Abstract
Specific toxicity test is a major quality control test for acellular pertussis (aP) vaccines performed by manufacturers and regulatory authorities. The ‘mouse body weight gain test (MWGT)’, the ‘leukocytosis-promoting test (LPT)’ and the ‘histamine sensitization test (HIST)’ have been conducted to check the specific toxicity of all batches of aP vaccines used in Korea through the national quality control program, which requires a lot of animals, labor and time. In this study, test results obtained in the past 9 y from a total of 258 lots of aP vaccines were examined retrospectively to evaluate the three test methods. A pairwise comparison of the test results indicated a good correlation between LPT and HIST, whereas MWGT showed no correlation with either LPT or HIST. Moreover, the reversion to toxicity was higher than the residual toxicity in the majority of lots tested by HIST, which indicated that the histamine-sensitizing toxicity, although rated within a safe range, increased during the vaccine storage. Thus, the vaccine safety test results accumulated in the past might be useful for the improvement of test protocols.
Abstract
Introduction
Pertussis, also called whooping cough, is a highly contagious bacterial disease caused by Bordetella pertussis. It is a severe respiratory disease and results in high mortality and morbidity among children. Two types of vaccines, whole-cell pertussis (wP) vaccines and the acellular pertussis (aP) vaccines, are currently used worldwide. In South Korea, wP vaccines have been used since 1956 but were replaced with aP vaccines in 1986. Currently, aP vaccines are available as either a trivalent combination vaccine against diphtheria, tetanus and pertussis (DTaP) or as a quadrivalent combination vaccine that also contains the inactivated poliovirus (IPV). The combination vaccines distributed in South Korea are mainly imported from Japan, North America and Europe. Annually, more than 40 lots of these vaccines, equivalent to approximately 2.5 million doses, are examined for their quality by the Korea Food and Drug Administration (KFDA).
Because all of these vaccines contain inactivated pertussis toxin (PTx) as an important immunogenic component, testing for the specific toxicity is an essential part of the safety evaluation by regulatory authorities.Citation1-Citation4 The mouse body weight gain test (MWGT), the leukocytosis-promoting test (LPT), and the histamine sensitization test (HIST) are among the specific toxicity tests of aP vaccines. The MWGT compares the test vaccines with a standard reference preparation to determine the vaccines’ effects on the mouse body weight change after administration of the vaccine.Citation5 The LPT is a test based on the ability of the PTx to induce leukocytosis. The LPT compares the effect of the test vaccine with that of a standard reference preparation on the white blood cell count in the blood taken from the orbital veins three days after injection.Citation6 The HIST is another test based on the ability of PTx to sensitize animals to histamine. Two types of HIST are used. One method measures the reduction in the mouse body temperature upon challenge with histamine 4–5 d after the administration of a test vaccine or a reference preparation.Citation7,Citation8 The other method counts the number of mice that die from histamine challenge.Citation9 The WHO working group on the standardization and control of pertussis vaccines reported that the former method is more sensitive and quantitative than the latter method.Citation10
In the present study, we analyzed the results of specific PTx toxicity tests performed in South Korea from 2003 to 2011 on a total of 258 aP vaccine lots. As a result, we found a good correlation between the results of the HIST and the LPT. We also found that the HIST values of some vaccine lots increased during storage, although they remained within the safe range. Such information might be useful for the animal reduction and the improvement of the specific toxicity test in aP vaccine.
Results
Specific Toxicity Test Results for aP Vaccines
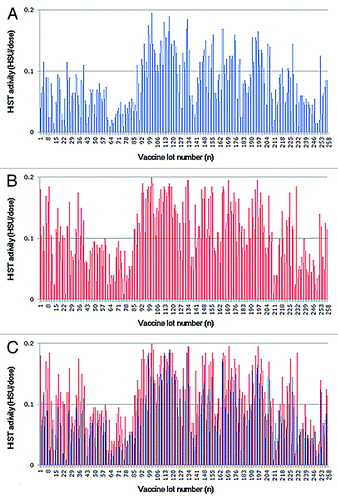
The MWGT, LPT and HIST were performed on 258 lots of commercial aP vaccine between 2003 and 2011. The specific toxicities of each lot were determined by comparing the test results with the results of a reference vaccine with the pre-determined toxicities. All of the batches of aP vaccine tested by the national quality control program exhibited toxicities within the safety limits of 5.0 body weight decreasing units (BWDU) for MWGT and 0.25 leukocytosis-promoting units (LPU) for LPT, which had been specified by the Korean Minimum Requirements. The vaccine toxicity varied from 0.1 to 4.9 BWDU, with a geometric mean of 1.6 (), and the LP toxicity ranged from 0.01 to 0.20 LPU, with a mean of 0.09 (). The safety limit is 0.25, and no sample showed an LP toxicity greater than 0.2 LPU over the past 9 y.
Figure 1. Results of the MWGT, the LPT, and HIST for DTaP vaccines (n = 258). The frequency indicates the number of vaccine lots showing the indicated BWDU (A), LPU (B), or HSU (C). The residual PTx toxicity is shown in blue bars, and the reversion to toxicity is shown in red bars. The dotted line represents the safety limit specified by the Korean Minimum Requirements.
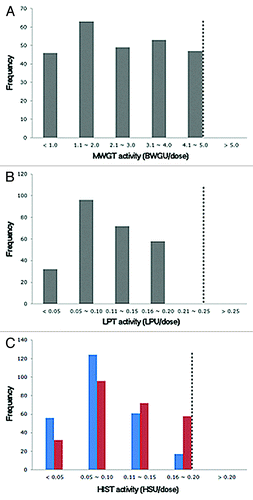
The HIST results showed that the residual toxicities of all of the tested vaccines were below 0.2 histamine-sensitizing units (HSU) (), ranging from 0.01 to 0.19 HSU, with a mean of 0.06. The reversion to toxicity also ranged from 0.01 to 0.20 HSU, with a geometric mean of 0.09. Although both the residual toxicity and reversion to toxicity were below the safety limit of 0.2 HSU, the apparent increase in the mean toxicity by 0.03 prompted us to compare the two specific toxicities (residual toxicity and reversion to toxicity) of each lot. As shown in , the reversion to toxicity was higher than the residual toxicity in 87.6% of the tested vaccines. This result suggests that the PTx toxicity has increased in the majority of the vaccine lots during storage.
Correlation between the HIST and LPT Results
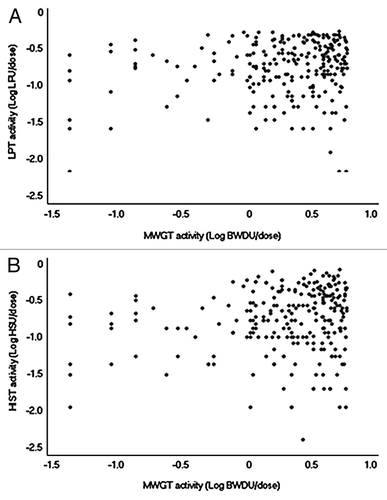
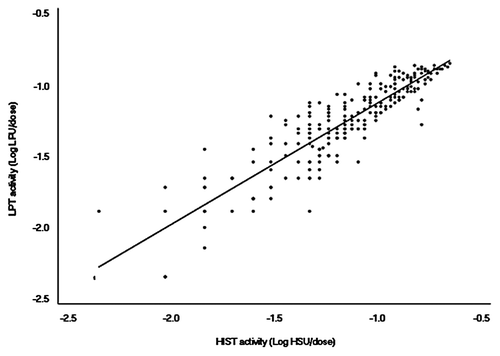
To identify relationships between the results of the specific toxicity tests, a correlation analysis was performed according to Pearson’s product-moment correlation method in the R program (www.r-project.org). No correlation was observed between the results of the MWGT and LPT () or between the results of the MWGT and HIST (). In contrast, a strong correlation was found between the results of the HIST and LPT. The regression coefficient was 0.8964 (p < 2.2e−16), with a 95% confidence interval from 0.8683 to 0.9188. In addition, the y-intercept of the regression line was near 0 ().
Discussion
Specific toxicity test is one of the key steps in the quality control of the aP vaccines. The MWGT, LPT and HIST are included in the Korean Minimum Requirements for the testing of the specific toxicity of pertussis vaccines.Citation1 Aim of the specific toxicity tests is to measure the residual PTx in the aP vaccines. Since 2003, the Laboratory Information Management System (LIMS) has enabled the systematic accumulation of national quality control data for the KFDA. In this study, we examined the information accumulated on the total of 258 aP vaccine lots to re-evaluate the validity of the specific toxicity tests. Overall, the results of the MWGT, LPT, and HIST were quite consistent. We did not note any peculiar results for specific manufactures or products.
The MWGT, which is primarily used for endotoxin detection in wP vaccine, is considered a general, nonspecific toxicity test method that measures the overall toxicity (and thus is not an optimal procedure for testing PTx toxicity).Citation11 On the other hand, it was also reported that the increased levels of active PTx may aid a pertussis vaccine to pass the MWGT.Citation12 The aP vaccines that we tested over the past several years showed BWDU values widely spread between 0.0 and 5.0. Most importantly, our MWGT results showed no correlation with those of the LPT or the HIST. These results are consistent with those of a previous study that suggested that the MWGT is not a useful procedure for aP vaccines.
The highlight of this study is the quantitative results of HIST that measures the mouse rectal temperature. Currently, HIST based on the temperature method is used effectively in Korea, Japan, and a few other countries but has not been used in Europe and North America where reliance is based on the HIST that measures the lethal end point. However, the lethal end point method does not provide any quantitative information about the residual PTx because the acceptance criteria of this test specify that “no mouse dies of histamine challenge on any vaccine lot.”Citation4 In contrast, the HIST based on the temperature measurement provides quantitative information on a specific vaccine lot ( and ). Moreover, the quantitative HIST based on temperature method showed the importance of testing ‘reversion to toxicity’ of the final products. Although all of the vaccines that we tested showed reversion to toxicity under 0.2 HSU, which is the safety limit, the observation that the value of reversion to toxicity was higher than that of the residual toxicity in almost all lots (226 of 258 lots) draws attention. Generally, a reversion to toxicity test is performed to ensure that the inactivated PTx will not recover its toxicity before the vaccine’s expiration date. Our results raise the possibility of toxicity recovery during vaccine storage. In many countries, reversion to toxicity is not mandatory for the release of each final lot but is considered only a part of the validation process by the vaccine manufacturer. However, our results indicate that the reversion to toxicity deserves more attention in vaccine quality control in the future.
The specific toxicity tests have been used consistently for decades to analyze the aP vaccine and is of enormous value. Our analysis suggests that MWGT and LPT may not be necessary for the measurement of specific toxicity in aP vaccine.
Materials and Methods
Pertussis vaccines tested in this study
A total of 258 lots of commercial DTaP vaccines were tested through the Korean National Vaccine Quality Control Program between 2003 and 2011. As a reference, the Korean national reference pertussis vaccine (06/016, KFDA) has been used since 2006.Citation1 The PTx activity of this vaccine was assigned 1466.4 BWDU, 23.76 LPU, and 38.4 HSU per vial. Prior to 2006, the Japanese reference vaccine (Lot2, NIID) was used; the PTx activity of the latter reference was assigned 1368 BWDU, 36.0 LPU, and 48.0 HSU per vial.Citation13 Both reference vaccines are formalin-inactivated, lyophilized whole-cell preparations of Bordetella pertussis (Tohama phase I).
Animals
Four-week-old female CD1 mice of specific-pathogen-free (SPF) grade were used. All of the animal tests were approved and conducted according to the national animal testing guidelines. The mice were housed in an SPF facility at 20–24°C and a relative humidity of 55 ± 10% with 15–20 air changes/hour in 12 h light/darkness cycles. The animals were fed ad libitum with free access to tap water. All of the mice were acclimatized for one week before testing.
Specific toxicity tests
The specific toxicity of aP vaccines was determined by the MWGT, LPT and HIST to ascertain whether the vaccines meet the Korean Minimum Requirements for biological products.Citation1 In brief, groups of ten CD1 mice were intraperitoneally injected with a single human dose (SHD) of a test vaccine or an aliquot of serially diluted reference preparation. The mouse body weight change between the time of inoculation and 16 h later was measured, and the specific toxicity was indicated in BWDU per dose.Citation5 For the LPT, peripheral leukocytes in the blood taken from the orbital veins 3 d after the vaccine inoculation were counted with a hematology analyzer (Hemavet, Drew Scientific Ltd., Cumbria, UK), and the specific toxicity was indicated in LPU per dose.Citation6 For the HIST, four days after the vaccine inoculation, the mice were injected intraperitoneally with 4 mg of histamine dihydrochloride in 0.5 mL of 0.85% saline, and the rectal temperature was measured 30 min later using an electronic thermometer with a probe for mice (BAT-10, Physitemp Instruments Inc., New Jersey, USA).Citation8 The residual toxicity was indicated in HSU per dose. The reversion to toxicity was measured by the same HIST protocol for the vaccines that had been incubated at 37°C for 4 weeks.
Statistical analysis
The PTx activities of the tested vaccines were determined relative to those of the reference preparations (06/016 or Lot 2) according to Finney’s parallel line assay (PLA)Citation14 with the statistical software, Bioassay Assist (version 3.0, NIID, Japan). The relationship between the results of the HIST and LPT was determined by the product moment correlation in the R program (version R-2.14.0) (www.r-project.org).
References
- Korea Food and Drug Administration notification, Korean Government. Minimum Requirements for Biological Products;2005.
- Ministry of Health, Labour and Welfare, Japanese Government. Minimum Requirements for Biological Products;2004.
- Pertussis vaccine, In: Chinese pharmacopoeia (volume III), Beijing, People’s Medical Publishing House. 2005; pp. 67-71.
- Diphtheria, tetanus and pertussis vaccine (adsorbed). In:European pharmacopeia (4th ed.) 2178-2200 (2002)
- Ishida S. Characterization of the body weight-decreasing toxicities in mice by the lymphocytosis-promoting factor and the heat-labile toxin of B. pertussis and endotoxin. Jpn J Med Sci Biol 1968; 21:115 - 35; PMID: 4299575
- Iwasa S, Ishida S, Asakawa M. Lymphocytosis-promoting factor produced by Bordetella pertussis.. Jpn J Med Sci Biol 1963; 21:363 - 8
- Ishida S, Kurokawa M, Asakawa S, Iwasa S. A sensitive assay method for the histamine-sensitizing factor using change in rectal temperature of mice after histamine challenge as a response. J Biol Stand 1979; 7:21 - 9; http://dx.doi.org/10.1016/S0092-1157(79)80034-7; PMID: 489619
- Ochiai M, Yamamoto A, Kataoka M, Toyoizumi H, Arakawa Y, Horiuchi Y. Highly sensitive histamine-sensitization test for residual activity of pertussis toxin in acellular pertussis vaccine. Biologicals 2007; 35:259 - 64; http://dx.doi.org/10.1016/j.biologicals.2007.01.004; PMID: 17363269
- World Health Organization. Guidelines for the production and control of the acellular pertussis component of monovalent or combined vaccines. WHO Technical Report Series 1998;878:57-76.
- Corbel MJ, Kreeftenberg JG, Knezevic I. WHO Working Group on the standardisation and control of pertussis vaccines-report of a meeting held on 6-7 May 2003, Ferney Voltaire, France. Vaccine 2004; 22:293 - 300; http://dx.doi.org/10.1016/j.vaccine.2003.08.016; PMID: 14670307
- Corbel MJ, Xing DKL. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines 2004; 3:89 - 101; http://dx.doi.org/10.1586/14760584.3.1.89; PMID: 14761246
- Horiuchi Y, Takahashi M, Asada S, Ishida S. Increased levels of active pertussis toxin may aid a pertussis vaccine to pass the mouse body weight gain test. Biologicals 1994; 22:243 - 8; http://dx.doi.org/10.1006/biol.1994.1035; PMID: 7811458
- Kataoka M, Yamamoto A, Ochiai M, Harashima A, Nagata N, Hasegawa H, et al. Comparison of acellular pertussis-based combination vaccines by Japanese control tests for toxicities and laboratory models for local reaction. Vaccine 2009; 27:1881 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.01.095; PMID: 19368767
- Finney DJ. Statistical methods in biological assay. 3 rd ed. London: Charles Griffin;1978.