Abstract
In this Extra View, we highlight recent Drosophila research that has uncovered a new role for the innate immune response. The research indicates that, in addition to combating infection, the innate immune response promotes neurodegeneration. Our publication (Petersen et al., 2012) reveals a correlative relationship between the innate immune response and neurodegeneration in a model of the human disease Ataxia-telangiectasia (A-T). We also found that glial cells are responsible for the innate immune response in the A-T model, and work by others implicates glial cells in neurodegeneration. Additionally, publications by Chinchore et al. (2012) and Tan et al. (2008) reveal a causative role for the innate immune response in models of human retinal degenerative disorders and Alzheimer disease, respectively. Collectively, these findings suggest that activation of the innate immune response is a shared cause of neurodegeneration in different human diseases.
Innate Immunity Genes are Misexpressed in Diverse Drosophila Neurodegeneration Disease Models
Our recent publication focuses on understanding why neurons die in the human disease Ataxia-telangiectasia (A-T).Citation1 A-T is caused by mutation of the A-T mutated (ATM) gene, which encodes a protein kinase that functions in the response to DNA damage.Citation2 To model A-T, we utilized a temperature-sensitive allele of ATM (ATM8) to generate adult flies deficient for ATM kinase activity.Citation3 At the non-permissive temperature, ATM8 flies have reduced lifespan, reduced climbing ability, holes in the brain and increased expression of an apoptosis marker in the brain—all indications of neurodegeneration.Citation1,Citation4 Unexpectedly, ATM8 flies also have increased expression of numerous innate immunity genes ().Citation1
Figure 1. The diagrams summarize the work of Petersen et al. (2012), Chinchore et al. (2012) and Tan et al. (2008) that examined the role of the innate immune response in Drosophila models of human neurodegenerative diseases, (A) A-T, (B) retinal degeneration and (C) Alzheimer disease, respectively. Each panel depicts the key membrane-bound and cytoplasmic factors in the two primary innate immune response pathways and indicates through color-coding (see legend) which factors have been genetically implicated in neurodegeneration. Each panel also indicates through color-coding whether the innate immune response occurs in glial cells and whether nuclear events (i.e., innate immunity gene transcription) have been shown to occur.
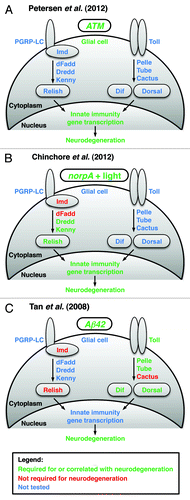
The innate immune response is an evolutionarily conserved mechanism in animals that serves to combat pathogens.Citation5 In flies, two main signaling pathways activate the transcription of innate immunity genes. One pathway primarily responds to eukaryotic pathogens (yeast and fungi) and gram-positive bacteria (). Recognition of pathogens by peptidoglycan recognition proteins (PGRPs) stimulates the proteolytic cleavage of the cytokine Spätzle, which binds and activates the Toll receptor. Through interactions with dMyD88, Pelle, Tube and other proteins, activated Toll stimulates the degradation of the Inhibitor of κB (IκB) protein Cactus. This allows translocation of Nuclear Factor-κB (NF-κB) transcription factors Dif and Dorsal to the nucleus. Dif and Dorsal then activate innate immunity gene transcription, notably the transcription of antimicrobial peptides (AMPs), which directly combat pathogens.
The second innate immune response pathway primarily responds to gram-negative bacteria.Citation5 PGRP proteins recognize pathogens and signal through Immune deficiency (Imd) to promote cleavage and activation of the NF-κB transcription factor Relish (Rel) (). Rel cleavage is elicited by the IκB Kinase (IKK) complex, consisting of Kenny and Ird5 and may be directly carried out by the caspase Dredd subsequent to its activation by dFadd. Rel is a homolog of mammalian p105-like NF-κB proteins that have an N-terminal NF-κB domain and a C-terminal IκB inhibitory domain. Cleavage by Dredd releases the NF-κB domain from its inhibitory domain, allowing it to translocate to the nucleus and activate the transcription of innate immunity genes, including AMPs.
In the case of ATM8 flies, upregulation of innate immunity gene expression was unexpected because there were no added pathogens. However, upregulation of innate immunity gene expression is not an oddity of ATM8 flies; instead, it is a feature of other Drosophila neurodegenerative disease models. AMP gene expression is also upregulated in models of Parkinson’s disease,Citation6 CAG-repeat RNA-based neurodegeneration,Citation7 and retinal degeneration.Citation8 Thus, a diverse array of triggers of neurodegeneration, including ATM mutation, parkin mutation, CAG-repeat mRNA misexpression and norpA mutation combined with light, result in activation of the innate immune response.
Canonical Innate Immune Response Pathway Factors are Required for Neurodegeneration
Chinchore et al. (2012) found that activation of the NF-κB factor Rel is required for neurodegeneration in a Drosophila model of human retinal disorders ().Citation8 This model involves light-induced degeneration of photoreceptor neurons in the eye of norpA mutant flies.Citation9 Neurodegeneration is thought to result from excessive light-dependent endocytosis of rhodopsin, a light-sensing protein.Citation10 The authors found that rel mutations almost completely block light-induced photoreceptor degeneration in norpA flies.Citation8 They also found that mutations in kenny and dredd, genes required for Rel activation, almost completely block photoreceptor degeneration. However, mutations in imd and dFadd, which function upstream of Dredd activation, have no effect on photoreceptor degeneration. Thus, it appears that in this model a non-canonical innate immune response signal activates Dredd to bring about photoreceptor degeneration.
Tan et al. (2008) found that activation of the NF-κB factors Dif and Dorsal is required for neurodegeneration in a Drosophila model of Alzheimer disease ().Citation11 This model involves misexpression of Aβ42, a protein associated with the onset of Alzheimer disease that accumulates in large aggregates in the brains of Alzheimer patients.Citation12 Misexpression of Aβ42 in the eye causes an external rough eye phenotype, the severity of which is likely to report the extent of internal photoreceptor neuron degeneration.Citation13 In a screen for modifiers of the rough eye phenotype, the authors identified a suppressor deficiency that included the Toll gene.Citation11 By systematically working through mutants of Toll signaling pathway genes, they determined that Toll, pelle, tube, dif and Dorsal mutants also suppress the rough eye phenotype. In contrast, cactus mutants do not affect the rough eye phenotype, indicating that Cactus is not involved in the signaling pathway or, more likely, that cactus is not haploinsufficient in this assay. Interestingly, mutations in Imd or rel, members of the other innate immune response pathway that is implicated by Chinchore et al. (2012) in retinal degeneration, have no effect on the rough eye phenotype. However, the caveat about haploinsufficiency also applies to this finding. Thus, it appears that each innate immune response pathway is required in a different context to promote neurodegeneration. The triggering event may specify which pathway is involved; however, additional data are required to draw this conclusion.
Glial Cells May Produce the Innate Immune Response That Causes Neurodegeneration
Identification of the cell type that produces the innate immune response is critical to understanding how the innate immune response causes neurodegeneration. Our expectation, based on studies of pathogen challenge, was that the fat body would produce the innate immune response.Citation5 The fat body is the primary source of the innate immune response that combats pathogens, and the fat body not only resides in the abdomen but also the head.Citation5,Citation14 However, in the A-T model, we found, using GFP reporters for AMP gene expression in ATM8 flies, that the innate immune response occurs exclusively in glial cells.Citation1 Moreover, we found that activation of the innate immune response in glial cells is cell autonomous. ATM knockdown in glial cells, but not in neurons, activates the innate immune response and causes neurodegeneration. Thus, activation of the innate immune response appears to occur in response to a toxic event in glial cells rather than a toxic event in neurons. In support of this possibility, glial cells are central players in other Drosophila neurodegenerative disease models. The neurodegenerative mutants swiss cheese and drop-dead have defective glial cell morphology that may contribute to the observed pathology.Citation15,Citation16 Also, overexpression of the disease-causing polyglutamine proteins Ataxin-1 or Huntington exclusively in glial cells induces neurodegeneration.Citation17 However, it remains to be determined in any of these systems that the innate immune response by glial cells causes neurodegeneration.
Summary and Future Directions
Taken together, the three highlighted publications [Petersen et al. (2012), Chinchore et al. (2012) and Tan et al. (2008)] suggest that activation of the innate immune response in glial cells drives neurodegeneration. However, because this is a new discovery, many of the details are unclear. Foremost, among the unknown details are the nature of the signal that activates the innate immune response in glial cells and the mechanism by which the innate immune response causes neurons to die. Drosophila is well suited to address these details because of the availability of genetic and molecular reagents with which to study the innate immune response. For example, reagents exist to determine the extent to which activation of the innate immune response at various points in the pathway is sufficient to cause neurodegeneration. To illustrate, Chinchore et al. (2012) showed, through misexpression of a constitutively active form of Rel in the eye, that Rel activation is sufficient to cause adult photoreceptor neuron degeneration. As a final point, it is likely that mechanistic links between the innate immune response and neurodegeneration in Drosophila are conserved in humans. In human neurodegenerative diseases such as Alzheimer and Parkinson, chronic inflammatory responses by microglial cells are thought to exacerbate the process of neurodegeneration.Citation18 Moreover, innate immune response pathways are conserved between Drosophila and mammals,Citation19 and Toll-like receptors have been specifically implicated in neurodegeneration in mammals.Citation20,Citation21 Because of the availability of reagents and the potential for uncovering therapeutic targets for human neurodegenerative diseases, we anticipate rapid progress in discovering the details of the mechanism by which the innate immune response causes neurodegeneration in Drosophila.
Acknowledgments
We thank Stacey Rimkus, Becky Katzenberger and Randy Tibbetts for their contributions to our research on the Drosophila A-T model. We also thank Grace Boekhoff-Falk, Stanislava Chtarbanova, Barry Ganetzky and Shigeki Miyamoto for their thoughtful comments on the manuscript. Work on the Drosophila A-T model was supported by a grant from the NIH (R01 NS059001 to D.A.W.) and a pre-doctoral fellowship from NIH training grant T32 GM08688 (to A.J.P.).
References
- Petersen AJ, Rimkus SA, Wassarman DA. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc Natl Acad Sci U S A 2012; 109:E656 - 64; PMID: 22355133
- McKinnon PJ. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol 2012; 7:303 - 21; http://dx.doi.org/10.1146/annurev-pathol-011811-132509; PMID: 22035194
- Pedersen M, Tiong S, Campbell SD. Molecular genetic characterization of Drosophila ATM conserved functional domains. Genome 2010; 53:778 - 86; http://dx.doi.org/10.1139/G10-067; PMID: 20962884
- Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet 2009; 10:359 - 70; http://dx.doi.org/10.1038/nrg2563; PMID: 19434080
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster.. Annu Rev Immunol 2007; 25:697 - 743; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141615; PMID: 17201680
- Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. . Hum Mol Genet 2005; 14:799 - 811; http://dx.doi.org/10.1093/hmg/ddi074; PMID: 15689351
- Shieh SY, Bonini NM. Genes and pathways affected by CAG-repeat RNA-based toxicity in Drosophila. Hum Mol Genet 2011; 20:4810 - 21; http://dx.doi.org/10.1093/hmg/ddr420; PMID: 21933837
- Chinchore Y, Gerber GF, Dolph PJ. Alternative pathway of cell death in Drosophila mediated by NF-κB transcription factor Relish. Proc Natl Acad Sci U S A 2012; 109:E605 - 12; http://dx.doi.org/10.1073/pnas.1110666109; PMID: 22328149
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron 2000; 28:129 - 38; http://dx.doi.org/10.1016/S0896-6273(00)00091-X; PMID: 11086989
- Orem NR, Dolph PJ. Loss of the phospholipase C gene product induces massive endocytosis of rhodopsin and arrestin in Drosophila photoreceptors. Vision Res 2002; 42:497 - 505; http://dx.doi.org/10.1016/S0042-6989(01)00229-2; PMID: 11853766
- Tan L, Schedl P, Song HJ, Garza D, Konsolaki M. The Toll-->NFkappaB signaling pathway mediates the neuropathological effects of the human Alzheimer’s Abeta42 polypeptide in Drosophila. PLoS One 2008; 3:e3966; http://dx.doi.org/10.1371/journal.pone.0003966; PMID: 19088848
- Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease: an update. Ann Med 2008; 40:562 - 83; http://dx.doi.org/10.1080/07853890802186905; PMID: 18608129
- Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci 2004; 26:365 - 75; http://dx.doi.org/10.1016/j.mcn.2004.03.001; PMID: 15234342
- Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, et al. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 2000; 13:737 - 48; http://dx.doi.org/10.1016/S1074-7613(00)00072-8; PMID: 11114385
- Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 1993; 10:839 - 50; http://dx.doi.org/10.1016/0896-6273(93)90200-B; PMID: 8494644
- Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci 1997; 17:7425 - 32; PMID: 9295388
- Tamura T, Sone M, Yamashita M, Wanker EE, Okazawa H. Glial cell lineage expression of mutant ataxin-1 and huntingtin induces developmental and late-onset neuronal pathologies in Drosophila models. PLoS One 2009; 4:e4262; http://dx.doi.org/10.1371/journal.pone.0004262; PMID: 19165334
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology 2010; 129:154 - 69; http://dx.doi.org/10.1111/j.1365-2567.2009.03225.x; PMID: 20561356
- Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science 2010; 330:1061 - 4; http://dx.doi.org/10.1126/science.1189468; PMID: 21097929
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 2003; 100:8514 - 9; http://dx.doi.org/10.1073/pnas.1432609100; PMID: 12824464
- Arroyo DS, Soria JA, Gaviglio EA, Rodriguez-Galan MC, Iribarren P. Toll-like receptors are key players in neurodegeneration. Int Immunopharmacol 2011; 11:1415 - 21; http://dx.doi.org/10.1016/j.intimp.2011.05.006; PMID: 21616174