Abstract
A pooled analysis was conducted of 1257 toddlers who received a fourth dose of Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY-TT) or Hib conjugate vaccine (Hib polysaccharide conjugated to N. meningitidis outer membrane protein) coadministered with measles-mumps-rubella (MMR) and varicella (VAR) vaccines (NCT00134719/NCT00289783). Noninferiority of immunological responses to MMR and VAR was demonstrated between groups and incidences of MMR- and VAR-specific solicited symptoms were similar, indicating that HibMenCY-TT can be coadministered with MMR and VAR.
Introduction
Previous studies showed that a novel, combined Haemophilus influenzae type b (Hib) and Neisseria meningitidis serogroups C and Y conjugate vaccine that uses tetanus toxoid as carrier protein (HibMenCY-TT) was immunogenic with an acceptable safety profile when administered to infants as a 3-dose primary series,Citation1-Citation5 with a fourth dose in the second year of life.Citation2,Citation4-Citation6
The vaccination schedule for HibMenCY-TT is consistent with the current Hib immunization schedule recommended by the United States (US) Advisory Committee on Immunization Practices, which includes a fourth dose of Hib at 12 to 15 mo of age.Citation7 In Australia, a fourth dose of Hib conjugate vaccine is routinely given at 1 y of age. According to these schedules, HibMenCY-TT could potentially be coadministered with the combined measles, mumps, and rubella vaccine (MMR) and monovalent varicella vaccine (VAR). To facilitate integration of HibMenCY-TT into toddler vaccination schedules, it is important to demonstrate a lack of immune interference and an acceptable safety profile when MMR, VAR, and HibMenCY-TT are given at the same time.
In 2 randomized controlled studies,Citation4,Citation5 infants received either HibMenCY-TT or Hib polysaccharide conjugated to tetanus toxoid (Hib-TT; ActHIB®, Sanofi Pasteur SA, Lyon, France) at 2, 4, and 6 mo of age, followed at 12 to 15 mo of age by 1 dose of HibMenCY-TT or Hib polysaccharide conjugated to N. meningitidis outer membrane protein (Hib-OMP; PedvaxHIB®, Merck and Co. Inc., Whitehouse Station, NJ, USA) coadministered with MMR (M-M-R® II; Merck and Co. Inc.) and VAR (Varivax®; Merck and Co. Inc.). Hib-OMP was used for the toddler dose at the request of the US Food and Drug Administration, because US prescribing information for Hib-TT does not include immunogenicity data for children aged between 12 and 15 mo.Citation8 The Hib, MenC, and MenY immunogenicity results from both studies were reported previously.Citation4,Citation5
The designs of the studies were sufficiently similar to allow a pooled analysis of immune responses to the coadministered MMR and VAR in the HibMenCY-TT group relative to those in the Hib-OMP control group at 12 to 15 mo of age.
Materials and Methods
Immunogenicity data available from Australian children included in a Phase II randomized controlled studyCitation4 (NCT00134719) and US children enrolled in a Phase III randomized controlled studyCitation5 (NCT00289783) were assessed. The studies, which were described in detail previously,Citation4,Citation5 were designed in a similar way within the context of each country’s immunization schedule, with the pre-specified objective of pooling data for the primary objective of assessing the non-inferiority of MMR and VAR co-administered with Hib-MenCY compared with co-administration with a licensed Hib vaccine. Conditions to pool the data were therefore defined prospectively, and were determined on the grounds of comparable immunogenicity between studies and were met if the point estimates of the difference between the HibMenCY-TT and Hib-OMP groups were above the predefined noninferiority limits in each study in terms of anti-measles seroconversion, anti-mumps seroconversion, anti-rubella seroresponse, and anti-varicella seroconversion.
Immunogenicity analyses were performed on the pooled according-to-protocol (ATP) cohorts for immunogenicity from each study, defined as vaccinated children who met all eligibility criteria, complied with protocol-defined procedures, and had antibody assay results available for at least 1 antigen component. Cut-offs for seropositivity were defined as follows: anti-measles, enzyme-linked immunosorbent assay (ELISA) value ≥ 150 mIU/mL; anti-mumps, neutralization test value ≥ 28 ED50; anti-rubella, ELISA value ≥ 4 IU/mL; anti-varicella fluorescent antibody membrane assay value ≥ 1:5. Seroconversion for antibodies to measles, mumps, and varicella was defined as the presence of detectable levels of the relevant antibodies at 42 d post-vaccination in subjects who were seronegative before vaccination. Seroresponse for rubella was defined as the appearance of anti-rubella virus antibodies to a concentration ≥ 10 IU/mL in subjects who were seronegative before vaccination (concentration < 4 IU/mL).
The coprimary objectives of the pooled immunogenicity analysis were to demonstrate noninferiority of MMR and VAR when coadministered with a dose of HibMenCY-TT compared with MMR and VAR coadministered with a dose of Hib-OMP in terms of anti-measles seroconversion, anti-mumps seroconversion, anti-rubella seroresponse, and anti-varicella seroconversion at 42 d (range 35–56 d) after administration of the vaccines. The pooling criterion prespecified in the study protocols stated that the data could be pooled if, in each individual study, the point estimate for the group difference in seroconversion/seroresponse rates (HibMenCY-TT group minus Hib-OMP group) was ≥ -5% for measles, mumps, and rubella, and ≥ -10% for varicella (note: the individual studies were not adequately powered to demonstrate a lower limit higher than the noninferiority criterion). Assessment of noninferiority was based on standardized asymptotic 95% confidence intervals (CIs) for the difference between groups (HibMenCY-TT group minus Hib-OMP control group) in percentage of subjects reaching the separate prespecified endpoints for each of the MMR and VAR components. Noninferiority would be demonstrated if the lower limit of the 95% CI was above -5% for measles, mumps, and rubella, and above -10% for varicella.
Parents/guardians of subjects completed diary cards with any solicited symptoms specific to MMR and VAR vaccination: fever ≥ 38°C measured by any route, rash/exanthem, parotid/salivary gland swelling, and any suspected meningeal signs or febrile convulsions over a 43-d follow-up period after vaccination. Subjects with symptoms of parotid swelling were to return to the study center to be medically examined to confirm the diagnosis of parotiditis. As planned in the study protocols, the reactogenicity and safety analysis of these symptoms was performed on subjects in the pooled total vaccinated cohorts (TVCs) from both studies with safety data available (i.e., diary cards returned). Relative risk (percentage of subjects reporting a specific symptom in HibMenCY-TT group over percentage in Hib-OMP group) was calculated with exact 95% CI for each symptom. P-values were calculated using a 2-sided Exact Stratified Test conditional to number of cases and taking into account the study effect.
Results
There were 1648 subjects in the pooled data set (); 1645 comprised the TVC (3 subjects were enrolled but did not receive study vaccines) and 1257 comprised the ATP cohort for immunogenicity. The demographic characteristics of the groups (TVC) were comparable in both countries. Mean age (± standard deviation) in the Australian study was 12.0 ± 0.3 and 12.0 ± 0.2 mo in the HibMenCY-TT and Hib-OMP groups, respectively, and 12.1 ± 0.5 and 12.3 ± 0.6 mo in the US HibMenCY-TT and Hib-OMP groups, respectively. In the Australian study, 49% of the HibMenCY-TT group and 47% of the Hib-OMP group were female; in the US cohort, 47% and 48%, respectively, were female. At least 94% in both Australian groups were white/Caucasian; 77–82% were white/Caucasian in both US groups, and 7% and 11% were African-American in the US HibMenCY-TT and Hib-OMP groups, respectively.
Table 1. Number of Subjects in Relevant Study Cohorts
Conditions to pool the data were met (), with seroconversion rates to measles, mumps, and varicella and the seroresponse rate to rubella in both the HibMenCY-TT and Hib-OMP groups of both studies exceeding 93% 42 d after the fourth dose (). The pooled data indicated that seroconversion or seroresponse levels in both groups were at least 95% for measles and at least 99% for mumps, rubella, and varicella, with antibody geometric mean concentrations/titers within the same range in both groups (). Statistical noninferiority of responses to MMR and VAR was demonstrated ().
Figure 1. Difference between the HibMenCY-TT and Hib-OMP groups in percentage of subjects who seroconverted for measles, mumps, and varicella and with a seroresponse to rubella 42 d post-fourth dose of HibMenCY-TT/Hib-OMP in the Australian study (Aus), US immunogenicity cohort (US), and pooled analysis (Pooled) (ATP cohorts for immunogenicity; subjects who were seronegative at baseline). Limits represent 95% confidence intervals. Footnote: Anti-measles seroconversion: post-vaccination antibody concentration ≥ 150 mIU/mL in initially seronegative subjects (< 150 mIU/mL); anti-mumps seroconversion: post-vaccination antibody titer ≥ 28 ED50 in initially seronegative subjects (< 28 ED50); anti-rubella seroresponse: post-vaccination antibody concentration ≥ 10 IU/mL in initially seronegative subjects (< 4 IU/mL); anti-varicella seroconversion: post-vaccination antibody titer ≥ 1:5 in initially seronegative subjects (< 1:5); * limit of non-inferiority varicella; ** limit of non-inferiority measles, mumps, and rubella.
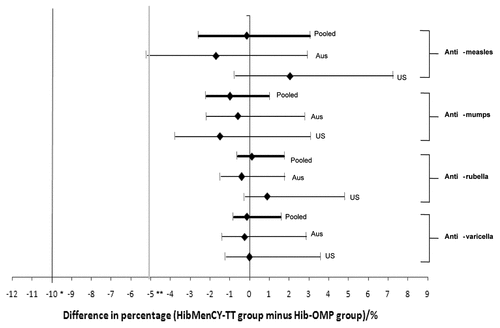
Table 2. Proportion of Subjects who Seroconverted to Measles, Mumps, or Varicella or achieved a Seroresponse to Rubella 42 d Post-Fourth Dose in the Australian, United States, and Pooled Study Groups (According-to-Protocol Cohort for Immunogenicity; Subjects who were Seronegative at Baseline)
There were no statistically significant differences between the HibMenCY-TT group and Hib-OMP group with respect to solicited symptoms specific to the coadministered MMR and VAR (p ≥ 0.05 for all relative risks) (). The most frequently reported solicited symptom during the 43-d period following administration of MMR and VAR was fever ≥ 38°C (45.8% and 48.7% of subjects in the HibMenCY-TT and Hib-OMP groups, respectively), with a peak in incidence at around day 9 in both groups (data not shown). Fever > 40.0°C was infrequent (< 2% in both groups). There were no reports of meningeal signs or febrile convulsions in the 43-d post-vaccination period. Parotid/salivary gland swelling was reported in 4 subjects in the HibMenCY-TT group (0.3%), with no reports in the Hib-OMP group; all reports were from Australia and all were considered by the investigator as related to vaccination. Two were categorized as grade 3, defined as swelling with accompanying general symptoms, occurred 12 to 14 and 14 to 17 d after vaccine administration, and were medically reviewed as a requirement of the study. All parotid/salivary gland swelling episodes resolved without sequelae. Rashes were reported in 25.0% and 22.6% of subjects in the HibMenCY-TT and Hib-OMP groups, respectively.
Table 3. Pooled Results: Incidence of Solicited General Adverse Events specific to Coadministered Measles-Mumps-Rubella and Varicella Vaccines reported within the 43-d Post-Vaccination Period following the Fourth Dose (Total Vaccinated Cohort)
Discussion
In 2 randomized controlled studies, 3 doses of HibMenCY-TT in infancy followed by 1 dose at 12 to 15 mo of age induced robust anti-Hib and anti-meningococcal serogroups C and Y immune responses and had an acceptable safety profile.Citation4,Citation5 Here, pooled analysis of data from these studies demonstrated noninferiority of immunological responses to MMR and VAR when concomitantly administered with the fourth dose of HibMenCY-TT compared with coadministration with a fourth dose of monovalent Hib-OMP. Previous analyses showed the percentages of children with anti-polyribosylribitol phosphate (PRP) antibody concentration ≥ 1.0 µg/mL were high after the fourth HibMenCY-TT dose and consistent with the Hib-OMP control group, and that anti-PRP geometric mean concentrations were significantly higher than in Hib-OMP recipients.Citation4,Citation5 These results were in line with a study of HibMenCY-TT vs. monovalent Hib-TT vaccine in which MMR and VAR were not coadministered with the fourth dose.Citation6 This suggests immune responses to HibMenCY-TT, MMR, and VAR are not compromised when administered together, although the possibility of interference cannot be completely excluded because no group in the current study received a fourth dose without MMR or VAR, or received MMR and VAR alone.
The reactogenicity profile of MMR and VAR was comparable when coadministered with HibMenCY-TT or Hib-OMP, as indicated by similar incidences of MMR- and VAR-specific solicited symptoms in both groups. Also, the incidence of fever > 38.5°C in the HibMenCY-TT and Hib-OMP groups was consistent with the incidence of fever ≥ 38.9°C reported in a study in which a group of children aged 12 to 23 mo received MMR and VAR alone.Citation9 Parotid/salivary gland swelling was reported in less than 1% of subjects in the HibMenCY-TT group, which was consistent with a review of various randomized studies involving 3 different MMR vaccines that also showed low incidence of this adverse event (≤ 1.8%).Citation10
The studies included in this pooled immunogenicity analyses had several limitations. Approximately one-third of enrolled infants in the US immunogenicity cohort were excluded from the ATP cohort for immunogenicity because of noncompliance with the blood sampling schedule or insufficient blood samples. Additionally, the majority of subjects in both studies were white/Caucasian. Future studies should further explore immunogenicity in other racial groups. Other potential limitations were the lack of a control group that did not receive M-M-R® II and Varivax® without the co-administration of licensed Hib vaccine, and lack of blinding of study personnel to treatment group, which could have influenced the assessment of reactogenicity, although in this instance one might expect the bias to be against the investigational vaccine, which includes two additional antigens. Also, the results of group comparisons should be interpreted with caution considering that there was no adjustment for multiplicity and the study was not powered for these comparisons, meaning that definite conclusions cannot be made regarding the relative risk of a given adverse event.
In conclusion, the results of this pooled analysis of data from 2 randomized controlled studies indicate that a dose of HibMenCY-TT at 12 to 15 mo of age, given as part of a 4-dose HibMenCY-TT series, can be administered with an acceptable safety profile without diminishing immune responses to MMR and VAR.
Disclosure of Potential Conflicts of Interest and Source of Funding
This study was supported by GlaxoSmithKline Biologicals (www.clinicaltrials.gov NCT00134719, NCT00289783). GlaxoSmithKline Biologicals was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals also underwrote all costs associated with the development and publishing of the present manuscript. All authors contributed to the study and writing of the manuscript and the corresponding author had full access to the data and final responsibility for submission of the publication. Emmanuel Aris, Narcisa Mesaros, and Jacqueline M. Miller are employees of GlaxoSmithKline (GSK) Biologicals. Jacqueline M. Miller owns stock options. Kristina A. Bryant, Jodie McVernon, Colin D. Marchant, Terry Nolan, Gary S. Marshall, Peter Richmond, Helen Marshall, Michael Nissen, and Stephen B. Lambert have provided consultant services and/or are investigators in studies funded by GSK, which also provided travel support (to Jodie McVernon, Terry Nolan, Peter Richmond, Helen Marshall, Michael Nissen, and Stephen B. Lambert) for presentation of scientific data, honoraria (to Terry Nolan) for membership in an independent DSMB for an unrelated vaccine or (to Kristina A. Bryant and Gary S. Marshall) for service on an advisory board and institutional funding (to Peter Richmond, Helen Marshall, and Stephen B. Lambert) for investigator-led epidemiological studies.
Acknowledgments
The authors thank the volunteers and their parents/guardians who participated in the studies, the study nurses, and other staff members without whom these studies would not have been possible. The authors thank the following for their valuable contributions. In the USA: AL, Dr William Johnston; AR, Dr Joseph Elser; CA, Dr Dean Blumberg, Dr Lawrence Sher, Dr Razia Sheikh, Dr William Hitchcock; IA, Dr Stephen Rinderknecht; ID, Dr Kenneth Cohen; KS, Dr Paul Klaassen; KY, Dr James Hedrick, Dr Michael Simon; MI, Dr Richard Hines; MN, Dr Arnold London, Dr Michael Severson; NC, Dr Emmanuel Walter; NE, Dr Meera Varman; NV, Dr Michael Levin; NY, Dr Leonard Weiner, Dr Michael Pichichero, Dr Sharon Nachman; OH, Dr Sherman Alter; OK, Dr Stanley Grogg; PA, Dr Michael Green, Dr Richard Kratz, Dr Mark Blatter; SC, Dr Michael Leonardi; TX, Dr Christine Turley, Dr Fernando Guerra, Dr John Fling, Dr Shari Medford; UT, Dr Michael Husseman, Dr Matthew Cox; VA, Dr David Johnson, Dr David Matson; WA: Dr Gerald F. Bader; WI, Dr Steven Manson. In Australia: Melbourne, Dr Karyn Alexander-Haikerwal, Dr Loretta Thorn, Dr Kerry-Ann O’Grady, Ms Marita Kefford, Ms Jacinta Sonego, Ms Jane Ryrie, Ms Elizabeth McGrath, Ms Alice Holloway, Ms Stephanie Lenko, Dr Marissa Trubidy Clark, Dr Nicole Rose, Dr Lana Horng, Dr Jenny Davey, Ms Erin Hill, Ms Mairead Phelan, Ms Marie West, Ms Elanna Nolan, Ms Clare Brophy, Ms Janet Briggs; Ms Betty Lim, Ms AnnMarie McEvoy; Adelaide, Dr Jan Walker, Dr Susan Evans, Dr Rachel Chen, Mrs Diana Weber, Mrs Christine Heath, Mrs Michelle Clarke, Mrs Jane Tidswell, Ms Susan Lee; Perth, Ms Jennifer Kent, Ms Larissa Rhind, Ms Jan Adams, Dr Tanya Stoney, Dr Karen Prosser; Brisbane, Mr Aaron Buckner, Dr Lai-man Raymond Chuk; Sydney, Dr John B Ziegler. From GSK they thank Drs Dominique Boutriau and Leonard Friedland for their valuable contributions to the study design, Drs Pascal Lestrate and Koen Maleux for testing assays, Dr Anne Sumbul for performing the statistical analyses, Dr Stephen Cross, Dr Jacqueline Vargas Castro, and Dr Heather Santiago for their valuable contribution to the study management, Dr Maja Galic for coordination and contacts with investigators. The authors also thank Dr Joanne Knowles (independent medical writer) for writing assistance and Dr Tatjana Mijatovic and Dr Wouter Houthoofd for editorial assistance and manuscript coordination on behalf of GSK.
Notes
† ActHIB is a registered trademark of Sanofi Pasteur SA; PedvaxHIB, M-M-R II, and Varivax are registered trademarks of Merck and Co. Inc.
References
- Nolan T, Lambert S, Roberton D, Marshall H, Richmond P, Streeton C, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine 2007; 25:8487 - 99; http://dx.doi.org/10.1016/j.vaccine.2007.10.013; PMID: 17996996
- Habermehl P, Leroux-Roels G, Sänger R, Mächler G, Boutriau D. Combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C (HibMenC) or serogroup C and Y-tetanus toxoid conjugate (and HibMenCY) vaccines are well-tolerated and immunogenic when administered according to the 2,3,4 months schedule with a fourth dose at 12-18 months of age. Hum Vaccin 2010; 6:640 - 51; http://dx.doi.org/10.4161/hv.6.8.12154; PMID: 20697200
- Marchant CD, Miller JM, Marshall GS, Blatter M, Aris E, Friedland LR, et al, HibMenCY-TT 005 Study Group. Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatr Infect Dis J 2010; 29:48 - 52; http://dx.doi.org/10.1097/INF.0b013e3181c3ce88; PMID: 20035207
- Nolan T, Richmond P, Marshall H, McVernon J, Alexander K, Mesaros N, et al. Immunogenicity and safety of an investigational combined haemophilus influenzae type B-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J 2011; 30:190 - 6; http://dx.doi.org/10.1097/INF.0b013e3181fcb2bf; PMID: 20948453
- Bryant KA, Marshall GS, Marchant CD, Pavia-Ruiz N, Nolan T, Rinderknecht S, et al. Immunogenicity and safety of H influenzae type b-N meningitidis C/Y conjugate vaccine in infants. Pediatrics 2011; 127:e1375 - 85; http://dx.doi.org/10.1542/peds.2009-2992; PMID: 21624883
- Marshall GS, Marchant CD, Blatter M, Aris E, Boutriau D, Poolman JT, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J 2010; 29:469 - 71; http://dx.doi.org/10.1097/INF.0b013e3181cdd379; PMID: 20072077
- Advisory Committee on Immunization Practices. 2011 Child & Adolescent Immunization Schedules. http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm#printable. Accessed July 03, 2011.
- ActHIB [package insert]. Swiftwater, PA: Sanofi Pasteur Inc.; 2009.
- Lieberman JM, Williams WR, Miller JM, Black S, Shinefield H, Henderson F, et al, Consistency Lot Study Group for ProQuad. The safety and immunogenicity of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children: a study of manufacturing consistency and persistence of antibody. Pediatr Infect Dis J 2006; 25:615 - 22; http://dx.doi.org/10.1097/01.inf.0000220209.35074.0b; PMID: 16804432
- Wellington K, Goa KL. Measles, mumps, rubella vaccine (Priorix; GSK-MMR): a review of its use in the prevention of measles, mumps and rubella. Drugs 2003; 63:2107 - 26; http://dx.doi.org/10.2165/00003495-200363190-00012; PMID: 12962524