Abstract
Using a population based self-controlled case series study design we examined data on 834 740 children in the province of Ontario, Canada. We observed that when comparing to SGA10 term children (term children born in the lowest 10th percentile of weight for a given gestational age), relative incidence of emergency room visits and admission in the 3 d post 2 mo vaccination progressively decreased in near term [relative incidence ratio 0.89 (95% CI 0.74–1.07)] and very premature children [0.67(0.49–0.93)]. When compared with all term children this decrease in risk is not statistically significant. We speculate that the immune response is reduced in premature children resulting in reduced adverse events. This is masked when comparing to all term children because the reduced birth weight of premature children results in a comparatively increased dose of vaccine. This in turn results in an increased immune response and risk of immediate adverse reactions. Future studies of immune response in premature children should examine the impact of weight at the time of immunization.
Introduction
Vaccine schedules for premature children, with the exception of hepatitis B vaccine for infants of HBsAg positive mothers, are recommended at a chronological age consistent with term children with no adjustment for degree of prematurity.Citation1,Citation2 A potential limitation of this approach is that the reduced immune response in premature children may impair response to the vaccine.Citation3,Citation4 Evidence, however, suggests that development of an adequate immune response and safety are similar in premature children compared with term children.Citation1,Citation4-Citation6 This seems contrary to current understanding of neonatal immunology.
In a previous analysis we examined the risk of adverse events following immunization in term children.Citation7 We found that higher birth weight in children was associated with lower event rates following vaccination at 2 mo of age. In this paper we examine rates of emergency room visits and hospital admissions following 2 mo vaccination in three categories of gestational age. We hypothesized that controlling for birth weight in premature children may identify different patterns of adverse events following vaccinationCitation7 so we repeated this analysis using term children who were in the lowest decile for birth weight controlling for their gestational age (SGA10), instead of all term children. In the province of Ontario, Canada, standard 2 mo vaccines consist of diphtheria, pertussis, tetanus, polio, hemophilus influenzae type b (Hib) and pneumococcus. We present the results of this analysis and propose a potential explanation for our findings.
Results
We examined data on 714,841 term children, 63,287 SGA10 term children, 49,220 near term and 7,392 very premature children. Of these, 26,413 term children, 2,550 SGA10 term children 2,784 near term children and 608 very premature children had an event in the follow-up period and were included in the SCCS analysis. Mean birth weight and gestational age can be found in . demonstrates the pattern of events peri-vaccination in all children analyzed. The relative incidence of events in all term children, SGA10 term children, near term and very premature children were 0.81 (95% CI 0.77–0.84), 0.98 (0.86–1.12), 0.88 (0.77–1.00), 0.66 (0.49–0.88) respectively (). Compared with all term children, the relative incidence ratio of events for near term children was 1.09 (0.95–1.25) and for very premature children 0.82 (0.60–1.10) demonstrating no significant decrease in events. When compared with SGA10 term children as the reference group, the relative incidence ratio was 0.89 (0.74–1.07) in near term children and 0.67 (0.49–0.93) in very premature children, demonstrating a progressive reduction in events with increasing category of prematurity. The overall test for differences in incidence ratios among the groups was significant (p = 0.0463) (). This finding was persistent in our sensitivity analyses using a shorter risk period (p-int = 0.0097) and longer control period (p-int = 0.0498) ( and ). ER visits comprised the large majority of events for the composite endpoint in all analyses.
Table 1. Characteristics of children in analysis
Figure 1. ER visits and hospital admissions before and after 2 mo vaccination in all children excluding extremely premature (day 0 is day of vaccination).
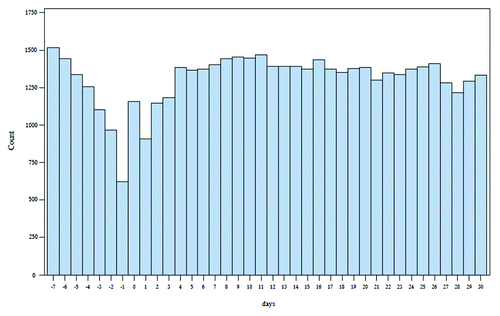
Table 2. Relative incidence of events and relative incidence ratio based on category of prematurity
Table 3. Relative incidence of events and relative incidence ratio based on category of prematurity: sensitivity analysis with longer control period (day 5–21)
Table 4. Relative incidence of events and relative incidence ratio based on category of prematurity: sensitivity analysis with shorter risk period (day 1 or 2)
Discussion
Our analysis of adverse events as defined by ER visits and hospital admissions within 3 d of 2 mo vaccination, found that in all categories of prematurity examined the relative incidence of events was less than 1.0. This is consistent with our previous analyses of health service utilization following immunization and is attributable to the healthy vaccinee effect, the fact that unhealthy children are unlikely to be vaccinated. This effect results in a lower than baseline rate of events in the immediate post-vaccination period and thus relative incidences of less than 1.0.Citation8 By using relative incidence ratios to compare relative incidences across different sub-populations, we have in effect adjusted for the healthy vaccinee bias, and we can discern the differential effect of the vaccination on adverse events in these sub-populations, in this case categories of prematurity. By doing this, we identified that when using term children as the reference group, a trend to lower incidences of events based on category of prematurity is not evident. However, when we partially adjust for weight by using SGA10 term children, the trend toward reductions in events with increasing degree of prematurity becomes much more evident and statistically significant. In particular, in very premature children we observed a 33% reduction in relative incidence of events compared with SGA10 term children. While SGA10 term children had approximately the same mean weight as near term children, very premature children have even lower weight. Our finding of reduced rates of adverse events in this population may therefore be an underestimate of the degree of reduction in events that would be observed if we were able to fully adjust for weight.
These results are intended to be hypothesis generating and stimulate further research. In a previous analysis we identified that when term children were divided into birth weight quintiles there was a trend toward increase in risk for ER visits and hospital admissions in the 3 d post 2 mo vaccination the lower the birth weight quintile. We also observed a significantly higher risk of events in term SGA10 children than non SGA10 children as well as in SGA10 children vaccinated comparatively earlier, and thus presumably smaller at the time of vaccination.Citation7 Similar correlations between birth weight and adverse events have been observed in animals.Citation9 We hypothesize that the comparatively lower levels of non-specific adverse events are related to less immunogenicity in a population. Thus comparatively heavier children who receive the same dose of vaccine as less heavy children may be experiencing less of an immune response and thus fewer adverse events immediately following immunization.
Limitations of our analysis include the choice of risk and control periods. The risk period of 3 d post vaccination is based on the understanding of the immunological reaction caused by the vaccine which usually manifests within 24 h.Citation7 It is possible that reactions could occur beyond the 3 d risk interval although these events would likely be comparatively infrequent. We are restricted as to the period after vaccination we could use as a control period because if it were too proximal to the next vaccination the rates may be influenced by the healthy vaccinee effect. Furthermore baseline rates of ER visits and admissions to hospital generally decline as the child ages. Thus rates of these events would decline the further removed from the vaccination and disproportionately so the more premature a child is thus inflating the relative incidence of events in these children. Fortunately, graphical analysis demonstrates that the rates have stabilized during the control period we chose. Our findings persist in sensitivity analyses using a shorter risk period and a longer control period. Another limitation to our study is the absence of vaccine specific codes.
We speculate that a reduced immune response in premature children results in fewer adverse reactions immediately following vaccination. Previous studies on premature children have demonstrated a reduced yet adequate immune response in premature children supporting this explanation.Citation4 However, the lower weight of premature children, and the comparative increased dose of vaccine, results in this effect being masked in near term infants. Our observation potentially reconciles the understanding that premature children have depressed immune responses yet have the same immunogenic response to vaccines. Future research should further examine the immune response to pediatric vaccines adjusting for both weight and prematurity.
Methods
Using historical data, and the Vaccine and Immunization Surveillance in Ontario (VISION) system, we examined whether rates of emergency room (ER) visits and hospital admissions in the 3 d post 2-mo vaccination varied differed between term (37+ weeks), near term (33–36 weeks) and very premature (28–32 weeks) infants. We repeated this analysis by using term SGA10 children (term children born in the lowest 10th percentile of weight for a given gestational age) instead of all term children. We utilized the self-controlled case series (SCCS) method where individuals serve as their own control and the rate of events per day in an “at risk” period (immediate 3 d post vaccination) is compared with the rate of events in a control period(s) defined as a period during which it would be unlikely that the exposure produced the outcome (9–18 d post-vaccination)Citation7,Citation8 In the self-controlled case series model, only individuals who were both vaccinated and had an event of interest during the observation period contribute to the analysis. The date of vaccination serves as the index date for exposure for each patient. Each individual’s follow-up period is divided up into distinct intervals: an initial 3-d interval classified as exposed, followed by days 9–18 classified as unexposed with a washout period in between the exposed and unexposed periods. Our choice of the control period was based on the fact that it would be highly unlikely that acute inflammation from the vaccine would result in health service increases this far removed from the date of vaccination. Further we did not want to choose a control period so far removed from the vaccination that it could be influenced by the subsequent vaccination event, typically occurring at 4 mo of age. The relative incidence rate of the composite end point of ER visit or hospitalization during the exposed period compared with the unexposed period was computed using a fixed effects conditional Poisson regression model that included indicator variables for exposure period and for individual patients, allowing each individual to serve as his or her own control, in a very similar manner to a case crossover analysis. To address the correlation of multiple events close together in time (e.g., an ER visit leading to an admission, or serial ER visits), the occurrence of events were classified as “one or more events” or “no events” in each of the risk and control periods.
The SCCS model implicitly adjusts for all fixed confounding variables. The choice of the at-risk period was based on the expected physiological reaction to the vaccine and the recognition that adverse events typically manifest within 48 h of vaccination.Citation10 The choice of the control period was based on our previous analysis which demonstrated that rates of events were stable at a baseline during this period.Citation8 We conducted sensitivity analyses using a shorter risk interval (48 h) and a longer control period (days 5 to 21). In two separate analyses, we compared incidence rates across categories of prematurity by calculating relative incidence ratios with either SGA10 term children or all term children serving as the reference group.Citation11 We conducted a test for interaction in the SCCS model using a likelihood ratio test to examine whether the relative incidence changed significantly across the levels of prematurity. The study included all children born in Ontario between April 1st, 2002 and March 31st, 2009, who were present in the Institute for Clinical Evaluative Sciences Registered Persons Database. Vaccination at 2 mo of age vaccination was identified using Ontario Health Insurance Plan billing codes for general vaccination occurring on the exact due date (60 d) as well as occurring up to14 d before, or 40 d after the due date. We identified data on hospital admissions from the Canadian Institute for Health Information’s Discharge Abstract Database and data on emergency department visits from the National Ambulatory Care Registration System.
Acknowledgments
Funding: This study was supported by the Canadian Foundation for Innovation, the Population Health Improvement Research Network (PHIRN), and by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, Ontario MOHLTC or PHIRN is intended or should be inferred. Dr. Wilson holds the Canada Research Chair in Public Health Policy. This study received ethics approval from the Ottawa Hospital Research Institute’s Research Ethics Board.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Saari TN, American Academy of Pediatrics Committee on Infectious Diseases. Immunization of preterm and low birth weight infants. Pediatrics 2003; 112:193 - 8; http://dx.doi.org/10.1542/peds.112.1.193; PMID: 12837889
- Public Health Agency of Canada. Canadian Immunization Guide. Seventh Edition - 2006. Part 3. Recommended Immunization-Immunization of Infants Born Prematurely. 2006.
- D’Angio CT. Active immunization of premature and low birth-weight infants: a review of immunogenicity, efficacy, and tolerability. Paediatr Drugs 2007; 9:17 - 32; http://dx.doi.org/10.2165/00148581-200709010-00003; PMID: 17291134
- Schloesser RL, Fischer D, Otto W, Rettwitz-Volk W, Herden P, Zielen S. Safety and immunogenicity of an acellular pertussis vaccine in premature infants. Pediatrics 1999; 103:e60; http://dx.doi.org/10.1542/peds.103.5.e60; PMID: 10224204
- Conway S, James J, Balfour A, Smithells R. Immunisation of the preterm baby. J Infect 1993; 27:143 - 50; http://dx.doi.org/10.1016/0163-4453(93)94674-Z; PMID: 8228295
- Pullan CR, Hull D. Routine immunisation of preterm infants. Arch Dis Child 1989; 64:1438 - 41; http://dx.doi.org/10.1136/adc.64.10.1438; PMID: 2554822
- Wilson K, Hawken S, Kwong JC, Deeks SL, Manuel DG, Henningsen KH, et al. Impact of birth weight at term on rates of emergency room visits and hospital admissions following vaccination at 2 months of age. Vaccine 2011; 29:8267 - 74; http://dx.doi.org/10.1016/j.vaccine.2011.08.107; PMID: 21907256
- Wilson K, Hawken S, Potter BK, Chakraborty P, Kwong J, Crowcroft N, et al. Patterns of emergency room visits, admissions and death following recommended pediatric vaccinations—a population based study of 969,519 vaccination events. Vaccine 2011; 29:3746 - 52; http://dx.doi.org/10.1016/j.vaccine.2011.03.044; PMID: 21443964
- Moore GE, Guptill LF, Ward MP, Glickman NW, Faunt KK, Lewis HB, et al. Adverse events diagnosed within three days of vaccine administration in dogs. J Am Vet Med Assoc 2005; 227:1102 - 8; http://dx.doi.org/10.2460/javma.2005.227.1102; PMID: 16220670
- Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol 2007; 137:Suppl 1 S46 - 50; http://dx.doi.org/10.1016/j.jcpa.2007.04.012; PMID: 17559869
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006; 25:1768 - 97; http://dx.doi.org/10.1002/sim.2302; PMID: 16220518