; PMID: 16864782
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, et al. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 2006; 124:495 - 506; http://dx.doi.org/10.1016/j.cell.2005.12.034; PMID: 16469697
- Morales-Ruiz T, Ortega-Galisteo AP, Ponferrada-Marín MI, Martínez-Macías MI, Ariza RR, Roldán-Arjona T. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc Natl Acad Sci U S A 2006; 103:6853 - 8; http://dx.doi.org/10.1073/pnas.0601109103; PMID: 16624880
- Bennett MT, Rodgers MT, Hebert AS, Ruslander LE, Eisele L, Drohat AC. Specificity of human thymine DNA glycosylase depends on N-glycosidic bond stability. J Am Chem Soc 2006; 128:12510 - 9; http://dx.doi.org/10.1021/ja0634829; PMID: 16984202
- Cortázar D, Kunz C, Saito Y, Steinacher R, Schär P. The enigmatic thymine DNA glycosylase. DNA Repair (Amst) 2007; 6:489 - 504; http://dx.doi.org/10.1016/j.dnarep.2006.10.013; PMID: 17116428
- Zhu B, Zheng Y, Angliker H, Schwarz S, Thiry S, Siegmann M, et al. 5-Methylcytosine DNA glycosylase activity is also present in the human MBD4 (G/T mismatch glycosylase) and in a related avian sequence. Nucleic Acids Res 2000; 28:4157 - 65; http://dx.doi.org/10.1093/nar/28.21.4157; PMID: 11058112
- Zhu B, Zheng Y, Hess D, Angliker H, Schwarz S, Siegmann M, et al. 5-methylcytosine-DNA glycosylase activity is present in a cloned G/T mismatch DNA glycosylase associated with the chicken embryo DNA demethylation complex. Proc Natl Acad Sci U S A 2000; 97:5135 - 9; http://dx.doi.org/10.1073/pnas.100107597; PMID: 10779566
- Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 2010; 463:1101 - 5; http://dx.doi.org/10.1038/nature08829; PMID: 20098412
- Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 2010; 463:1042 - 7; http://dx.doi.org/10.1038/nature08752; PMID: 20027182
- Bhutani N, Decker MN, Brady JJ, Bussat RT, Burns DM, Corbel SY, et al. A critical role for AID in the initiation of reprogramming to induced pluripotent stem cells. FASEB J 2013; 27:1107 - 13; http://dx.doi.org/10.1096/fj.12-222125; PMID: 23212122
- Wossidlo M, Arand J, Sebastiano V, Lepikhov K, Boiani M, Reinhardt R, et al. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J 2010; 29:1877 - 88; http://dx.doi.org/10.1038/emboj.2010.80; PMID: 20442707
- Kress C, Thomassin H, Grange T. Active cytosine demethylation triggered by a nuclear receptor involves DNA strand breaks. Proc Natl Acad Sci U S A 2006; 103:11112 - 7; http://dx.doi.org/10.1073/pnas.0601793103; PMID: 16840560
- Barreto G, Schäfer A, Marhold J, Stach D, Swaminathan SK, Handa V, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007; 445:671 - 5; http://dx.doi.org/10.1038/nature05515; PMID: 17268471
- Schmitz KM, Schmitt N, Hoffmann-Rohrer U, Schäfer A, Grummt I, Mayer C. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell 2009; 33:344 - 53; http://dx.doi.org/10.1016/j.molcel.2009.01.015; PMID: 19217408
- Le May N, Fradin D, Iltis I, Bougnères P, Egly JM. XPG and XPF endonucleases trigger chromatin looping and DNA demethylation for accurate expression of activated genes. Mol Cell 2012; 47:622 - 32; http://dx.doi.org/10.1016/j.molcel.2012.05.050; PMID: 22771116
- Le May N, Mota-Fernandes D, Vélez-Cruz R, Iltis I, Biard D, Egly JM. NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol Cell 2010; 38:54 - 66; http://dx.doi.org/10.1016/j.molcel.2010.03.004; PMID: 20385089
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009; 324:929 - 30; http://dx.doi.org/10.1126/science.1169786; PMID: 19372393
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324:930 - 5; http://dx.doi.org/10.1126/science.1170116; PMID: 19372391
- Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466:1129 - 33; http://dx.doi.org/10.1038/nature09303; PMID: 20639862
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011; 333:1303 - 7; http://dx.doi.org/10.1126/science.1210944; PMID: 21817016
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011; 333:1300 - 3; http://dx.doi.org/10.1126/science.1210597; PMID: 21778364
- Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res 2011; 21:1670 - 6; http://dx.doi.org/10.1038/cr.2011.189; PMID: 22124233
- Pfaffeneder T, Hackner B, Truss M, Münzel M, Müller M, Deiml CA, et al. The discovery of 5-formylcytosine in embryonic stem cell DNA. Angew Chem Int Ed Engl 2011; 50:7008 - 12; http://dx.doi.org/10.1002/anie.201103899; PMID: 21721093
- Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem 2011; 286:35334 - 8; http://dx.doi.org/10.1074/jbc.C111.284620; PMID: 21862836
- Cortázar D, Kunz C, Selfridge J, Lettieri T, Saito Y, MacDougall E, et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature 2011; 470:419 - 23; http://dx.doi.org/10.1038/nature09672; PMID: 21278727
- Shen L, Wu H, Diep D, Yamaguchi S, D’Alessio AC, Fung HL, et al. Genome-wide Analysis Reveals TET- and TDG-Dependent 5-Methylcytosine Oxidation Dynamics. Cell 2013; 153:692 - 706; http://dx.doi.org/10.1016/j.cell.2013.04.002; PMID: 23602152
- Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, et al. Genome-wide Profiling of 5-Formylcytosine Reveals Its Roles in Epigenetic Priming. Cell 2013; 153:678 - 91; http://dx.doi.org/10.1016/j.cell.2013.04.001; PMID: 23602153
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 2011; 145:423 - 34; http://dx.doi.org/10.1016/j.cell.2011.03.022; PMID: 21496894
- Nabel CS, Jia H, Ye Y, Shen L, Goldschmidt HL, Stivers JT, et al. AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nat Chem Biol 2012; 8:751 - 8; http://dx.doi.org/10.1038/nchembio.1042; PMID: 22772155
- Rangam G, Schmitz KM, Cobb AJ, Petersen-Mahrt SK. AID enzymatic activity is inversely proportional to the size of cytosine C5 orbital cloud. PLoS One 2012; 7:e43279; http://dx.doi.org/10.1371/journal.pone.0043279; PMID: 22916236
- Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, et al. Replacement of Oct4 by Tet1 during iPSC Induction Reveals an Important Role of DNA Methylation and Hydroxymethylation in Reprogramming. Cell Stem Cell 2013; 12:453 - 69; http://dx.doi.org/10.1016/j.stem.2013.02.005; PMID: 23499384
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 2011; 477:606 - 10; http://dx.doi.org/10.1038/nature10443; PMID: 21892189
- Iqbal K, Jin SG, Pfeifer GP, Szabó PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A 2011; 108:3642 - 7; http://dx.doi.org/10.1073/pnas.1014033108; PMID: 21321204
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun 2011; 2:241; http://dx.doi.org/10.1038/ncomms1240; PMID: 21407207
- Inoue A, Zhang Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science 2011; 334:194; http://dx.doi.org/10.1126/science.1212483; PMID: 21940858
- Münzel M, Lischke U, Stathis D, Pfaffeneder T, Gnerlich FA, Deiml CA, et al. Improved synthesis and mutagenicity of oligonucleotides containing 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine. Chemistry 2011; 17:13782 - 8; http://dx.doi.org/10.1002/chem.201102782; PMID: 22069110
- Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 2013; 339:448 - 52; http://dx.doi.org/10.1126/science.1229277; PMID: 23223451
- Smiley JA, Kundracik M, Landfried DA, Barnes VR Sr., Axhemi AA. Genes of the thymidine salvage pathway: thymine-7-hydroxylase from a Rhodotorula glutinis cDNA library and iso-orotate decarboxylase from Neurospora crassa. Biochim Biophys Acta 2005; 1723:256 - 64; http://dx.doi.org/10.1016/j.bbagen.2005.02.001; PMID: 15794921
- Schiesser S, Hackner B, Pfaffeneder T, Müller M, Hagemeier C, Truss M, et al. Mechanism and stem-cell activity of 5-carboxycytosine decarboxylation determined by isotope tracing. Angew Chem Int Ed Engl 2012; 51:6516 - 20; http://dx.doi.org/10.1002/anie.201202583; PMID: 22644704
- Niehrs C. Active DNA demethylation and DNA repair. Differentiation 2009; 77:1 - 11; http://dx.doi.org/10.1016/j.diff.2008.09.004; PMID: 19281759
- Niehrs C, Schäfer A. Active DNA demethylation by Gadd45 and DNA repair. Trends Cell Biol 2012; 22:220 - 7; http://dx.doi.org/10.1016/j.tcb.2012.01.002; PMID: 22341196
- Sytnikova YA, Kubarenko AV, Schäfer A, Weber AN, Niehrs C. Gadd45a is an RNA binding protein and is localized in nuclear speckles. PLoS One 2011; 6:e14500; http://dx.doi.org/10.1371/journal.pone.0014500; PMID: 21249130
- Hollander MC, Fornace AJ Jr.. Genomic instability, centrosome amplification, cell cycle checkpoints and Gadd45a. Oncogene 2002; 21:6228 - 33; http://dx.doi.org/10.1038/sj.onc.1205774; PMID: 12214253
- Smith ML, Kontny HU, Zhan Q, Sreenath A, O’Connor PM, Fornace AJ Jr.. Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to u.v.-irradiation or cisplatin. Oncogene 1996; 13:2255 - 63; PMID: 8950993
- Engel N, Tront JS, Erinle T, Nguyen N, Latham KE, Sapienza C, et al. Conserved DNA methylation in Gadd45a(-/-) mice. Epigenetics 2009; 4:98 - 9; http://dx.doi.org/10.4161/epi.4.2.7858; PMID: 19229137
- Jin SG, Guo C, Pfeifer GP. GADD45A does not promote DNA demethylation. PLoS Genet 2008; 4:e1000013; http://dx.doi.org/10.1371/journal.pgen.1000013; PMID: 18369439
- Schäfer A, Schomacher L, Barreto G, Döderlein G, Niehrs C. Gemcitabine functions epigenetically by inhibiting repair mediated DNA demethylation. PLoS One 2010; 5:e14060; http://dx.doi.org/10.1371/journal.pone.0014060; PMID: 21124914
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009; 323:1074 - 7; http://dx.doi.org/10.1126/science.1166859; PMID: 19119186
- Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell 2008; 135:1201 - 12; http://dx.doi.org/10.1016/j.cell.2008.11.042; PMID: 19109892
- Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 2011; 146:67 - 79; http://dx.doi.org/10.1016/j.cell.2011.06.020; PMID: 21722948
- Jenuwein T, Allis CD. Translating the histone code. Science 2001; 293:1074 - 80; http://dx.doi.org/10.1126/science.1063127; PMID: 11498575
- Koch CM, Andrews RM, Flicek P, Dillon SC, Karaöz U, Clelland GK, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res 2007; 17:691 - 707; http://dx.doi.org/10.1101/gr.5704207; PMID: 17567990
- Schäfer A, Karaulanov E, Stapf U, Döderlein G, Niehrs C. Ing1 functions in DNA demethylation by directing Gadd45a to H3K4me3. Genes Dev 2013; 27:261 - 73; http://dx.doi.org/10.1101/gad.186916.112; PMID: 23388825
- Cheung KJ Jr., Mitchell D, Lin P, Li G. The tumor suppressor candidate p33(ING1) mediates repair of UV-damaged DNA. Cancer Res 2001; 61:4974 - 7; PMID: 11431327
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 2006; 442:86 - 90; PMID: 16728976
- Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 2005; 121:859 - 72; http://dx.doi.org/10.1016/j.cell.2005.03.036; PMID: 15960974
- Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 2013; 152:1146 - 59; http://dx.doi.org/10.1016/j.cell.2013.02.004; PMID: 23434322