Abstract
A 20-year-old male presented with symptoms of meningococcal sepsis and died despite appropriate medical interventions. Blood cultures grew N. meningitidis serogroup Y. The patient had received the meningococcal quadrivalent (A,C,W-135,Y) polysaccharide vaccine 15 months previously. Because the patient had a history of meningococcal meningitis at age 10, archived serum was obtained for further analysis. Complement component C7 was found to be deficient and antibody levels to meningococcal polysaccharides were undetectable for two serogroups and low for the infecting serogroup 10 months post-vaccination. This case highlights the fact that some individuals with terminal complement component deficiencies mount an impaired or short-lived response to vaccination with meningococcal capsular polysaccharides and underscores the appropriateness of a more aggressive vaccination strategy in this patient population.
Patient Presentation
A 10 y old male presented to his pediatrician with fever, headache and body aches. The following day he developed photophobia, neck stiffness, and petechiae. He presented to a hospital emergency room where lumbar puncture showed pleocytosis and Gram-negative diplococci. His platelet count was 94 × 103/mm3, but prothrombin and activated partial thromboplastin times were normal. He was treated with dexamethasone for 4 d and a total of 7 d of intravenous antibiotics (ampicillin, switched to meropenem because of a rash) and recovered without sequelae. Cerebrospinal fluid cultures grew Neisseria meningitidis (serogroup not specified). A CH50 level performed 1 y later was normal (40 U/mL, reference range 31–66).
At age 19 he received the tetravalent polysaccharide meningococcal vaccine when he joined the military. Fifteen months after vaccination he presented to the local military clinic with a 1-d history of fevers; chills; aching in the muscles of his legs, upper back and neck; and a pounding frontal headache with light sensitivity. At this initial clinic visit he was diagnosed with a probable viral syndrome, prescribed ibuprofen, and excused from duty for the day. About 8 h later he developed nausea and vomiting and a generalized purplish macular rash. His roommate called the clinic and reported that the patient said he felt “like he did when he had meningitis.” The patient was taken immediately to an emergency room.
On arrival he was in respiratory distress, prompting endotracheal intubation. Admission labs showed a white blood cell count of 5.2 × 103/mm3, platelet count 23 × 103/mm3, creatinine 2.42 mg/dL, prothrombin time 31.6 sec, activated partial thromboplastin time 131.9 sec and arterial pH of 7.20. He was started on several broad-spectrum antibiotics, including ceftriaxone, and dexamethasone. His CH50, drawn while he was in the throes of sepsis, was < 10 U/mL; C3 was low at 62 mg/dL (normal 82–235) and C4 was low at 10 mg/dL (normal 16–70). Despite pressor support, transfusions of fresh frozen plasma and hemodialysis, he expired due to disseminated intravascular coagulation and intracranial hemorrhage on hospital day 6. Blood cultures grew N. meningitidis at 24 h, which was subsequently shown to be serogroup Y.
Serum collected while in good health (routinely archived upon joining the military and periodically thereafter) was obtained from the Armed Forces Health Surveillance Center repository and assayed for complement levels as follows:
C6: 10.4 mg/dL (reference range 7.1–12.8);
C7: undetected (reference range 4–11 mg/dl);
C8: 19.8 mg/dL (reference range 10.7–24.9);
C9: 31 mg/dL (reference range 6–29).
An additional archived serum sample, drawn 10 mo post-vaccination (5 mo before his terminal illness), showed the following IgG levels to capsular polysaccharides:
group A capsule: 3.5 µg/mL;
group C capsule: < 0.5 µg/mL;
group Y capsule: 0.8 µg/mL;
group W-135 capsule: < 0.5 µg/mL.
Discussion
A significant proportion of individuals with sporadic meningococcal infection have deficiencies of the complement system.Citation1,Citation2 A recent study found that 15?23% of adults with meningococcal meningitis had an underlying complement deficiency.Citation3 The most common and most recently described deficiencies predisposing to meningococcal disease result in defective pathogen recognition via mannose-binding lectin (MBL).Citation4-Citation6 Under normal circumstances, MBL recognizes carbohydrate patterns on the surface of bacteria, including meningococci, mediating phagocytosis and complement-mediated lysis.Citation7 Although individuals lacking this function are predisposed to meningococcal infection, protection via the classical complement pathway can bypass the defect once an antibody response has developed. Recurrent meningococcal infection and infection with unusual serogroupsCitation6 is therefore not typical with MBL deficiencies.
Deficiencies of various components and regulatory proteins of the classical (IgG,Citation8 C1q,Citation9 C2,Citation9 C4Citation10) and alternative (properdin,Citation11 factor D,Citation12 and factor BCitation9) complement pathways predispose to meningococcal disease. Defects of the classical pathway can be compensated for by the alternative pathway and, once an antibody response has developed, vice versa (). Recurrent meningococcal disease is therefore less likely with these deficiencies,Citation9 though still occasionally reported.Citation13
Figure 1. The complement system as it pertains to meningococcus. Triangles represent factors bound to or within the bacterial surface; squares/rectangles represent factors that are bound to other factors; circles represent circulating factors; parallelograms represent factors that support serum levels by inhibiting complement activation on host cells (factor I and factor H). Factors associated with recurrent meningococcal infection are shown in red; those associated with meningococcal infection, but not with recurrent infection, are shown in orange. Abbreviations: MBL, mannose binding lectin; MASP2, mannose binding lectin-associated serine protease-2; Ig, immunoglobulin.
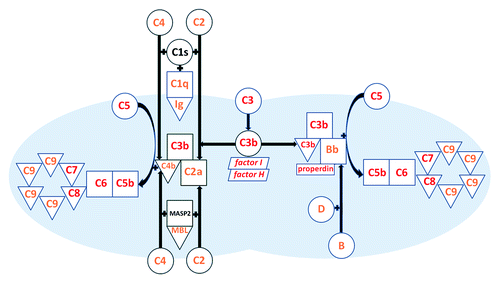
At the convergence of classical and alternative pathways is complement component C3. When C3 is lacking, not only is opsonization of the organism by C3b impaired, but so is the formation of both types of C5 convertase, resulting in impaired formation of the membrane attack complex and deficient bacterial lysis. In this case, one pathway cannot compensate for the other, so deficiency of C3,Citation14 deficiencies of regulatory proteins that prevent overconsumption of C3 (factor HCitation15 and factor ICitation16), and autoantibodies that promote overconsumption of C3 (C3 nephritic factorCitation17) are all associated with recurrent meningococcal infection.Citation9 When components of the terminal complement system (C5,Citation18 C6,Citation19 C7,Citation20 C8,Citation21 and possibly C9Citation22) are lacking, bacterial lysis is also impaired regardless of whether the meningococci are recognized by innate or acquired mechanisms. Significant hemolytic activity is retained in complete C9 deficiency and the association with meningococcal disease is less obvious,Citation22 but individuals with C5 through C8 deficiencies (TCCD) depend on phagocytosis for immune protection. Antibodies directed against the polysaccharide capsule increase serum bactericidal activity in complement-sufficient individuals and are good opsonins,Citation23 but they do not always develop as a result of natural infection or colonization.Citation2 Antibodies against sub-capsular antigens, such as outer membrane proteinsCitation24 and lipooligosaccharidesCitation25 enhance bactericidal activity against the infecting strain and provide varying degrees of cross-protection to strains from other serogroups in complement-sufficient individuals, but they are less effective at mediating opsonophagocytosis.Citation23 As a result, recurrent infectionCitation9 and infection with unusual serogroupsCitation26,Citation27 is commonly reported with TCCD.
Serum bactericidal activity (SBA) has long been considered a marker for immunologic protection from meningococcal disease,Citation28 and an increased meningococcal SBA titer is the standard efficacy end-point in meningococcal vaccine trials.Citation29 However this test is performed by adding complement pooled from laboratory animals or from immunocompetent human donors, and would over-estimate efficacy in individuals with complement deficiencies who are not able to mount a bactericidal response in vivo.Citation9 Such individuals must rely on other immunologic mechanisms, principally opsonophagocytosis,Citation30 for immune protection. Anti-capsular antibody induced by polysaccharide vaccination has been shown to protect individuals with TCCD,Citation30 however, the antibody response varies. Biselli et al. found that 11 individuals with homozygous C7, C8 or factor H deficiencies had significantly decreased antibody responses to both group A and group C capsular polysaccharides at 45 d post-vaccination vs. 24 controls.Citation31 Eleven heterozygous individuals appeared to have an intermediate response. Andreoni and colleagues found no significant differences among eight survivors of meningococcal infection with absent CH50 activity, eight heterozygous family members, and three controls at 3–4 weeks post-vaccination.Citation23 Platonov et al. did not find significant differences between 18 patients with C7 or C8 deficiency, 7 of their healthy relatives, or 38 health controls at 54 weeks post-vaccination,Citation32 nor did they find differences 4 y out between 54 complement-deficient individuals and controls.Citation33 Drogari-Apiranthitou and colleagues found trends toward a lower response to group Y 7 y post-vaccination in 17 patients with TCCD, but this fell short of statistical significance (p = 0.07).Citation34 Herva et al. reported an individual with low levels of several complement components whose response to group A vaccination was low despite a normal response to a 14-valent pneumococcal polysaccharide vaccine.Citation35 Taken together, these reports suggest that impaired anti-capsular antibody formation is not a uniform feature of TCCD, but is nevertheless significant in some groups.
The anti-capsular IgG levels of the patient in this case report, compared with typical responses to meningococcal polysaccharide vaccination, in which the 95% confidence intervals for total antibody to groups A and C remained above 2 µg/mL up to ten years out,Citation36 appear to be modest for group A, but clearly deficient to group C. His antibodies to group W-135 were undetectable and to group Y were clinically inadequate. This case report represents the earliest time to vaccine failure in a C7-deficient individual in which the infecting serogroup and prior antibody levels are known. Platonov et al. reported on meningococcal disease occurring in a C7-deficient individual at 9 mo post-vaccination.Citation32,Citation37 The serogroup of the infecting organism was not reported, but the individual showed an increase in antibody level to the group C polysaccharide during convalescence, suggesting that this, too, was a vaccine failure. Andreoni et al. reported a case of recurrent meningococcal disease in a C7-deficient patient 2.5 y post-vaccination. This patient initially had a good antibody response to the group C polysaccharide. The antibody response at the time of recurrent infection was 0.38 µg/mL, but it is not clear if this was the level when he was first re-exposed or reflected consumption due to active infection.Citation23 Fijen and colleagues documented infection with group Y in C8β-deficient individuals at 3.5 and 5 y post-vaccination.Citation38 Based on this experience, they recommended repeat vaccination after 3 y in individuals with TCCD, a recommendation that was, until recently,Citation39 reflected in national guidelines.Citation40
It has been suggested, based on in vitro studies, that anti-capsular antibody levels are appropriate surrogate markers for protection in individuals with TCCD, and that levels as low as 1 to 2 µg/mL are adequate for protection.Citation23 Our case, in which an anti-Y antibody level of 0.8 µg/mL 5 mo before the terminal infection was not protective, is consistent with this suggestion. Defining a precise minimum protective cutoff is probably not possible due to differences in antibody binding avidities, and anti-capsular antibody may underestimate the degree of protection.Citation30 Nevertheless, this test is readily available and may help identify those with an inadequate response to vaccination.
This case highlights the fact that some individuals with TCCD demonstrate an impaired or short-lived response to vaccination with meningococcal capsular polysaccharide, and underscores the appropriateness of a more aggressive vaccination strategy. Current guidelines from the US Centers for Disease Control and Prevention recommend that individuals with complement deficiencies receive a two-dose primary series of meningococcal conjugate vaccine followed by boosting every 5 y.Citation39 Whether or not this will be adequate for this population remains to be seen.Citation41 Vaccines developed for group B meningococcus based on sub-capsular antigens do elicit opsonophagocytic as well as bactericidal activity in normal hosts,Citation42 and therefore have the potential to induce cross-protecting antibodies in individuals with TCCD. Since individuals with TCCD are also susceptible to serogroups not available in vaccines and are less likely to develop cross-protection from antibodies to sub-capsular antigens that develop as a result of colonization, the use of sub-capsular meningococcal vaccines, when they become available, will likely be appropriate. Until such vaccines are available and demonstrate full protection in individuals with TCCD, additional strategies, such as antibiotic prophylaxis or self-treatment of prodromal symptoms, seem prudent.
The normal CH50 in this patient at age 11 appears to have been a laboratory error, although some TCCD mutations are associated with subtotal deficiencies that allow for detectable CH50 activity.Citation43 Unfortunately, no more archived samples are available from this patient to further characterize the precise defect associated with his lack of detectable serum C7. The CH50 test should detect most of those defects that predispose to recurrent meningococcal infection. However, given the high prevalence of complement deficiencies among individuals with sporadic meningococcal disease, the expected increase in this prevalence as the overall incidence of meningococcal disease declinesCitation44 and the possibility of identifying other family members who may be at risk (properdin deficiency is X-linked; C7 and other hereditary complement defects are autosomal co-dominantCitation44), a more thorough workup of the complement system in survivors of meningococcal disease, to include AH50 and/or tests for individual complement components, could be justified.
| Abbreviations: | ||
| AH50 | = | alternative complement pathway hemolytic assay |
| CH50 | = | classical complement pathway hemolytic assay |
| Ig | = | immunoglobulin |
| MASP2 | = | mannose binding lectin-associated serine protease |
| MBL | = | mannose binding lectin |
| TCCD | = | terminal complement component deficiency |
Acknowledgments
Tests of archived sera were funded by the Walter Reed Army Medical Center and made possible by the staff of the Armed Forces Health Surveillance Center. The views expressed in this work are those of the authors and do not reflect the official policy of the Department of the Navy, Department of the Army, Department of Defense, or the US Government. Approved for public release; distribution is unlimited. This research has been conducted in compliance with all applicable federal regulations governing the protection of human subjects in research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984; 63:243 - 73; PMID: 6433145
- D’Amelio R, Agostoni A, Biselli R, Brai M, Caruso G, Cicardi M, et al. Complement deficiency and antibody profile in survivors of meningococcal meningitis due to common serogroups in Italy. Scand J Immunol 1992; 35:589 - 95; http://dx.doi.org/10.1111/j.1365-3083.1992.tb03258.x; PMID: 1579859
- Kallel-Sellami M, Abdelmalek R, Zerzeri Y, Laadhar L, Blouin J, Zitouni M, et al. Déficits héréditaires en protéines du complément au cours des méningites purulentes: étude de 61 patients adultes tunisiens et revue de la littérature. Arch Inst Pasteur Tunis 2006; 83:25 - 34; PMID: 19388594
- Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M, Meningococcal Research Group. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 1999; 353:1049 - 53; http://dx.doi.org/10.1016/S0140-6736(98)08350-0; PMID: 10199352
- Bax WA, Cluysenaer OJ, Bartelink AK, Aerts PC, Ezekowitz RA, van Dijk H. Association of familial deficiency of mannose-binding lectin and meningococcal disease. Lancet 1999; 354:1094 - 5; http://dx.doi.org/10.1016/S0140-6736(99)02563-5; PMID: 10509505
- Kuijper EJ, Fijen CA, Dankert J, Thiel S. Mannose-binding lectin and meningococcal disease. Lancet 1999; 354:338; http://dx.doi.org/10.1016/S0140-6736(05)75244-2; PMID: 10440336
- Eisen DP, Minchinton RM. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis 2003; 37:1496 - 505; http://dx.doi.org/10.1086/379324; PMID: 14614673
- Salit IE. Meningococcemia caused by serogroup W135. Association with hypogammaglobulinemia. Arch Intern Med 1981; 141:664 - 5; http://dx.doi.org/10.1001/archinte.1981.00340050110026; PMID: 6784688
- Densen P. Complement deficiencies and infection. In: Volanakis JE, Frank MM, eds. The Human Complement System in Health and Disease. M Dekker, 1998: 409-421.
- Bishof NA, Welch TR, Beischel LS. C4B deficiency: a risk factor for bacteremia with encapsulated organisms. J Infect Dis 1990; 162:248 - 50; http://dx.doi.org/10.1093/infdis/162.1.248; PMID: 2355198
- Densen P, Weiler JM, Griffiss JM, Hoffmann LG. Familial properdin deficiency and fatal meningococcemia. Correction of the bactericidal defect by vaccination. N Engl J Med 1987; 316:922 - 6; http://dx.doi.org/10.1056/NEJM198704093161506; PMID: 3102964
- Sprong T, Roos D, Weemaes C, Neeleman C, Geesing CL, Mollnes TE, et al. Deficient alternative complement pathway activation due to factor D deficiency by 2 novel mutations in the complement factor D gene in a family with meningococcal infections. Blood 2006; 107:4865 - 70; http://dx.doi.org/10.1182/blood-2005-07-2820; PMID: 16527897
- Cunliffe NA, Snowden N, Dunbar EM, Haeney MR. Recurrent meningococcal septicaemia and properdin deficiency. J Infect 1995; 31:67 - 8; http://dx.doi.org/10.1016/S0163-4453(95)91550-8; PMID: 8522838
- S Reis E, Falcão DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol 2006; 63:155 - 68; http://dx.doi.org/10.1111/j.1365-3083.2006.01729.x; PMID: 16499568
- Nielsen HE, Christensen KC, Koch C, Thomsen BS, Heegaard NH, Tranum-Jensen J. Hereditary, complete deficiency of complement factor H associated with recurrent meningococcal disease. Scand J Immunol 1989; 30:711 - 8; http://dx.doi.org/10.1111/j.1365-3083.1989.tb02480.x; PMID: 2532396
- Thompson RA, Lachmann PJ. A second case of human C3b inhibitor (KAF) deficiency. Clin Exp Immunol 1977; 27:23 - 9; PMID: 849647
- Teisner B, Elling P, Svehag SE, Poulsen L, Lamm LU, Sjöholm A. C3 nephritic factor in a patient with recurrent Neisseria meningitidis infections. Acta Pathol Microbiol Immunol Scand C 1984; 92:341 - 9; PMID: 6570081
- Ducret F, Decoux M, Pointet P, Lambert C, Grosperrin E, Sédaillan A. Déficit héréditaire en C5 et méningite récidivante à Neisseria meningitidis. Rev Med Interne 1988; 9:534 - 7; http://dx.doi.org/10.1016/S0248-8663(88)80021-3; PMID: 3067301
- Lim D, Gewurz A, Lint TF, Ghaze M, Sepheri B, Gewurz H. Absence of the sixth component of complement in a patient with repeated episodes of meningococcal meningitis. J Pediatr 1976; 89:42 - 7; http://dx.doi.org/10.1016/S0022-3476(76)80924-9; PMID: 819642
- Lee TJ, Utsinger PD, Snyderman R, Yount WJ, Sparling PF. Familial deficiency of the seventh component of complement associated with recurrent bacteremic infections due to Neisseria. J Infect Dis 1978; 138:359 - 68; http://dx.doi.org/10.1093/infdis/138.3.359; PMID: 100562
- Petersen BH, Lee TJ, Snyderman R, Brooks GF. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Ann Intern Med 1979; 90:917 - 20; PMID: 109025
- Zoppi M, Weiss M, Nydegger UE, Hess T, Späth PJ. Recurrent meningitis in a patient with congenital deficiency of the C9 component of complement. First case of C9 deficiency in Europe. Arch Intern Med 1990; 150:2395 - 9; http://dx.doi.org/10.1001/archinte.1990.00390220127027; PMID: 2241452
- Andreoni J, Käyhty H, Densen P. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Infect Dis 1993; 168:227 - 31; http://dx.doi.org/10.1093/infdis/168.1.227; PMID: 8515116
- Wedege E, Høiby EA, Rosenqvist E, Bjune G. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect Immun 1998; 66:3223 - 31; PMID: 9632589
- Schmiel DH, Moran EE, Keiser PB, Brandt BL, Zollinger WD. Importance of antibodies to lipopolysaccharide in natural and vaccine-induced serum bactericidal activity against Neisseria meningitidis group B. Infect Immun 2011; 79:4146 - 56; http://dx.doi.org/10.1128/IAI.05125-11; PMID: 21768280
- Fijen CA, Kuijper EJ, Hannema AJ, Sjöholm AG, van Putten JP. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet 1989; 2:585 - 8; http://dx.doi.org/10.1016/S0140-6736(89)90712-5; PMID: 2570284
- Fijen CA, Kuijper EJ, Tjia HG, Daha MR, Dankert J. Complement deficiency predisposes for meningitis due to nongroupable meningococci and Neisseria-related bacteria. Clin Infect Dis 1994; 18:780 - 4; http://dx.doi.org/10.1093/clinids/18.5.780; PMID: 8075270
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307 - 26; http://dx.doi.org/10.1084/jem.129.6.1307; PMID: 4977280
- Jodar L, Cartwright K, Feavers IM. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A and C vaccines. Biologicals 2000; 28:193 - 7; http://dx.doi.org/10.1006/biol.2000.0253; PMID: 10964447
- Schlesinger M, Greenberg R, Levy J, Kayhty H, Levy R. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J Infect Dis 1994; 170:449 - 53; http://dx.doi.org/10.1093/infdis/170.2.449; PMID: 8035035
- Biselli R, Casapollo I, D’Amelio R, Salvato S, Matricardi PM, Brai M. Antibody response to meningococcal polysaccharides A and C in patients with complement defects. Scand J Immunol 1993; 37:644 - 50; http://dx.doi.org/10.1111/j.1365-3083.1993.tb01677.x; PMID: 8316762
- Platonov AE, Beloborodov VB, Pavlova LI, Vershinina IV, Käyhty H. Vaccination of patients deficient in a late complement component with tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol 1995; 100:32 - 9; http://dx.doi.org/10.1111/j.1365-2249.1995.tb03600.x; PMID: 7697919
- Platonov AE, Vershinina IV, Kuijper EJ, Borrow R, Käyhty H. Long term effects of vaccination of patients deficient in a late complement component with a tetravalent meningococcal polysaccharide vaccine. Vaccine 2003; 21:4437 - 47; http://dx.doi.org/10.1016/S0264-410X(03)00440-7; PMID: 14505927
- Drogari-Apiranthitou M, Fijen CA, Van De Beek D, Hensen EF, Dankert J, Kuijper EJ. Development of antibodies against tetravalent meningococcal polysaccharides in revaccinated complement-deficient patients. Clin Exp Immunol 2000; 119:311 - 6; http://dx.doi.org/10.1046/j.1365-2249.2000.01130.x; PMID: 10632668
- Herva E, Leinonen M, Käyhty H, Mäkelä PH, Vetoniemi-Korhonen SL. Recurrent meningococcal meningitis due to partial complement defects and poor anti-meningococcal antibody response. J Infect 1983; 6:55 - 60; http://dx.doi.org/10.1016/S0163-4453(83)95636-0; PMID: 6411822
- Zangwill KM, Stout RW, Carlone GM, Pais L, Harekeh H, Mitchell S, et al. Duration of antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis 1994; 169:847 - 52; http://dx.doi.org/10.1093/infdis/169.4.847; PMID: 8133100
- Platonov AE, Beloborodov VB, Vershinina IV. Meningococcal disease in patients with late complement component deficiency: studies in the U.S.S.R. Medicine (Baltimore) 1993; 72:374 - 92; http://dx.doi.org/10.1097/00005792-199311000-00002; PMID: 8231787
- Fijen CA, Kuijper EJ, Drogari-Apiranthitou M, Van Leeuwen Y, Daha MR, Dankert J. Protection against meningococcal serogroup ACYW disease in complement-deficient individuals vaccinated with the tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol 1998; 114:362 - 9; http://dx.doi.org/10.1046/j.1365-2249.1998.00738.x; PMID: 9844044
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of meningococcal conjugate vaccines---Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep 2011; 60:72 - 6; PMID: 21270745
- Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54:RR-7 1 - 21; PMID: 15917737
- Committee on Infectious Diseases. Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics 2011; 128:1213 - 8; http://dx.doi.org/10.1542/peds.2011-2380
- Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol 2009; 16:785 - 91; http://dx.doi.org/10.1128/CVI.00007-09; PMID: 19339487
- Rameix-Welti MA, Régnier CH, Bienaimé F, Blouin J, Schifferli J, Fridman WH, et al. Hereditary complement C7 deficiency in nine families: subtotal C7 deficiency revisited. Eur J Immunol 2007; 37:1377 - 85; http://dx.doi.org/10.1002/eji.200636812; PMID: 17407100
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359 - 95; PMID: 1889047