Abstract
It was previously demonstrated that miR-199a was downregulated in testicular germ cell tumor (TGCT), probably due to hypermethylation of its promoter. Further study found that re-expression of miR-199a in testicular cancer cells (NT2) led to suppression of cell growth, cancer migration, invasion and metastasis. More detailed analyses showed that these properties of miR-199a could be assigned to miR-199a-5p, one of its two derivatives. The biological role of the other derivative, miR-199a-3p in TGCT, remains largely uncharacterized. In this report, we identified DNA (cytosine-5)-methyltransferase 3A (DNMT3A), the de novo methyltransferase, as a direct target of miR-199a-3p using a 3′-UTR reporter assay. Transient expression of miR-199a-3p in NT2 cells led to decrease, while knocking down of miR-199a-3p in a normal human testicular cell line (HT) led to elevation, of DNMT3A2 (DNMT3A gene isoform 2) mRNA and protein levels. In clinical samples, DNMT3A2 was significantly overexpressed in malignant testicular tumor, and the expression of DNMT3A2 was inversely correlated with the expression of miR-199a-3p. However, DNMT3A did not affect miR-199a expression in NT2 cells. Further characterization of miR-199a-3p revealed that it negatively regulated DNA methylation, partly through targeting DNMT3A. Overexpression of miR-199a-3p restored the expression of APC and MGMT tumor-suppressor genes in NT2 cells by affecting DNA methylation of their promoter regions. Our studies demonstrated the deregulation of miR-199a-3p expression in TGCT may provide novel mechanistic insights into TGCT carcinogenesis and suggested a potentially therapeutic use of synthetic miR-199a-3p oligonucleotides as effective hypomethylating compounds in the treatment of TGCT.
Introduction
Epigenetic mechanisms, including methylation, histone modification, nucleosome positioning and non-coding RNAs, are essential for normal development and maintenance of gene expression patterns in mammals. Abnormalities of these epigenetic processes have been regarded as closely related to disease development, including human cancer.Citation1 Currently, three DNA methyltransferases, namely, DNMT1, DNMT3A, and DNMT3B, have been identified to be responsible for DNA methylation in mammals. All of them facilitate the transfer of the methyl group from S-adenosyl-methionine (SAM) to the C in CpG site. DNMT1 is responsible for the maintenance of methylation, and it has a 30- to 40-fold preference for hemimethylated DNA, while DNMT3A and DNMT3B play a role in de novo methylation, being responsible for establishing the methylation pattern necessary during embryonic development.Citation2
Testicular germ cell tumors (TGCTs) are the most frequent solid tumor of Caucasian adolescents and young adult males and are a diverse group of neoplasms that can also be present in extragonadal sites.Citation3 Histologically, TGCTs are divided into seminomas, which resemble primordial germ cells (PGCs) and non-seminomas, which are either undifferentiated (embryonal carcinoma) or differentiated (embryonic [teratoma] or extra-embryonic [yolk sac choriocarcinoma]).Citation4 In the previous genome-wide DNA methylation profiling study on TGCT conducted in our lab, larger numbers of differentially methylated regions (DMRs) were identified in NT2 cells and clinical TGCT samples, compared with normal human testicular cells (HT) and normal testis tissues.Citation5 Intriguingly, DNMT1 was upregulated in embryonal carcinoma,Citation6 DNMT3B was overexpressed in non-seminomas,Citation7,Citation8 and significant upregulation of DNMT3A2 but not DNMT3A was observed in TGCT (seminomas and non-seminomas).Citation9 Although aberrant DNA methylation and overexpressed DNMTs were also observed in other cancer types,Citation1 the causes of the change of methylation pattern and the overexpression of DNMTs have not been fully elucidated.
The DNMT3A2 isoform of DNMT3A gene was first reported by Chen et al.Citation10 in 2002. The DNMT3A genomic locus contains two genes, DNMT3A1 and DNMT3A2. Transcription of the DNMT3A2 isoform is initiated from a different promoter in the sixth intron of the DNMT3A gene; thus, the DNMT3A2 protein (approximately 82 kDa) lacks the N-terminal 223 (human) or 219 (mouse) amino acid residues of the full-length DNMT3A1 (termed as the long isoform, approximately 120 kDa). Recombinant DNMT3A2 protein displayed similar cytosine methyltransferase activity as DNMT3A in vitro.Citation10 A high-throughput gene-specific DNA methylation analysis of HEK 293T cells stably transfected with two DNMT3A isoforms separately, revealed that DNMT3A1 and DNMT3A2 shared 93% DNA methylation target sites.Citation11 Suetake et al.Citation12 found that the N-terminal sequence from residues 1–211 of full-length DNMT3A1 isoform was able to bind to DNA, but could not distinguish methylated and unmethylated CpG, and contribute to higher DNA-binding and DNA-methylation activities for DNMT3A1 compared with DNMT3A2.
miRNAs, typically around 21 nucleotides in length, are a well-studied class of short ncRNAs in mammalian cells. The precise biological effects of miRNAs are far from being understood, even though miRNAs are known to bind to the 3′UTR of their target gene mRNAs and induce their translational repression, deadenylation or degradation.Citation13 Recent studies indicated that some specific miRNAs could play an important role in the control of global methylation pattern through targeting the DNA methylation machinery. These include the miR-29 family,Citation14 miR-152,Citation15 mir-126,Citation16 and miR-450a.Citation17 A newcomer is hsa-miR-199a, a miRNA transcribed as antisense of DYNAMIN 3 (chromosome 1q24.3), shown to be downregulated in TGCT.Citation5 pre-miR-199a is encoded by two loci in the human genome, miR-199a-1 in chromosome 19 and miR-199a-2 in chromosome 1. Both loci encode the same mature hsa-miR-199a. Previous studies showed that the promoters of both miR-199a-1 and miR-199a-2 were hypermethylated resulting in silencing of the expression of miR-199a in TGCTs (refs. Citation5 and Citation18 and Shen Gu et al., unpublished). Treatment of NT2 cells with 5-aza upregulated the expression of hsa-mir-199a by 42-fold.Citation18 Further studies found that re-expression of miR-199a in testicular cancer cells (NT2) led to suppression of cell growth, cancer migration, invasion and metastasis.Citation18 More detailed analyses showed that these properties of miR-199a could be assigned to miR-199a-5p, one of its two derivatives. The biological role of the other derivative, miR-199a-3p in TGCT, remains largely uncharacterized. Interestingly, as predicted by several in silico methods for target gene prediction, including PicTar,Citation19 TargetScan,Citation20 miRNA.org,Citation21 and DIANA LAB,Citation22 DNMT3A was identified as one of the high-scoring candidate targets of miR-199a-3p. Thus, it is possible that miR-199a-3p can regulate global DNA methylation pattern through direct targeting of DNMT3A, leading to the speculation that downregulation of miR-199a-3p may be a potential cause of overexpression of DNMT3A. Since hsa-miR-199a is downregulated in TGCT due to its hypermethylated promoter, it is also questionable whether DNMT3A could negatively regulate the expression of hsa-miR-199a.
Results
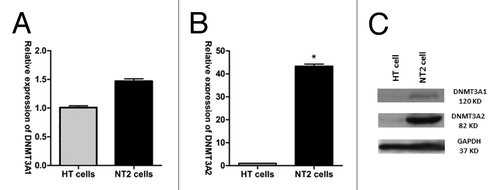
DNMT3A2 is significantly upregulated in NT2 cells compared with HT cells
The expression of DNMT3A (isoform 1 and 2) in HT cells and NT2 cells was compared by RT-qPCR. DNMT3A1 showed 1.5-fold upregulation in NT2 cells, whereas DNMT3A2 showed 44-fold upregulation (). Results of western blot analysis corroborated with this observation ().
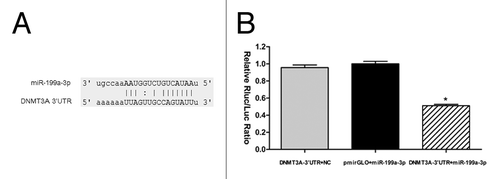
DNMT3A is the direct target of miR-199a-3p
DNMT3A was predicted to be one of the miR-199a-3p targets with high probability by several in silico methods. DNMT3A1 and DNMT3A2 share the same 3′-UTR. Therefore, both mRNAs are putative targets of miR-199a-3p (). To validate the miRNA-target interactions, the fragment of DNMT3A-3′-UTR containing the putative miR-199a-3p recognition sequence was cloned into the luciferase reporter vector pmirGLO. The recombinant plasmid was co-transfected with miR-199a-3p mimics or negative control RNA, or empty pmirGLO with miR-199a-3p mimics, into NT2 cells. As shown in , When co-transfected with miR-199a-3p mimics, the relative luciferase activities of pmirGLO-DNMT3A-3UTR luciferase reporter in NT2 cells were significantly suppressed by almost 50% compared with the transfection with negative control and empty pmirGLO vector.
Figure 2. miR-199a-3p directly targets DNMT3A. (A) The putative human DNMT3A 3′-UTR fragment containing miR-199a-3p binding sequence (837bp ~843bp) was inserted into the luciferase report vector pmirGLO downstream. (B) Dual luciferase assay of NT2 cells co-transfected with the luciferase constructs containing the DNMT3A 3′-UTR as well as miR-199a-3p mimics or scrambled oligonucleotides as the negative control.
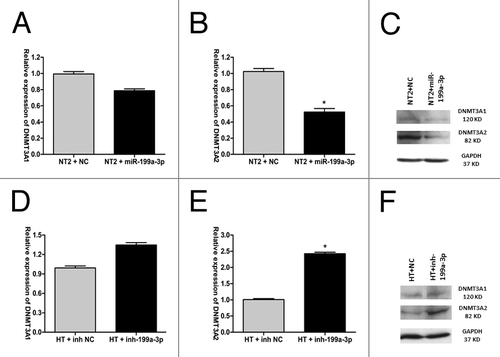
miR-199a-3p regulates endogenous DNMT3A expression, especially DNMT3A2 expression
To test the hypothesis that miR-199a-3p downregulates DNMT3A expression in NT2 cells, mRNA levels (as measured by RT-qPCR) and protein levels (as measured by western blot analysis) of DNMT3A1/2 isoforms were measured 48 h after transient transfection of miR-199a-3p mimics into NT2 cells. The results showed that miR-199a-3p expression caused a significant decrease of DNMT3A1 and DNMT3A2 expression at both mRNA and protein levels in comparison with the negative control in NT2 cells. The reduction of DNMT3A2 expression was more significant (p = 0.0104) (). Knocking down of miR-199a-3p expression in HT cells using an anti-miRNA inhibitor specific for miR-199a-3p resulted in significant upregulation of DNMT3A1/2 mRNA and protein expression, especially DNMT3A2 (p = 0.0017) ().
Figure 3. miR-199a-3p regulates DNMT3A expression. (A) DNMT3A1 mRNA and (B) DNMT3A2 mRNA expression after the transfection with miR-199a-3p mimics or negative control (NC) in NT2 cells. (C) DNMT3A1/2 protein expression of NT2 cells transfected with miR-199a-3p mimics or NC. (D) DNMT3A1 mRNA (E) DNMT3A2 mRNA expression after the transfection with an anti-miRNA inhibitor specific for miR-199a-3p or negative control (NC) in HT cells. (F) DNMT3A1/2 proteins expression of HT cells transfected with an anti-miRNA inhibitor specific for miR-199a-3p or NC.
miR-199a-3p and DNMT3A2 are reciprocally regulated in clinical samples
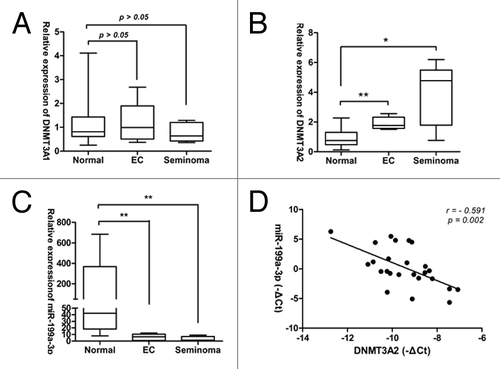
The expression levels of miR-199a-3p and DNMT3A (1/2) in 25 clinical samples was tested. DNMT3A2 was significantly upregulated in tumor tissues (either embryonal carcinoma or seminoma) compared with normal tissues (), but no significant difference of DNM3A1 expression was observed between tumor tissues (neither in embryonal carcinoma nor in seminoma) and normal tissues (). Significantly downregulated expression of miR-199a-3p was observed in tumor tissues (either in embryonal carcinoma or in seminoma) (). In addition, a statistically significant inverse correlation was observed between DNMT3A2 mRNA and miR-199a-3p in clinical samples (n = 25, r = -0.591, p = 0.002. Pearson’s correlation; ). These data showed the reciprocal regulation of the tumor suppressor miR-199a-3p and its target DNMT3A2 in human TGCTs and suggested that downregulation of miR-199a-3p may play a causal role in aberrant DNA methylation leading to TGCT development.
Figure 4. miR-199a-3p expression and DNMT3A2 mRNA levels inversely correlate in human TGCT tissues. (A) DNM3A1 mRNA expression (B) DNMT3A2 mRNA expression (C) miR-199a-3p expression in TGCT tissues compared with Normal testis tissues. (D) miR-199a-3p and DNMT3A2 mRNA expression levels inversely correlated in 25 clinical samples. Statistical analysis to evaluate correlation was performed using Pearson's correlation analysis (r = −0.591, p = 0.002).
DNMT3A does not negatively regulate pre-miR-199a expression via promoter methylation in NT2 cells
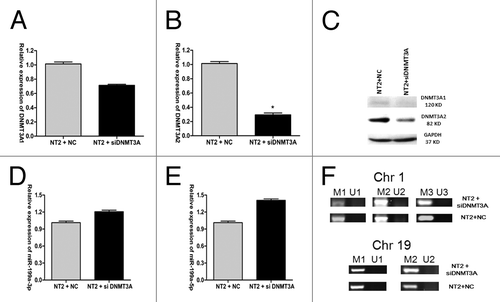
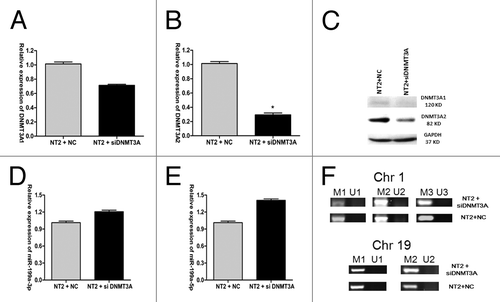
Since hsa-miR-199a-3p is silenced in NT2 cells, and a significant inverse correlation was observed between DNMT3A2 mRNA expression and miR-99a-3p mRNA expression, the possibility remains that DNMT3A expression could downregulate pre-miR-199a expression via promoter hypermethylation. To test this hypothesis, DNMT3A expression was inhibited by transfecting siDNMT3A (small interfering RNA specifically targeting DNMT3A) in NT2 cells. Similar to the observation made with miR-199a-3p overexpression, siDNMT3A significantly downregulated DNMT3A1 and DNMT3A2 expression at both mRNA and protein levels, and the reduction of DNMT3A2 expression was more significant () (two-tailed Student’s t test, p = 0.0188 for , p = 0.0008 for , triplicate samples). After 48h of transfection, the expression of two mature miRNAs of pre-miR-199a, miR-199a-3p and 5p, were significantly upregulated (Student’s test, p = 0.0165 for and p = 0.0021 for Fig. 5E, triplicate samples) (). Methylation-specific PCR (MSP) was used to examine the promoter methylation changes of pre-miR-199a. Five sets of MSP primers were designed, of which two sets of primers focus on two CpG rich regions of miR-199a-1, and three sets of primers focus on three CpG rich regions of miR-199a-2. Each set of primers contains two pairs of primers to amplify either methylated or unmethylated alleles. miR-199a-1 is localized in chromosome 19, with a size of 71 bp (+1 to +71) in human genome (10 928 102–10 928 172). The two regions selected for MSP analysis, based on their CpG content and being at the 5′ end of the gene,Citation23 are (-472 to +97) for M1/U1 and (-472 to -11) for M2/U2. miR-199a-2 is localized in chromosome 1, with a size of 110 bp (+1 to +110) in human genome (172 113 675–172 113 784). The three regions selected for MSP analysis are (-21 to +396) for M1/U1, (+65 to +374) for M2/U2 and (-21 to +100) for M3/U3. These three regions were previously identified to be in the potential promoter of miR-199a-2.Citation18 MSP results showed that the promoter methylation levels of pre-miR-199a in the two loci were not significantly changed after transfection with siDNMT3A (). Taken together, these results indicated that DNMT3A expression did not negatively regulate pre-miR-199a expression via promoter methylation in NT2 cells.
Figure 5. DNMT3A does not negatively regulate miR-199a expression via promoter methylation in NT2 cells. DNM3A1 mRNA expression (A), DNMT3A2 mRNA expression (B) and their protein levels (C) after transfection of siDNMT3A into NT2 cells. (D) MiR-199a-3p mRNA expression and (E) miR-199a-5p mRNA expression after transfection of siDNMT3A into NT2 cells. (F) pre-miR-199a promoter methylation on chromosome 1 and chromosome 19 in NT2 cells after siDNMT3A transfection.
Changes of miR-199a-3p affect the expression of APC and MGMT tumor-suppressor genes (TSGs), as well as the DNA methylation patterns of their promoter regions in NT2 cells
DNA hypermethylation contributes to the silencing of TSGs involved in testicular tumorigenesis.Citation24 Downregulation of miR-199a-3p allowed for increased DNMT3A2 expression, which could cause increased methylation. It was reasoned that the increased DNA methylation through this mechanism would hypermethylate some TSGs and facilitate the development of TGCT. Several TSGs have been identified as being frequently hypermethylated in TGCT, including PRSS21, RASSF1A, MGMT, SCGB3A1, HIC1, HOXA9, APC, and RUNX1.Citation25,Citation26 To assess whether miR-199a-3p could affect the mRNA expression of these 8 TSGs, miR-199a-3p was overexpressed in NT2 cells, and RT-qPCR was used to quantitate the expression level of the TSGs. showed that 5 of 8 tested TSGs (MGMT, SCGB3A1, HIC1, HOXA9 and APC) were significantly upregulated in the miR-199a-3p mimics group compared with negative control group. Next, the expression of miR-199a-3p was suppressed with an anti-miRNA inhibitor specific for miR-199a-3p in HT cells, RT-qPCR results revealed that three of the five TSGs were significantly downregulated in the miR-199a-3p inhibitor group, compared with the control group (). These results suggested that in TGCT, APC, MGMT, and SCGB3A1 were indirectly affected by miR-199a-3p, probably through the action of DNMT3A. In fact, the expression of APC, MGMT and SCGB3A1 was upregulated in the siDNMT3A group in which DNMT3A was knocked down, when compared with the NC group (). It indicated that APC, MGMT and SCGB3A1 were affected by DNMT3A. To examine whether miR-199a-3p regulated the expression of these three TSGs through affecting their promoter methylation, the promoter methylation patterns of APC, MGMT, and SCGB3A1 genes in NT2 and HT cells were assessed using bisulfite sequencing PCR (BSP) method. Consistent with previous observation, all three TSGs were hypermethylated in NT2 cells compared with HT cells (APC, 38.1% vs. 3.75%; MGMT, 40.7% vs. 12.7%; SCGB3A1, 42.2% vs. 17.8%. (Fig. S1). Furthermore, after transfection of miR-199a-3p mimics into NT2 cells, promoters methylation of APC and MGMT were significantly decreased in the miR-199a-3p mimics group compared with control (APC, 24.4% vs. 40.6%; MGMT, 14.3% vs. 39.2%), while the promoter methylation of SCGB3A1 was not significantly changed ().
Figure 6. Enforced miR-199a-3p expression decreases methylation of some gene promoters in NT2 cell lines. (A) PRSS21, RASSF1A, MGMT, SCGB3A1, HIC1, HOXA9, APC, and RUNX1 mRNA expression levels in NT2 cells after transfection of miR-199a-3p mimics. (B) APC, HIC1, HOXA9, MGMT and SCGB3A1 mRNA expression levels in HT cells after transfection of miR-199a-3p inhibitor. (C) APC, HIC1, HOXA9, MGMT and SCGB3A1 mRNA expression levels in NT2 cells after transfection of siDNMT3A. (D) The promoter methylation pattern of APC, MGME and SCGB3A1 in NT2 cells after transfection of miR-199a-3p mimics.
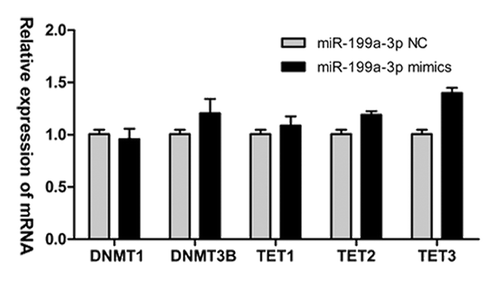
miR-199a-3p does not significantly affect the expression of other DNMTs and TETs in NT2 cells
Recent studies have shown that the TET family proteins can catalyze 5-methylcytosine (5mC) oxidation and generate 5-hydroxymethylcytosine (5hmC), which is essential for DNA demethylation.Citation27 Thus, there is a possibility that miR-199a-3p affects promoter methylation of some TSGs via the other DNMTs and TETs instead of DNMT3A alone. To rule out the involvement of the other DNMTs (DNMT1/3B) and TETs (TET1/2/3), the effects of miR-199a-3p on these epigenetic enzymes were investigated. RT-qPCR results indicated that enhanced miR-199a-3p expression did not significantly affect the expression of DNMT1, DNMT3B and the three members of the TET family ().
Discussion
Significant changes of global DNA methylation have been observed in cultured cancer cells and primary human tumor tissues. Aberrant hypermethylated CpG islands have been reported in almost every tumor type, including testicular germ cell tumors.Citation5 As discussed earlier, aberrant DNA methylation and overexpressed DNMTs were observed in TGCT or its subtypes. However, the underlying mechanisms remained not unclear. The current studies focused on the relationship between miR-199a-3p and DNMT3A in seminoma and embryonal carcinoma, a subtype of non-seminomas. Results obtained are relevant to these two subtypes of TGCT.
Ishii and colleaguesCitation9 recently examined the expression of DNMT3A mRNA and protein in TGCTs, RT-PCR analysis revealed that DNMT3A and DNMT3A2 were significantly overexpressed in seminomas as well as non-seminomas, while western blot results revealed that DNMT3A2 protein level but not DNMT3A1 protein level was several times higher than control levels in both seminomatous and non-seminomatous TGCTs. The upregulation of DNMT3A2 in TGCT could partially contribute to the aberrant DNA methylation of TGCT and account for the hypermethylation of CpG islands observed in the promoters of many genes. In mammals, global de novo methylation occurs in the primordial germ cells at the early stage during germ cells development and plays a critical role in the establishment of genomic imprinting.Citation28 As a de novo DNA methyltransferase, DNMT3A2 was predominantly observed at this stage together with the co-expressed DNMT3L. Dnmt3a2 recruits Dnmt3l to chromatin and induces regional DNA methylation in male germ cells.Citation29,Citation30 Moreover, Dnmt3a2 transcripts were at its highest level in type A spermatogonia, and decreased dramatically in type B spermatogonia in mouse.Citation31 These evidences suggested that a tightly regulated expression pattern of DNMT3A2 is critical for normal germ cell development, which, when altered, will lead to pathological changes, such as embryonal carcinoma tumorigenesis.
In the current study, we confirmed that DNMT3A1 isoform showed slight upregulation in NT2 cell lines but no upregulation in clinical non-seminomas and seminomas, while DNMT3A2 did show significant upregulation in NT2 cell lines as well as in non-seminomas and seminomas tissues. The dual luciferase reporter assay results showed that miR-199a-3p directly targets DNMT3A, and miR-199a-3p expression inversely correlated with DNMT3A2 expression in clinical samples (r = −0.591, p = 0.002). Furthermore, miR-199a-3p expression significantly downregulated the DNMT3A2 expression in NT2 cells, while suppressed miR-199a-3p expression allowed significant upregulation of DNMT3A2 in HT cells. These observations suggest an epigenetic regulatory role for miR-199a-3p in TGCT. The observation of the difference in the knockdown efficiency of miR-199a-3p on the DNMT3A isoforms could be the result of the difference in the tertiary structure of the mRNA of the isoforms, which affects the binding efficiency of the miRNA to the DNMT3A mRNAs, even though they have the same 3′UTR. Since miR-199a-3p normally functions as an upstream inhibitor of DNMT3A2 expression, downregulation of miR-199a-3p will allow for upregulation of DNMT3A2. This provides the mechanism of the overexpressed DNMT3A2 in TGCT and suggests a tumor suppressor role for miR-199a-3p via regulating methylation in TGCT. To this end, we characterized the role of miR-199a-3p in the restoration of hypermethylated TSGs in NT2 cell lines. Specifically, three of 8 selected TSGs, namely, APC, MGMT, and SCGB3A1, were identified as indirectly affected by miR-199a-3p, probably through the action of DNMT3A. These three TSGs were shown previously to exhibit hypermethylation in TGCT.Citation24,Citation32,Citation33 Consistent with previous study, our data showed that all three genes were hypermethylated in NT2 cells compared with HT cells. Further, overexpressed miR-199a-3p in NT2 cells reduced the promoter methylation of APC and MGMT genes. However, no significant change of SCGB3A1 promoter methylation was observed, which may suggest that miR-199a-3p regulates SCGB3A1 via methylation at a different region of the gene.Citation34 Moreover, since miR-199a-3p expression did not significantly affect the expression of DNMT1, DNMT3B and the three members of the TET family, it confirmed that the change of DNA methylation level caused by miR-199a-3p was credited to targeting of DNMT3A.
In conclusion, our data demonstrated that miR-199a-3p directly targeted DNMT3A, and that miR-199a-3p significantly regulated the expression of DNMT3A2 isoform in NT2 cells. Further correlation study revealed a statistically significant inverse correlation between miR-199a-3p mRNA level and DNMT3A2 mRNA level in clinical samples. In addition, restoration of APC and MGMT, induced by miR-199a-3p overexpression in NT2 cells, coincided with significant DNA hypomethylation of their promoter regions, suggesting the hypomethylation function of miR-199a-3p, which implicated a potential therapeutic use of synthetic miR-199a-3p oligonucleotides as effective hypomethylating compounds in the treatment of TGCT. However, since DNMT3A expression did not significantly change pre-miR-199a expression, the mechanism of pre-miR-199a hypermethylation in TGCT needs further investigation.
Methods
Primary TGCT specimens
Genomic RNA (11 cases, 6 embryonal carcinoma and 5 seminoma) samples obtained from TGCT patients were purchased from Oncomatrix. Normal testicular RNA (14 cases) were purchased from Zyagen. Each RNA samples was isolated from a single individual.
Cell culture and transfection
Ntera 2 (NT2), an established pluripotent human testicular embryonal carcinoma cell lines,Citation35 as well as normal human testis cell line Hs 1.Tes (CRL-7002™) were purchased from ATCC (Manassas, VA, USA) supplemented with 10% FBS and incubated in a 37 °C humidified incubator supplied with 5% CO2. Synthetic double-stranded miR-199a-3p mimics, miR-199a-3p inhibitor or scramble oligonucleotides used as negative control (GenePharma) at a final concentration of 20 nM were introduced into NT2 cells or HT cells by Lipofectamine 2000 kit (Invitrogen) according to the manufacturer’s instructions. Transfected cells were harvested at 24, 48, or 72 h.
Inhibition of DNMT3A expression
The small interfering RNA (siRNA) specifically targeting DNMT3A (siDNMT3A) was obtained commercially (GenePharma). The synthetic siDNMT3A or negative control siRNA at a final concentration of 20 nM were introduced into NT2 cells by using Lipofectamine 2000 kit (Invitrogen) as previously described by cell culture and transfection methods. Transfected cells were harvested at 24 or 48 h.
Construction of luciferase reporter vector
The fragment (+562 bp ~+1202 bp) of DNMT3A 3′-untranslated region (3′-UTR, total of 1303 bp) containing an intact miR-199a-3p recognition sequence were amplified by PCR from genomic DNA and inserted into the Firefly/Renilla dual reporter vector pmirGLO (Promega) at the 3′-end of the firefly luciferase gene (luc2). The primers for construction of pmirGLO-DNMT3A-3′-UTR vector was shown in .
Table 1. Primers for clone PCR, real-time RT-PCR, methylation specific PCR (MSP) and bisulfite sequencing PCR (BSP) assays
Reverse-Transcription reaction (RT) and quantitative real-time PCR (qPCR)
The harvested cells were placed in TRIzol® Reagent (Invitrogen), and total RNAs were extracted according to the TRIzol manufacturer’s instructions. Total RNA (1 μg) was primed by random hexamers and converted into cDNA using SuperScript III (Invitrogen). The SYBR green-based real-time PCR was performed in an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems), and GAPDH was used as an endogenous control to normalize the amount of total mRNA in each sample. The expression of mature miR-199a-3p and miR-199a-5p were measured using the well-established stem-loop RT-qPCR method as previously described,Citation36 and the level of miRNAs expression were normalized by U6 RNA. Real-time PCR was performed with a standard SYBR-Green PCR kit protocol on ABI 7900HT Fast Real Time PCR system (Applied Biosystems). The primer sequences were summarized in .
Luciferase reporter assay
An amount of 3 × 104 NT2 cells were plated in each well of a 24-well plate the day before transfection. NT2 cells were co-transfected with pmirGLO-DNMT3A-3′UTR construct and miR-199a-3p mimics or a negative control, or co-transfected with empty pmirGLO plasmid and miR-199a-3p mimics by Lipofectamine 2000 (Invitrogen) as previously described by cell culture and transfection methods. Four wells for each group in a single independent experiment. A luciferase activity assay was performed 48 h after transfection with the dual luciferase reporter assay system (Promega). The relative luciferase activity was normalized with Renilla luciferase activity.
Western blot analysis
Total cell lysate was prepared in a 1x sodium dodecyl sulfate buffer. Proteins in the same amount were separated by sodium dodecyl sulfate–PAGE and transferred onto polyvinylidene fluoride membranes. After incubation with antibodies specific for either DNMT3A (monoclonal antibody 64B1446) (Abeam) or GAPDH (Abeam), the blots were incubated with goat anti-mouse (Santa Cruz Biotechnology) and visualized with enhanced chemiluminescence. The monoclonal antibody 64B1446 detects both isoforms of DNMT3A1 and DNMT3A2, as the two polypeptides have a common carboxyl-terminal catalytic domain.Citation10
Methylation specific PCR (MSP) and bisulfite sequencing PCR (BSP)
DNA was extracted using Invitrogen™ genomic DNA extraction kits. The bisulfite conversion of gDNA (400 ng) was conducted with EZ DNA Methylation-Gold™ Kit (Zymo Research Corporation). MSP and BSP were performed as previously described.Citation37,Citation38 The bisulfite-treated DNA (80–100 ng) was amplified with the methylation-specific primers or the unmethylated-specific primers, and the amplified products were analyzed on agarose gel. To perform the BSP, the bisulfite-treated DNA (80–100 ng) was amplified using primers that are specific for the modified DNA but do not contain any CpG sites in their sequence. The resulting PCR product was cloned into TOPO® TA Cloning® Kit (Invitrogen) and 8–10 positive clones were directly sequenced. The primer sequences were summarized in .
Statistical analysis
The expressions of miR-199a-3p, DNMT3A1 and DNMT3A2 in primary TGCT specimens and normal testis tissues were compared by the Mann-Whitney Test. The relationship of miR-199a-3p and DNMT3A2 mRNA expression levels was analyzed by Pearson’s correlation. The differences for luciferase assay and RT-qPCR data were determined by two-tailed Student’s t test, data are expressed as means and standard deviations (SD) from at least three independent experiments. All p values were two-sided and were obtained with the SPSS 16.0 software package (SPSS). A p value < 0.05 was considered statistically significant.
Additional material
Download Zip (369.8 KB)Acknowledgments
This research was supported by the start-up fund provided by the Chinese University of Hong Kong.
Submitted
06/13/2013
Revised
07/14/2013
Accepted
07/18/2013
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/25799
References
- Taby R, Issa JP. Cancer epigenetics. CA Cancer J Clin 2010; 60:376 - 92; http://dx.doi.org/10.3322/caac.20085; PMID: 20959400
- Lan J, Hua S, He XN, Zhang Y. DNA methyltransferases and methyl-binding proteins of mammals. Acta Biochim Biophys Sin (Shanghai) 2010; 42:243 - 52; http://dx.doi.org/10.1093/abbs/gmq015; PMID: 20383462
- Gilbert D, Rapley E, Shipley J. Testicular germ cell tumours: predisposition genes and the male germ cell niche. Nat Rev Cancer 2011; 11:278 - 88; http://dx.doi.org/10.1038/nrc3021; PMID: 21412254
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 2005; 5:210 - 22; http://dx.doi.org/10.1038/nrc1568; PMID: 15738984
- Cheung HH, Lee TL, Davis AJ, Taft DH, Rennert OM, Chan WY. Genome-wide DNA methylation profiling reveals novel epigenetically regulated genes and non-coding RNAs in human testicular cancer. Br J Cancer 2010; 102:419 - 27; http://dx.doi.org/10.1038/sj.bjc.6605505; PMID: 20051947
- Omisanjo OA, Biermann K, Hartmann S, Heukamp LC, Sonnack V, Hild A, Brehm R, Bergmann M, Weidner W, Steger K. DNMT1 and HDAC1 gene expression in impaired spermatogenesis and testicular cancer. Histochem Cell Biol 2007; 127:175 - 81; http://dx.doi.org/10.1007/s00418-006-0234-x; PMID: 16960727
- Almstrup K, Hoei-Hansen CE, Nielsen JE, Wirkner U, Ansorge W, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Genome-wide gene expression profiling of testicular carcinoma in situ progression into overt tumours. Br J Cancer 2005; 92:1934 - 41; http://dx.doi.org/10.1038/sj.bjc.6602560; PMID: 15856041
- Korkola JE, Houldsworth J, Dobrzynski D, Olshen AB, Reuter VE, Bosl GJ, Chaganti RS. Gene expression-based classification of nonseminomatous male germ cell tumors. Oncogene 2005; 24:5101 - 7; http://dx.doi.org/10.1038/sj.onc.1208694; PMID: 15870693
- Ishii T, Kohu K, Yamada S, Ishidoya S, Kanto S, Fuji H, Moriya T, Satake M, Arai Y. Up-regulation of DNA-methyltransferase 3A expression is associated with hypomethylation of intron 25 in human testicular germ cell tumors. Tohoku J Exp Med 2007; 212:177 - 90; http://dx.doi.org/10.1620/tjem.212.177; PMID: 17548962
- Chen T, Ueda Y, Xie S, Li E. A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J Biol Chem 2002; 277:38746 - 54; http://dx.doi.org/10.1074/jbc.M205312200; PMID: 12138111
- Choi SH, Heo K, Byun HM, An W, Lu W, Yang AS. Identification of preferential target sites for human DNA methyltransferases. Nucleic Acids Res 2011; 39:104 - 18; http://dx.doi.org/10.1093/nar/gkq774; PMID: 20841325
- Suetake I, Mishima Y, Kimura H, Lee YH, Goto Y, Takeshima H, Ikegami T, Tajima S. Characterization of DNA-binding activity in the N-terminal domain of the DNA methyltransferase Dnmt3a. Biochem J 2011; 437:141 - 8; http://dx.doi.org/10.1042/BJ20110241; PMID: 21510846
- Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis 2007; 28:2 - 12; http://dx.doi.org/10.1093/carcin/bgl185; PMID: 17028302
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 2007; 104:15805 - 10; http://dx.doi.org/10.1073/pnas.0707628104; PMID: 17890317
- Huang J, Wang Y, Guo Y, Sun S. Down-regulated microRNA-152 induces aberrant DNA methylation in hepatitis B virus-related hepatocellular carcinoma by targeting DNA methyltransferase 1. Hepatology 2010; 52:60 - 70; http://dx.doi.org/10.1002/hep.23660; PMID: 20578129
- Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, Yin H, Lu Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum 2011; 63:1376 - 86; http://dx.doi.org/10.1002/art.30196; PMID: 21538319
- Weng Z, Wang D, Zhao W, Song M, You F, Yang L, Chen L. microRNA-450a targets DNA methyltransferase 3a in hepatocellular carcinoma. Exp Ther Med 2011; 2:951 - 5; PMID: 22977604
- Cheung HH, Davis AJ, Lee TL, Pang AL, Nagrani S, Rennert OM, Chan WY. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene 2011; 30:3404 - 15; http://dx.doi.org/10.1038/onc.2011.60; PMID: 21383689
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003; 115:787 - 98; http://dx.doi.org/10.1016/S0092-8674(03)01018-3; PMID: 14697198
- Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al. Combinatorial microRNA target predictions. Nat Genet 2005; 37:495 - 500; http://dx.doi.org/10.1038/ng1536; PMID: 15806104
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res 2008; 36:Database issue D149 - 53; http://dx.doi.org/10.1093/nar/gkm995; PMID: 18158296
- Maragkakis M, Vergoulis T, Alexiou P, Reczko M, Plomaritou K, Gousis M, Kourtis K, Koziris N, Dalamagas T, Hatzigeorgiou AG. DIANA-microT Web server upgrade supports Fly and Worm miRNA target prediction and bibliographic miRNA to disease association. Nucleic Acids Res 2011; 39:Web Server issue W145-8; http://dx.doi.org/10.1093/nar/gkr294; PMID: 21551220
- Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci 2003; 60:1647 - 58; http://dx.doi.org/10.1007/s00018-003-3088-6; PMID: 14504655
- Koul S, Houldsworth J, Mansukhani MM, Donadio A, McKiernan JM, Reuter VE, Bosl GJ, Chaganti RS, Murty VV. Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol Cancer 2002; 1:8; http://dx.doi.org/10.1186/1476-4598-1-8; PMID: 12495446
- Lind GE, Skotheim RI, Lothe RA. The epigenome of testicular germ cell tumors. APMIS 2007; 115:1147 - 60; http://dx.doi.org/10.1111/j.1600-0463.2007.apm_660.xml.x; PMID: 18042148
- Okamoto K, Kawakami T. Epigenetic profile of testicular germ cell tumours. Int J Androl 2007; 30:385 - 92, discussion 392; http://dx.doi.org/10.1111/j.1365-2605.2007.00754.x; PMID: 17521367
- Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 2012; 139:1895 - 902; http://dx.doi.org/10.1242/dev.070771; PMID: 22569552
- Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet 2012; 28:33 - 42; http://dx.doi.org/10.1016/j.tig.2011.09.004; PMID: 22019337
- Nimura K, Ishida C, Koriyama H, Hata K, Yamanaka S, Li E, Ura K, Kaneda Y. Dnmt3a2 targets endogenous Dnmt3L to ES cell chromatin and induces regional DNA methylation. Genes Cells 2006; 11:1225 - 37; http://dx.doi.org/10.1111/j.1365-2443.2006.01012.x; PMID: 16999741
- Sakai Y, Suetake I, Shinozaki F, Yamashina S, Tajima S. Co-expression of de novo DNA methyltransferases Dnmt3a2 and Dnmt3L in gonocytes of mouse embryos. Gene Expr Patterns 2004; 5:231 - 7; http://dx.doi.org/10.1016/j.modgep.2004.07.011; PMID: 15567719
- La Salle S, Trasler JM. Dynamic expression of DNMT3a and DNMT3b isoforms during male germ cell development in the mouse. Dev Biol 2006; 296:71 - 82; http://dx.doi.org/10.1016/j.ydbio.2006.04.436; PMID: 16725135
- Smith-Sørensen B, Lind GE, Skotheim RI, Fosså SD, Fodstad O, Stenwig AE, Jakobsen KS, Lothe RA. Frequent promoter hypermethylation of the O6-Methylguanine-DNA Methyltransferase (MGMT) gene in testicular cancer. Oncogene 2002; 21:8878 - 84; http://dx.doi.org/10.1038/sj.onc.1205978; PMID: 12483540
- Lind GE, Skotheim RI, Fraga MF, Abeler VM, Esteller M, Lothe RA. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1). J Pathol 2006; 210:441 - 9; http://dx.doi.org/10.1002/path.2064; PMID: 17029216
- Ramachandran K, Gopisetty G, Gordian E, Navarro L, Hader C, Reis IM, Schulz WA, Singal R. Methylation-mediated repression of GADD45alpha in prostate cancer and its role as a potential therapeutic target. Cancer Res 2009; 69:1527 - 35; http://dx.doi.org/10.1158/0008-5472.CAN-08-3609; PMID: 19190346
- Pleasure SJ, Lee VM. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res 1993; 35:585 - 602; http://dx.doi.org/10.1002/jnr.490350603; PMID: 8411264
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 2005; 33:e179; http://dx.doi.org/10.1093/nar/gni178; PMID: 16314309
- Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 1996; 93:9821 - 6; http://dx.doi.org/10.1073/pnas.93.18.9821; PMID: 8790415
- Li Y, Tollefsbol TO. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 2011; 791:11 - 21; http://dx.doi.org/10.1007/978-1-61779-316-5_2; PMID: 21913068