Abstract
In India, pneumococcal diseases are major causes of child mortality, and effective vaccines against Streptococcus pneumoniae are needed. This single-blind, randomized study assessed the immunogenicity, reactogenicity, and safety of the 10-valent pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV) co-administered with DTPw-HBV/Hib in Indian infants as 3-dose primary vaccination course. A total of 360 infants were randomized (2:1) to receive either PHiD-CV co-administered with DTPw-HBV/Hib (PHiD-CV group) or a Hib vaccine co-administered with DTPw-HBV (control group) at 6, 10, and 14 weeks of age. For each vaccine pneumococcal serotype, the percentage of infants in the PHiD-CV group with antibody concentrations ≥ 0.2 µg/mL one month after the third vaccine dose was at least 98.3%, except for serotypes 6B (77.7%) and 23F (89.5%), and opsonophagocytic activity titers ≥ 8 were measured in at least 95.7% of infants, except for serotypes 1 (90.5%) and 6B (84.5%). In addition, all the infants in the PHiD-CV group were seroprotected against diphtheria, tetanus, Hib, and hepatitis B or seropositive for antibodies against pertussis and NTHi protein D (except one infant). Incidences of solicited local and general symptoms were comparable between groups, except for fever (axillary temperature ≥ 37.5°C), which seemed to occur more frequently in the PHiD-CV group. In conclusion, PHiD-CV was shown to be immunogenic and well-tolerated when co-administered with DTPw-HBV/Hib in Indian infants. This study has been registered at www.clinicaltrials.gov NCT00814710.
Introduction
Streptococcus pneumoniae is a major cause of bacterial pneumonia, meningitis, and sepsis in children across the world and is responsible for 700,000 to 1 million child deaths every year.Citation1 It has been estimated that South Asia alone accounts for one quarter of all pneumococcal infections worldwide, resulting in approximately 142,000 deaths of children below 5 y of age in India, 27,000 in Pakistan and 21,000 in Bangladesh in the year 2000.Citation1
The incidence of invasive pneumococcal disease (IPD) in India is difficult to estimate due to the large population and geographic size and the absence of a national surveillance system for IPD.Citation2-Citation4 However, a retrospective study conducted in 2006 in Bangalore estimated that the annual incidence of hospitalizations with invasive bacterial disease-associated syndromes (pneumonia, sepsis, and meningitis) was 1,500 per 100,000 children younger than five years, with a mortality rate of 8%.Citation3 This is at the level of incidence rates previously reported for IPD in AfricaCitation5-Citation7 but contrasts with results from population based surveillance studies in Bangladesh, which estimated the incidence of IPD to be 86 to 447 cases per 100,000 children under 5 y of age in rural and urban communities, respectively.Citation8,Citation9 Variations in the composition of the capsular polysaccharides (PS) of S. pneumoniae have lead to the identification of more than 90 different serotypes.Citation10 In 2008, it was estimated that the most common disease causing serotype in Asia was serotype 14 (11.6%), followed by serotypes 6B (11.5%), 23F (9.7%), 1 (9.5%), 19F (8.1%) and 5 (6.7%).Citation11
S. pneumoniae along with non-typeable Haemophilus influenzae (NTHi) are two of the leading causes of acute otitis media (AOM) in children.Citation12 AOM is a common childhood infection, which is usually clinically mild but can nevertheless result in complications such as hearing loss, spontaneous tympanic membrane rupture, acute mastoiditis, and meningitis.Citation13 Pneumococcal serogroups 6, 14, 19 and 23 are commonly associated with childhood otitis mediaCitation14 and are responsible for persistent rhinorrhoea in Indian children, which appears to be associated with chronic otitis media.Citation15
The severity of pneumococcal diseases in infants and children and growing antibiotic resistance support the development of new vaccines against pneumococci.Citation16-Citation18 Three pneumococcal conjugate vaccines are currently available in many countries: a 7-valent (7vCRM) and a 13-valent (13vCRM) vaccine using mutated diphtheria toxoid protein (CRM) as carrier, and a 10-valent vaccine (PHiD-CV) using NTHi derived protein D, tetanus toxoid (TT), and diphtheria toxoid (DT) as carrier proteins. In PHiD-CV, 8 of the 10 serotypes are conjugated to NTHi protein D, which was shown in a previous clinical trial with a predecessor 11-valent formulation to provide protection against AOM caused by NTHi in addition to protection against pneumococcal AOM.Citation19 Previous studies showed that PHiD-CV was well-tolerated and induced a good immune response in infants and toddlers.Citation20-Citation23 PHiD-CV has obtained regulatory approval in more than 100 countries and has also been pre-qualified by the World Health Organization (WHO), allowing purchase by United Nations organizations, and financing through Advanced Market Commitment for use in countries that are eligible for support from the Global Alliance for Vaccines and Immunization (GAVI), such as India.
The co-administration of PHiD-CV with routinely administered pediatric vaccines would facilitate its implementation into vaccination schedules without increasing the number of visits required. In previous studies, PHiD-CV was shown to be immunogenic when co-administered with different pediatric vaccines, with no clinically relevant interactions.Citation20-Citation24 Since diphtheria-tetanus-whole cell pertussis (DTPw)-based combination vaccines are still widely used, studies evaluating the immunogenicity and safety of PHiD-CV when co-administered with these combination vaccines were needed.Citation25 The immunogenicity and the good safety profile of PHiD-CV co-administered with a combined DTPw-hepatitis B/H. influenzae type b vaccine (DTPw-HBV/Hib) were previously shown in the Philippines, Poland,Citation21 and Mali and Nigeria.Citation26 The aim of the present study was to evaluate the immunogenicity, safety, and reactogenicity of a 3-dose PHiD-CV primary immunization course administered to Indian infants at 6, 10 and 14 weeks of age. The immune response to a booster dose of PHiD-CV is currently being evaluated in this population and results will be published separately.
Results
Study participants
A total of 360 infants were enrolled in the study and 349 of them completed the last study visit (). The according to protocol (ATP) immunogenicity cohort included 345 infants ([29 who received PHiD-CV co-administered with DTPw-HBV/Hib (PHiD-CV group) and 116 who received a vaccine against H. influenzae type b (Hib) co-administered with DTPw-HBV (control group)]. One infant in the PHiD-CV group did not complete the study due to a fatal serious adverse event (SAE) (aspiration pneumonia), which was not considered to be related to vaccination. The baseline demographic characteristics of the infants in the ATP immunogenicity cohort were comparable between the two groups ().
Figure 1. Participant flow diagram. Note: PHiD-CV group, infants who received PHiD-CV co-administered with DTPw-HBV/Hib; Control group, infants who received the Hib vaccine co-administered with DTPw-HBV; N, number of infants; TVC, total vaccinated cohort; ATP, according to protocol
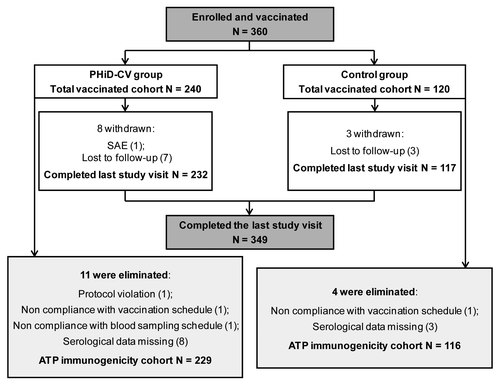
Table 1. Summary of demographic characteristics at the time of administration of the first study vaccine dose (ATP immunogenicity cohort)
Immunogenicity
Immune response to pneumococcal polysaccharide
Prior to vaccination, the percentages of infants with antibody concentration ≥ 0.2 µg/mL ranged from 17.5% for serotype 1 to 90.8% for serotype 14 in the PHiD-CV group and from 17.2% to 91.4% for the same serotypes in the control group (). For each of the 10 vaccine pneumococcal serotypes, at least 98.3% of the infants in the PHiD-CV group reached antibody concentrations ≥ 0.2 µg/mL one month after the third primary dose, except for serotypes 6B (77.7%) and 23F (89.5%), and robust increases in geometric mean antibody concentrations (GMCs) were observed from pre- to post-vaccination. For the cross-reactive serotypes 6A and 19A, the percentages of infants with antibody concentrations ≥ 0.2 µg/mL following PHiD-CV vaccination were 41.5% and 63.8%, respectively.
Table 2. ELISA antibody concentrations for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A at pre-vaccination and one month after the third primary vaccine dose (ATP immunogenicity cohort)
For each of the 10 vaccine pneumococcal serotypes, at least 95.7% of the infants in the PHiD-CV group had opsonophagocytic activity (OPA) titers ≥ 8 one month after the third primary vaccination, except for serotypes 1 (90.5%) and 6B (84.5%) (). The percentages of infants reaching an OPA titer ≥ 8 against the cross-reactive pneumococcal serotypes 6A and 19A following PHiD-CV vaccination were 49.1% and 32.1%, respectively.
Table 3. Opsonophagocytic titers for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A one month after the third primary vaccine dose (ATP immunogenicity cohort)
Post-vaccination antibody GMCs and OPA geometric mean titers (GMTs) were low in the control group for all tested pneumococcal serotypes ( and ).
Immune response to protein D
All infants in the PHiD-CV group except one were seropositive for antibodies against NTHi protein D following vaccination, and antibody GMCs increased markedly from pre- to post-vaccination (). Post-vaccination anti-protein D antibody GMCs remained low in the control group.
Table 4. Anti-protein D antibody responses at pre-vaccination and one month after the third primary vaccine dose (ATP immunogenicity cohort)
Immune response to co-administered DTPw-HBV/Hib vaccine
All infants in both groups had seroprotective antibody concentrations against diphtheria, tetanus, Hib and hepatitis B, except one infant in the control group for antibodies against Hib (). Anti-TT antibody GMCs seemed to be higher in the PHiD-CV group than in the control group (non-overlapping 95% confidence intervals [CIs]). All infants were seropositive for antibodies against B. pertussis, with a trend for higher antibody GMCs in the control group.
Table 5. Antibody responses against co-administered DTPw-HBV and Hib antigens one month after the third primary vaccine dose (ATP immunogenicity cohort)
Evaluation of safety and reactogenicity
One SAE with fatal outcome (aspiration pneumonia) was reported 14 d after the third dose in one infant of the PHiD-CV group. In addition, at least one non-fatal SAE was reported in four infants of the PHiD-CV group (chikungunya virus infection, gastroenteritis, septicemia and bronchopneumonia, and bronchopneumonia) and in one infant of the control group (bronchopneumonia). None of these SAEs were considered to be causally related to vaccination, and all non-fatal SAEs resolved without sequelae.
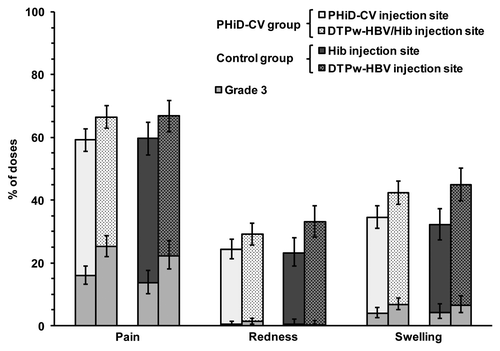
Local adverse events (AEs) were more frequently reported than general AEs in both groups, and there was no increase in the incidence of solicited local and general AEs with successive doses during the primary vaccination course. High incidences of pain with grade 3 intensity were observed in both groups (following 27.7% of doses [95% CI: 24.4–31.1] in the PHiD-CV group and 24.9% of doses [95% CI: 20.4–29.7] in the control group) (). Pain was more frequently reported at the DTPw-HBV/Hib injection site compared with the PHiD-CV injection site in the PHiD-CV group.
Figure 2. Incidence of solicited local symptoms reported during the four-day post-vaccination period (overall/dose; total vaccinated cohort). Notes: Error bars represent 95% CIs; Grade 3 = symptom with grade 3 intensity (pain if the child cried when the limb was moved or if the limb was spontaneously painful; redness and swelling if the diameter was > 30 mm).
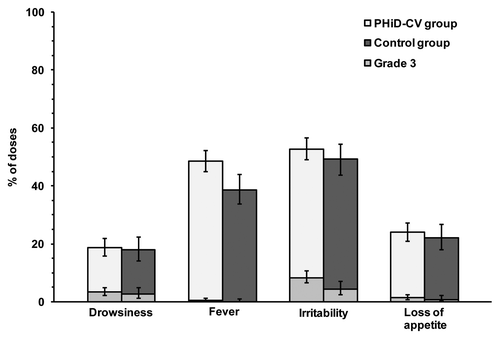
Irritability was the most frequently reported solicited general AE in both groups [following 52.8% of doses (95% CI: 49.1–56.6) in the PHiD-CV group and 49.2% of doses (95% CI: 43.8–54.5) in the control group]. The incidence of grade 3 solicited general AEs ranged from 0.0% to 8.3% of doses. Except for fever, the incidences of solicited general AEs were within the same ranges in both groups (). Although fever tended to be more frequent in the PHiD-CV group [following 48.6% of vaccine doses (95% CI: 44.8–52.3)] than in the control group [following 38.7% of vaccine doses (95% CI: 33.6–44.0)], the incidence of grade 3 fever was within the same range in both groups.
Figure 3. Incidence of solicited general symptoms reported during the four-day post-vaccination period (overall/dose; total vaccinated cohort). Notes: Error bars represent 95% CIs; Grade 3 = symptom with grade 3 intensity (fever if axillary temperature was > 39.5°C; loss of appetite if the child did not eat at all; irritability if the child cried and could not be comforted; and drowsiness if it prevented normal everyday activity).
Unsolicited AEs were reported following 6.0% of vaccine doses (95% CI: 4.4–8.0) in the PHiD-CV group and 4.8% of vaccine doses (95% CI: 2.8–7.5) in the control group. Grade 3 unsolicited AEs were reported following two of the vaccine doses (0.3%) in the PHiD-CV group and none in the control group. None of the grade 3 unsolicited AEs were considered to be causally related to vaccination. The most frequently reported unsolicited symptoms were cough (following 2.0% of doses), rhinitis (following 1.8% of doses), and pyrexia (following 1.7% of doses) in the PHiD-CV group and rhinitis (following 1.7% of doses) and cough (following 1.1% of doses) in the control group.
Discussion
Co-administration of new vaccines with those currently recommended by the Indian Academy of Pediatrics would maximize the likelihood that Indian children will receive new vaccines against different diseases. However, before allowing such co-administration, it is important to evaluate the risks of immune interference or significant worsening of the safety profile. In this study, the immunogenicity, the reactogenicity, and the safety of PHiD-CV co-administered with DTPw-HBV/Hib at 6, 10 and 14 weeks of age were evaluated in Indian infants.
At one month after the third primary dose administration, PHiD-CV was shown to be highly immunogenic in terms of antibody concentrations and opsonophagocytic activity induced against the 10 vaccine pneumococcal serotypes. For each of the vaccine pneumococcal serotypes, the post-vaccination geometric mean antibody concentrations were higher in Indian infants (range 0.71–15.23 µg/mL) when compared with those measured in previous studies conducted in Europe (range 0.33–5.30 µg/mL) despite the more challenging schedule in India (6–10–14 weeks) compared with Europe (2–3–4 mo or 2–4–6 mo).Citation21-Citation23 Antibody concentrations measured in the present study were in line with those measured in previous studies with PHiD-CV conducted in the Philippines (range 1.19–11.56 µg/mL)Citation21 and in Mali and Nigeria (range 0.95–10.01 µg/mL).Citation26 It remains unclear why differences exist between different global regions and ethnic groups in terms of immune responses to bacterial capsular PS protein conjugate vaccines, with usually the lowest responses measured in European and North American infants.Citation27 Although early exposure or nasopharyngeal carriage of pneumococcal serotypes has been mentioned in the past as one potential explanation for the observed differences between populations,Citation27 more recent data seems to suggest that early pneumococcal nasopharyngeal colonization could actually have a negative impact on immune responses induced by pneumococcal conjugate vaccines in early infancy.Citation28-Citation31 In the present study, high percentages of infants had pre-vaccination pneumococcal antibody concentrations ≥ 0.2 µg/mL. This most probably reflects high maternal antibody levels as a consequence of high environmental prevalence and intense circulation of pneumococci in the Indian population,Citation32,Citation33 but did not seem to hamper PHiD-CV immunogenicity in Indian infants.
As in previous studies with PHiD-CV, post-vaccination concentrations of antibodies against vaccine pneumococcal serotypes were the lowest for serotypes 6B and 23F.Citation24-Citation26 Lower post-vaccination antibody concentrations were also measured for these serotypes in previous studies with 7vCRM,Citation21-Citation23 an experimental 7-valent pneumococcal polysaccharide meningococcal outer membrane protein conjugate vaccine (PncOMPC),Citation34 or an 11-valent pneumococcal protein D conjugate PHiD-CV-predecessor vaccine.Citation19 Despite the lower concentrations of antibodies against these serotypes, efficacy against AOM caused by serotypes 6B and 23F was demonstrated for each of these vaccines (range 52–88%).Citation19,Citation34-Citation36
Immune responses to cross-reactive serotypes 6A and 19A in terms of antibody concentrations and OPA titers were observed after PHiD-CV vaccination. For serotype 6A, cross-reactive antibody responses following 7vCRM vaccination were shown to correlate with functional OPA responses and resulted in protection against and significant reduction of serotype 6A disease.Citation35,Citation37 It is interesting to see that some functional OPA responses against serotype 19A could also be measured with the GSK Biologicals assay following PHiD-CV vaccination, in line with previous PHiD-CV studies,Citation21-Citation23 whereas no 19A OPA responses could be measured with this assay following 7vCRM vaccination in previous studies,Citation21-Citation23 and no reduction in IPD caused by serotype 19ACitation35,Citation37 was seen following inclusion of 7vCRM in routine vaccination.
Another important finding of our study, which is in line with results of previous studies, is that PHiD-CV induced antibodies against the NTHi protein D carrier.Citation21-Citation23 It has been shown in a previous AOM efficacy trial that a predecessor 11-valent protein D-conjugate vaccine provided 35.3% (95% CI: 1.8–57.4) protection against AOM episodes caused by NTHi.Citation19 In addition, the DTPw-HBV/Hib vaccine was also shown to be immunogenic when co-administered with PHiD-CV and no negative interference between the co-administered vaccines was observed in terms of immune response to DTPw-HBV/Hib antigens. The observed higher anti-tetanus antibody concentration in the PHiD-CV group compared with the control group may be explained by the higher dose of TT that infants received since PHiD-CV contains TT as carrier protein for capsular PS from serotype 18C.
The acceptance of new vaccines depends also on their reactogenicity and safety profile. The present study showed that the co-administration of PHiD-CV with vaccines currently recommended by the Indian Academy of Pediatrics did not result in major changes in the reactogenicity profile, except for fever that was more frequently reported in infants who received PHiD-CV. However, this observation can be considered of limited clinical relevance, since grade 3 fever (axillary temperature > 39.5°C) was infrequent in both groups. In previous studies, co-administration of pneumococcal conjugate vaccines with diphtheria-tetanus-acellular pertussis (DTPa)-based vaccines was associated with an increase in low grade fever (axillary temperature between 37.5°C and 38.5°C) compared with DTPa-based vaccines alone,Citation38-Citation41 and this seems also to be the case for PHiD-CV co-administered with DTPw-HBV/Hib vs. DTPw-based vaccine alone.Citation20 Pain was frequently reported in both study groups in the present study. The incidence of grade 3 pain seemed to be higher here than in the previous studies conducted in the Philippines, Poland and Mali and Nigeria with the same vaccines.Citation20,Citation26 This difference may derive from cultural perceptions and intensity of surveillance for vaccine reactions across populations, as suggested in a previous study in which different rates of local reactions were reported in Chilean and Belgian children who received the same acellular pertussis vaccine.Citation42
A potential limitation of this study was the absence of investigator blinding, which is unlikely to have influenced immunogenicity assessment but may have biased the safety analyses toward increased reporting of AEs in the infants who received the PHiD-CV vaccine, since the investigator was aware that the child received a new vaccine in addition to the antigens received by the infants in the control group. However, if such a bias occurred, it did not result in large differences of reactogenicity between the two study groups. The absence of a control group vaccinated with 7vCRM could also be seen as a limitation of this study, but we decided to administer vaccines recommended by the Indian Academy of Pediatrics to the control group, in order to evaluate the impact of the addition of PHiD-CV in the Indian vaccination program.
In summary, this study showed that PHiD-CV is immunogenic and well tolerated when co-administered with DTPw-HBV/Hib vaccine at 6, 10 and 14 weeks of age in Indian infants. Considering the importance of pneumococcal infections in this population and the robust immune responses observed following PHiD-CV vaccination in our study, we conclude that substantial public health benefits can be expected if PHiD-CV would be implemented in the Indian vaccination program.
Materials and Methods
Study design
This was a phase III, single-blind, randomized, controlled study conducted in four centers in India between March 2009 and November 2009. Healthy infants were randomized (2:1 treatment allocation ratio) to receive either PHiD-CV co-administered with DTPw-HBV/Hib (PHiD-CV group) or a Hib vaccine co-administered with DTPw-HBV (control group). The vaccines were administered as a 3-dose primary immunization course at 6, 10 and 14 weeks of age.
Treatment allocation was performed at each investigator site using a central randomization system. The randomization list was generated using a standard Statistical Analysis System (SAS) program and a randomization blocking scheme (block size of 3) was used to ensure that balance between treatment groups was maintained. The study was conducted in a single-blinded manner meaning that the investigator was aware of the treatment assignment but the infant’s parents/guardians were not.
The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice, appropriate Indian standards as recommended by the Indian Council of Medical Research, and all applicable regulatory requirements, including the Declaration of Helsinki. Written informed consent was obtained from each infant’s parent/guardian prior to the performance of any study-specific procedure. The study protocol and the informed consent were reviewed and approved by the local regulatory authorities (Drugs Controller General of India) and the relevant institutional Independent Ethics Committees. The study has been registered at www.clinicaltrials.gov NCT00814710. A summary of the protocol is available at http://www.gsk-clinicalstudyregister.com (GSK study ID 111188).
Study objectives
The primary objective of this study was to evaluate the immunogenicity of a 3-dose PHiD-CV primary immunization course in Indian infants. The secondary objectives were to assess the safety and reactogenicity of PHiD-CV and to evaluate the immunogenicity of the DTPw-HBV/Hib vaccine when co-administered with PHiD-CV.
Study participants
Eligible participants were healthy infants aged between and including 6 and 10 weeks at the time of the first vaccine dose administration, for whom the investigator believed that their parents/guardians would comply with the requirements of the protocol. Infants were excluded from participation if they had received other investigational products or had planned their use during the study period, had received immunoglobulin or blood products since birth, were immunosuppressed for any reason, had previous vaccination against or history of diphtheria, tetanus, pertussis, hepatitis B, Hib or S. pneumoniae disease (with the exception of hepatitis B vaccination at least 30 d before the study), had allergic disease or reactions likely to be exacerbated by any component of the vaccines, had a birth weight less than 2 kg if known at the time of the first vaccine dose administration, had a major congenital defect, a serious chronic illness, or a history of any neurological disorders or seizures.
Study vaccines
PHiD-CV [Synflorix™, GlaxoSmithKline (GSK) Biologicals, Rixensart, Belgium] is a 10-valent pneumococcal conjugate vaccine containing 1 µg of each capsular PS for serotypes 1, 5, 6B, 7F, 9V, 14 and 23F and 3 µg of PS for serotype 4 conjugated individually to NTHi protein D carrier, 3 µg of PS for serotype 18C conjugated to TT carrier and 3 µg of PS for serotype 19F conjugated to DT carrier. Each conjugate is adsorbed separately onto aluminum phosphate adjuvant.
The commercially available Hib vaccine (Hiberix™, GSK Biologicals, Rixensart, Belgium), DTPw-HBV/Hib (Tritanrix™ HepB/Hib, GSK Biologicals) and DTPw-HBV (Tritanrix™ HepB, GSK Biologicals) have been described previously.Citation43
All vaccines were administered intramuscularly; PHiD-CV and Hib in the right thigh and DTPw-HBV/Hib and DTPw-HBV in the left thigh.
Immunogenicity assessment
Blood samples were collected before the first vaccine dose and one month after the third vaccine dose administration and were stored at -20°C until analyzed. Serum anti-pneumococcal serotype specific antibody concentrations were measured by 22F-inhibition enzyme-linked immunosorbent assay (22F-ELISA).Citation44,Citation45 Purified serotype-specific pneumococcal polysaccharides (PS) sourced from GSK Biologicals were mixed with methylated human serum albumin and adsorbed onto microtiter plates overnight at 4°C. Serum samples were diluted in PBS buffer containing 10% FBS, 10 µg/mL of CPS, and 2 µg/mL of PS of serotype 22F and further diluted on the microtiter plates with the same buffer. Bound antibodies were detected by using peroxidase-conjugated anti-human IgG monoclonal antibodies. The antibody concentration was determined by logistic log comparison of the ELISA curves with a standard reference serum 89-SF available from the US Food and Drug Administration for which concentrations of antibodies to the pneumococcal serotypes were known. The cut-off of the assay was set at 0.05 µg/mL for all serotypes and the percentages of infants with antibody concentrations ≥ 0.2 µg/mL were calculated to assess immune responses. It was previously established that an antibody concentration of 0.2 µg/mL as determined by GSK’s 22F-inhibition ELISA is equivalent to an antibody concentration of 0.35 µg/mL as determined by the non-22F ELISA used by the WHO reference laboratory.Citation45
The functional capacity of pneumococcal antibodies was measured in a randomly selected subset of 50% of the post-vaccination serum samples by OPA testing using a killing-assay. Serum samples were heated to inactivate any remaining endogenous complement and were diluted in OPA buffer and incubated with a mixture of differentiated human promyelocytic leukemia (HL-60) cells, pneumococcal working seed, and baby rabbit complement in microtiter plates for 2h at 37°C to promote the phagocytic process. The reaction was stopped on ice and the plates were then incubated overnight at 37°C and 5% CO2 after addition of Todd-Hewitt Broth-0.9% agar. Pneumococcal colonies were counted using an automated image analysis system. The mean number of colonies of the control wells was determined and used for the calculation of the killing activity for each serum sample. The OPA titer for the serum samples was determined by the reciprocal dilution of serum able to induce 50% killing of the pneumococci. The cut-off of the assay was an OPA titer of 8.Citation46-Citation48
In addition, antibody concentrations against the NTHi protein D carrier were measured in human serum by an in-house ELISA. A coating solution with the non-lipidated protein D antigen diluted at approximately 2 µg/mL in PBS (pH 7.2) was adsorbed onto a polystyrene microtiter plate overnight at 4°C. The dilutions of human sera (1:16) in assay diluent (PBS-FBS 10%-PVA 0.1%) were freshly prepared and were added to the protein D coated plate for 30 min at room temperature. The bound antibodies were detected using a peroxidase-coupled goat anti-human IgG antibody followed by the addition of tetramethyl benzidine. The level of the specific anti-protein D IgG antibodies present in the serum under testing was determined by comparison to the “in-house” reference serum (arbitrarily defined) and was expressed in ELISA units per mL (EL.U/mL). The assay cut-off was 100 EL.U/mL.
Anti-DT and anti-TT antibody concentrations were also measured by ELISA with a cut-off for seroprotection of 0.1 IU/mL.Citation49,Citation50 For anti-polyribosylribitol phosphate (anti-PRP) antibodies against Hib, the percentages of infants with seroprotective antibody concentrations ≥ 0.15 µg/mL and ≥ 1.0 µg/mL were evaluated.Citation51 Concentrations of antibodies against hepatitis B surface antigen (anti-HBs) were measured using an in-house ELISA with a cut-off for seroprotection of 10 mIU/mL.Citation52,Citation53 For antibodies against B. pertussis, titers were measured using a commercially available kit (IgG EIA test kit, Labsystems) with an assay cut-off of 15 EL.U/mL. As per protocol, all the infants in the PHiD-CV group who were not selected for the OPA analysis and all the infants in the control group were included in the subset of infants for the testing of the co-administered vaccine.
Serum samples were analyzed at GSK Biologicals’ laboratory in Rixensart, Belgium, except for anti-HBs responses that were measured at the CEVAC laboratory in Ghent, Belgium. All methods and equipment were validated as required. Both laboratories had an established quality system, established quality control procedures, and were audited regularly for quality assessment.
Safety and reactogenicity assessment
Solicited local (pain, redness, and swelling) and general [irritability, drowsiness, loss of appetite, and fever (defined as axillary temperature ≥ 37.5°C)] AEs, commonly associated with injectable childhood vaccines, were recorded during a 4-d follow-up period after each vaccination. Unsolicited AEs were reported for a period of 31 d after each vaccination. The intensity of each symptom was graded on a 0–3 scale. Pain at the injection site was considered to have a grade 3 intensity if the child cried when the limb was moved or if the limb was spontaneously painful, redness and swelling at the injection site if the diameter was > 30 mm, fever if axillary temperature was > 39.5°C, loss of appetite if the child did not eat at all, and irritability if the child cried and could not be comforted. Drowsiness and unsolicited AEs were considered of grade 3 intensity if they prevented normal everyday activity.
SAEs were recorded throughout the entire study period. SAEs were defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in disability or incapacity, or was an important medical event. All solicited local AEs were considered causally related to vaccination; causality for all other AEs was assessed by the investigators using their clinical judgment.
Statistical analyses
To obtain 300 evaluable infants (200 in the PHiD-CV group and 100 in the control group) in the ATP immunogenicity cohort, 360 infants (240 in the PHiD-CV group and 120 in the control group) were planned to be enrolled.
The immunogenicity analyses were performed on the ATP immunogenicity cohort, which included eligible infants, complying with the procedures and intervals defined in the protocol, with no elimination criteria during the study, and for whom data concerning immunogenicity endpoint measures were available. The safety analyses were performed on the total vaccinated cohort, which included all the infants with at least one vaccine administration documented.
The percentages of infants with concentrations/titers equal to or greater than the seropositivity/seroprotection cut-offs were calculated with exact 95% CI for each antigen/serotype. The antibody GMCs/OPA GMTs were calculated by taking the anti-log of the mean of the log antibody concentration/OPA titer transformations. Antibody concentrations/OPA titers below the cut-off of the assay were given an arbitrary value of half the cut-off for the purpose of GMC/GMT calculation.
The overall per dose incidence of solicited and unsolicited AEs was calculated with exact 95% CI by group and according to the intensity. SAEs and withdrawal due to AE(s) were described in detail throughout the study.
As the sample size was not powered to formally compare study groups, non-overlapping 95% CIs were used to detect potential differences between study groups. Observed differences should be interpreted with caution since there was no adjustment for multiple comparisons of the various endpoints. The software SAS® (version 9.2) and StatXact version 8.1 were used for the analyses.
Synflorix, Hiberix, Tritanrix HepB/Hib and Tritanrix HepB are trademarks of the GlaxoSmithKline group of companies.
This study has been registered at www.clinicaltrials.gov NCT00814710.
| Abbreviations: | ||
| AE | = | adverse event |
| AOM | = | acute otitis media |
| ATP | = | according to protocol |
| CI | = | confidence interval |
| CRM | = | mutated diphtheria toxoid protein |
| DT | = | diphtheria toxoid |
| DTPa | = | diphtheria-tetanus-acellular pertussis |
| DTPw-HBV/Hib | = | diphtheria tetanus whole cell pertussis- hepatitis B/H. influenzae type b vaccine |
| ELISA | = | enzyme-linked immunosorbent assay |
| GAVI | = | Global Alliance for Vaccines and Immunization |
| GMC | = | geometric mean concentration |
| GMT | = | geometric mean titer |
| GSK | = | GlaxoSmithKline |
| HBs | = | hepatitis B surface antigen |
| Hib | = | Haemophilus influenzae type b |
| IPD | = | invasive pneumococcal disease |
| NTHi | = | non-typeable Haemophilus influenzae |
| OPA | = | opsonophagocytic activity |
| PHiD-CV | = | 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine |
| PncOMPC | = | 7-valent pneumococcal polysaccharide meningococcal outer membrane protein conjugate vaccine |
| PRP | = | polyribosylribitol phosphate |
| PS | = | polysaccharide |
| SAE | = | serious adverse event |
| TT | = | tetanus toxoid |
| WHO | = | World Health Organization |
| 7vCRM | = | 7-valent pneumococcal conjugate vaccine |
| 13vCRM | = | 13-valent pneumococcal conjugate vaccine |
Acknowledgments
The authors are indebted to the study participants and their parents, clinicians, nurses, and laboratory technicians at the study site as well as to clinical investigators Dr Sonali Palkar and Dr Nandini Malshe (Bharati Vidyapeeth University, Pune), Dr Atul Goel (Christian Medical College, Ludhiana), Dr Anna Simon and Dr Leni Grace Mathew (Christian Medical College, Vellore) for their contribution to this study. We would like to thank the sponsor’s project staff for their support and contributions throughout the study, in particular Sanjoy Datta for critical reading of the manuscript and very helpful suggestions and Nancy François for her contribution in terms of statistical input to the protocol and assistance with data management. We are also grateful to all teams of GSK Biologicals for their contribution to this study. Finally we thank Claire Verbelen (XPE Pharma and Science for GSK Biologicals) who provided medical writing services and Véronique Mouton (XPE Pharma and Science for GSK Biologicals) for editorial assistance and manuscript coordination.
Contributions
All authors participated in the implementation of the study including gathering of data. All authors had full access to the data and had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations, documentation and outline provided by the lead author. There were agreements concerning confidentiality of the data between the industry sponsor and the academic authors.
Funding
GSK Biologicals was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals also took responsibility for all costs associated with the development and publishing of the present manuscript.
Disclosure of Potential Conflicts of Interest
S.L., S.C., J.C., V.P.V. and their institutions received grants from GSK Biologicals to support this study. S.L. received support for attending the PEDICON 2011 meeting (Jaipur, India) to present the study results. D.B., M.M., S.M., F.S. and L.S. are employees of GSK Biologicals; D.B., M.M. and L.S. have stock options.
References
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al, Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893 - 902; http://dx.doi.org/10.1016/S0140-6736(09)61204-6; PMID: 19748398
- Lin TY, Shah NK, Brooks D, Garcia CS. Summary of invasive pneumococcal disease burden among children in the Asia-Pacific region. Vaccine 2010; 28:7589 - 605; http://dx.doi.org/10.1016/j.vaccine.2010.07.053; PMID: 20674872
- Shah AS, Nisarga R, Ravi Kumar KL, Hubler R, Herrera G, Kilgore PE. Establishment of population-based surveillance for invasive pneumococcal disease in Bangalore, India. Indian J Med Sci 2009; 63:498 - 507; http://dx.doi.org/10.4103/0019-5359.58879; PMID: 20075551
- Klugman K, Tam JS. Pneumococcal conjugate vaccine in Asia and the Pacific: Conference report. Vaccine 2001; 19:4579 - 87; http://dx.doi.org/10.1016/S0264-410X(01)00256-0; PMID: 11729814
- Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 2005; 352:39 - 47; http://dx.doi.org/10.1056/NEJMoa040275; PMID: 15635111
- Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, et al. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet 2006; 367:482 - 8; http://dx.doi.org/10.1016/S0140-6736(06)68180-4; PMID: 16473125
- Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, Sacarlal J, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 2009; 28:108 - 13; http://dx.doi.org/10.1097/INF.0b013e318187a87d; PMID: 19131902
- Brooks WA, Breiman RF, Goswami D, Hossain A, Alam K, Saha SK, et al. Invasive pneumococcal disease burden and implications for vaccine policy in urban Bangladesh. Am J Trop Med Hyg 2007; 77:795 - 801; PMID: 17984328
- Arifeen SE, Saha SK, Rahman S, Rahman KM, Rahman SM, Bari S, et al. Invasive pneumococcal disease among children in rural Bangladesh: results from a population-based surveillance. Clin Infect Dis 2009; 48:Suppl 2 S103 - 13; http://dx.doi.org/10.1086/596543; PMID: 19191605
- Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol 2007; 45:1225 - 33; http://dx.doi.org/10.1128/JCM.02199-06; PMID: 17267625
- GAVI's PneumoADIP. Pneumococcal Regional Serotype Distribution for Pneumococcal AMC TPP. Department of International Health Johns Hopkins Bloomberg School of Public Health. 30-11-2008; 1-36.
- Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J 2004; 23:1142 - 52; PMID: 15626953
- Bluestone CD. Clinical course, complications and sequelae of acute otitis media. Pediatr Infect Dis J 2000; 19:Suppl S37 - 46; http://dx.doi.org/10.1097/00006454-200005001-00007; PMID: 10821471
- Hausdorff WP, Yothers G, Dagan R, Kilpi T, Pelton SI, Cohen R, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J 2002; 21:1008 - 16; http://dx.doi.org/10.1097/00006454-200211000-00007; PMID: 12442021
- Cherian T, Bhattacharji S, Brahmadathan KN, Lalitha MK, Raghupathy P, Steinhoff MC. Persistent rhinorrhoea in rural Indian children: prevalence and consequences. J Trop Pediatr 2000; 46:365 - 7; http://dx.doi.org/10.1093/tropej/46.6.365; PMID: 11191150
- Adam D. Global antibiotic resistance in Streptococcus pneumoniae. J Antimicrob Chemother 2002; 50:Suppl 1 - 5; http://dx.doi.org/10.1093/jac/dkf801; PMID: 12077153
- World Health Organisation. Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec 2007; 82:93 - 104; PMID: 17380597
- Jain A, Kumar P, Awasthi S. High nasopharyngeal carriage of drug resistant Streptococcus pneumoniae and Haemophilus influenzae in North Indian schoolchildren. Trop Med Int Health 2005; 10:234 - 9; http://dx.doi.org/10.1111/j.1365-3156.2004.01379.x; PMID: 15730507
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740 - 8; http://dx.doi.org/10.1016/S0140-6736(06)68304-9; PMID: 16517274
- Chevallier B, Vesikari T, Brzostek J, Knuf M, Bermal N, Aristegui J, et al. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis J 2009; 28:Suppl S109 - 18; http://dx.doi.org/10.1097/INF.0b013e318199f62d; PMID: 19325447
- Bermal N, Szenborn L, Chrobot A, Alberto E, Lommel P, Gatchalian S, et al. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr Infect Dis J 2009; 28:Suppl S89 - 96; http://dx.doi.org/10.1097/INF.0b013e318199f901; PMID: 19325451
- Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène J-P, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28:Suppl S66 - 76; http://dx.doi.org/10.1097/INF.0b013e318199f8ef; PMID: 19325449
- Wysocki J, Tejedor JC, Grunert D, Konior R, Garcia-Sicilia J, Knuf M, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009; 28:Suppl S77 - 88; http://dx.doi.org/10.1097/INF.0b013e318199f609; PMID: 19325450
- Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, et al. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009; 28:Suppl S97 - 108; http://dx.doi.org/10.1097/INF.0b013e318199f61b; PMID: 19325452
- WHO. Pertussis vaccines--WHO position paper. Wkly Epidemiol Rec 2005; 80:31 - 9; PMID: 15717398
- Dicko A, Odusanya OO, Diallo AI, Santara G, Barry A, Dolo A, et al. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health 2011; 11:882; http://dx.doi.org/10.1186/1471-2458-11-882; PMID: 22112189
- Puumalainen T, Dagan R, Wuorimaa T, Zeta-Capeding R, Lucero M, Ollgren J, et al. Greater antibody responses to an eleven valent mixed carrier diphtheria- or tetanus-conjugated pneumococcal vaccine in Filipino than in Finnish or Israeli infants. Pediatr Infect Dis J 2003; 22:141 - 9; http://dx.doi.org/10.1097/01.inf.0000050459.74134.d5; PMID: 12586978
- Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 2010; 201:1570 - 9; http://dx.doi.org/10.1086/652006; PMID: 20384496
- Rodenburg GD, van Gils EJ, Veenhoven RH, Bogaert D, van den Dobbelsteen GP, Berbers GA, et al. Lower immunoglobulin G antibody responses to pneumococcal conjugate vaccination at the age of 2 years after previous nasopharyngeal carriage of Streptococcus pneumoniae. J Pediatr 2011; 159:965 - 70, e1; http://dx.doi.org/10.1016/j.jpeds.2011.06.011; PMID: 21813135
- Madhi SA, Violari A, Klugman KP, Lin G, McIntyre JA, von Gottberg A, et al, CIPRA 4 team. Inferior quantitative and qualitative immune responses to pneumococcal conjugate vaccine in infants with nasopharyngeal colonization by Streptococcus pneumoniae during the primary series of immunization. Vaccine 2011; 29:6994 - 7001; http://dx.doi.org/10.1016/j.vaccine.2011.07.035; PMID: 21787822
- Väkeväinen M, Soininen A, Lucero M, Nohynek H, Auranen K, Mäkelä PH, et al, ARIVAC Consortium. Serotype-specific hyporesponsiveness to pneumococcal conjugate vaccine in infants carrying pneumococcus at the time of vaccination. J Pediatr 2010; 157:778 - 83, e1; http://dx.doi.org/10.1016/j.jpeds.2010.04.071; PMID: 20547399
- Obaro SK, Steinhoff MC, Shahid N, Siber G. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 1996; 347:192 - 3; http://dx.doi.org/10.1016/S0140-6736(96)90376-1; PMID: 8544562
- Levine OS, Cherian T. Pneumococcal vaccination for Indian children. Indian Pediatr 2007; 44:491 - 6; PMID: 17684301
- Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, et al, Finnish Otitis Media Study Group. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis 2003; 37:1155 - 64; http://dx.doi.org/10.1086/378744; PMID: 14557958
- Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al, Finnish Otitis Media Study Group. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344:403 - 9; http://dx.doi.org/10.1056/NEJM200102083440602; PMID: 11172176
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al, Northern California Kaiser Permanente Vaccine Study Center Group. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000; 19:187 - 95; http://dx.doi.org/10.1097/00006454-200003000-00003; PMID: 10749457
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, et al, Active Bacterial Core Surveillance of the Emerging Infections Program Network. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354:1455 - 63; http://dx.doi.org/10.1056/NEJMoa051642; PMID: 16598044
- Knuf M, Habermehl P, Cimino C, Petersen G, Schmitt HJ. Immunogenicity, reactogenicity and safety of a 7-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a DTPa-HBV-IPV/Hib combination vaccine in healthy infants. Vaccine 2006; 24:4727 - 36; http://dx.doi.org/10.1016/j.vaccine.2006.03.032; PMID: 16616973
- Olivier C, Belohradsky BH, Stojanov S, Bonnet E, Petersen G, Liese JG. Immunogenicity, reactogenicity, and safety of a seven-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a fully liquid DTPa-IPV-HBV-Hib combination vaccine in healthy infants. Vaccine 2008; 26:3142 - 52; http://dx.doi.org/10.1016/j.vaccine.2007.11.096; PMID: 18502545
- Schmitt HJ, Faber J, Lorenz I, Schmöle-Thoma B, Ahlers N. The safety, reactogenicity and immunogenicity of a 7-valent pneumococcal conjugate vaccine (7VPnC) concurrently administered with a combination DTaP-IPV-Hib vaccine. Vaccine 2003; 21:3653 - 62; http://dx.doi.org/10.1016/S0264-410X(03)00389-X; PMID: 12922095
- Tichmann-Schumann I, Soemantri P, Behre U, Disselhoff J, Mahler H, Maechler G, et al. Immunogenicity and reactogenicity of four doses of diphtheria-tetanus-three-component acellular pertussis-hepatitis B-inactivated polio virus-Haemophilus influenzae type b vaccine coadministered with 7-valent pneumococcal conjugate Vaccine. Pediatr Infect Dis J 2005; 24:70 - 7; http://dx.doi.org/10.1097/01.inf.0000148923.46453.48; PMID: 15665713
- Lagos R, Hoffenbach A, Scemama M, Dupuy M, Schodel F, Hessel L, et al. Lot-to-lot consistency of a combined hexavalent diptheria-tetanus-acellular-pertussis, hepatitis B, Inactivated polio and haemophilus B conjugate vaccine, administered to healthy chilean infants at two, four and six months of age. Hum Vaccin 2005; 1:112 - 7; http://dx.doi.org/10.4161/hv.1.3.1848; PMID: 17012870
- Prymula R, Plisek S. Clinical experience with DTPw-HBV and DTPw-HBV/Hib combination vaccines. Expert Opin Biol Ther 2008; 8:503 - 13; http://dx.doi.org/10.1517/14712598.8.4.503; PMID: 18352853
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Vaccine Immunol 2001; 8:266 - 72; http://dx.doi.org/10.1128/CDLI.8.2.266-272.2001
- Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134 - 42; http://dx.doi.org/10.1128/CVI.00289-09; PMID: 19889940
- Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 2007; 25:2518 - 27; http://dx.doi.org/10.1016/j.vaccine.2006.09.029; PMID: 17034907
- Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 2006; 13:165 - 9; http://dx.doi.org/10.1128/CVI.13.2.165-169.2006; PMID: 16467321
- Rose CE, Romero-Steiner S, Burton RL, Carlone GM, Goldblatt D, Nahm MH, et al. Multilaboratory comparison of Streptococcus pneumoniae opsonophagocytic killing assays and their level of agreement for the determination of functional antibody activity in human reference sera. Clin Vaccine Immunol 2011; 18:135 - 42; http://dx.doi.org/10.1128/CVI.00370-10; PMID: 21084458
- Camargo ME, Silveira L, Furuta JA, Oliveira EP, Germek OA. Immunoenzymatic assay of anti-diphtheric toxin antibodies in human serum. J Clin Microbiol 1984; 20:772 - 4; PMID: 6386879
- Melville-Smith ME, Seagroatt VA, Watkins JT. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand 1983; 11:137 - 44; http://dx.doi.org/10.1016/S0092-1157(83)80038-9; PMID: 6345548
- Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 1999; 354:2063 - 8; http://dx.doi.org/10.1016/S0140-6736(99)04377-9; PMID: 10636384
- Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR United States 1991; 40:1 - 25
- World Health Organization (WHO). Progress in the control of viral hepatitis: memorandum from a WHO meeting. Bull World Health Organ 1988; 66:443 - 55; PMID: 2971466