Abstract
Allergy is the result of a disbalanced immune response to environmental innocuous antigens. Despite of accumulating data to define the pathomechanisms that take place in case of allergic diseases a detailed understanding of sequence of events that lead to the "normal" scenario of tolerance development are still under debate. Allergen-specific immunotherapy is the only causal treatment of allergic diseases. It modifies the immune response to a particular antigen to achieve tolerance against the symptom-causing allergen. This process is considered to mirror physiological peripheral tolerance induction. A number of immunological changes have been described to occur under allergen immunotherapy, including the generation of allergen-specific regulatory T cells, the induction of allergen-specific IgG4, an increase in the Th1/Th2 cytokine ratio and decreased activation and function of effector cells such as mast cells, basophils and eosinophils.
Introduction
The prevalence of allergic disease has increased dramatically over the past decades, representing a serious health problem that causes considerable morbidity worldwide. The reasons why some individuals generate an inflammatory response toward non-pathogenic innocuous environmental antigens are not entirely understood. To date specific immunotherapy represents the only causal treatment of allergic disease via restoration of peripheral tolerance to the symptom causing allergen.
The concept of allergen-specific immunotherapy (SIT) was first introduced 100 y ago by Noon and Freeman.Citation1 Since then, a wide range of treatment modalities and recommendations has evolved. The main underlying principle is the application of gradual increasing doses of allergen via subcutaneous (SCIT), the sublingual (SLIT). New routes of application have been recently proposed such as the intralymphatic route (ILIT) or epicutaneos route. In contrast to other anti-allergic treatments, SIT is an immunomodulatory approach, which establishes long-term tolerance to the symptom causing allergen and restores the immune balance. The present review will focus on the known mechanisms that take place during SIT while allergen specific tolerance is established.
Pathomechanisms of Allergic Disease
The type of immune response elicited by allergen exposure depends on various factors, including individual genetic susceptibility, route and dose of application and co-exposure of other immune-stimulating antigens. In allergic individuals, first contact with allergen is suggested to lead to IL4- and IL13-dependent production of allergen-specific IgE, with subsequent binding of these antibodies to the high affinity receptor for IgE (FcεRI) on the surface of basophils and mast cells (“sensitization phase”).
Allergen re-exposure (“effector phase”) results in cross linkage of membrane bound IgE on mast cells and basophils and subsequent mediator release that induce typical immediate type hypersensitivity reactions. In addition, the release of cytokines like IL4, IL5, IL9 and IL13 by Th2 type cells that promote the recruitment of inflammatory cells to sites of inflammation, eosinophilia, mucus secretion and activation of eosinophils and mast cells. Aside from Th2 type cells different T-cells subsets evolve from naïve T-cell subsets into other T-cell subsets, depending on the microenvironment. Th1-cells secrete IFN-γ which induces apoptosis in concert with TNFα in epithelial cells, smooth muscle cells in the lungs of asthmatic patients and in keratinocytes of patients with atopic dermatitisCitation2-Citation4 and activated Th1, but not Th2-cells. This may contribute to the limitation of inflammatory responses via elimination of cytokine and chemokine producing cells.Citation5 In addition IFN-γ and TNF-α induces IL32. IL32 is upregulated in atopic skin lesions, in sinus tissue from CRS tissue with polyposis and correlates with disease severity in serum of patients with asthma.Citation3,Citation6-Citation8 Recently a “new” Th2 type cytokine IL33 was described. It is able to act on precursor populations and dendritic cells that induce Th2 type responses and Th2 effector cell populations ultimately inducing Th2 type inflammation in the lung and the gut.Citation3,Citation9-Citation11 In analogy to IL32, IL33 is released via epithelial damage.Citation3
Antigen-Presenting Cells
Both SCIT and SLIT affect the regional antigen-presenting dendritic cells (DC) in local lymph nodes (SCIT) or directly at the application site (SLIT). DCs play a key role in defining the type of immune response. Whether they engage an immune triggering or regulatory function depends on their maturation stage. Both myeloid DCs (mDCs) and plasmacytoid dendritic cells (pDCs) are known to induce regulatory T-cells depending on their state of activation. Moreover, the induction of new populations of Treg cells by DCs after stimulation with various other exogenous factors, such as pathogen-derived molecules, Vitamin D3 metabolites or retinoid acid has been described.Citation12-Citation14 In the lung, partially mature DCs were shown to produce IL-10 and thereby promote the generation of type 1 T-regulatory (Treg) (Tr1) cells upon respiratory antigen exposure.Citation15 On the other hand maturing PDCs have the ability to generate T-regulatory cells and are able to suppress allergic inflammation in the lung upon adoptive transfer.Citation16,Citation17 Recently, tonsils have been linked to tolerance development that harbor functional allergen specific Treg populations, which are in close proximity to pDC populations and expand in vivo. Moreover pDCs from palatine and lingual tonsils have the ability to generate functional CD4+CD25+CD127-FOXP3+ Treg cells from naïve, tonsil derived, T-cells.Citation18 This mechanism may play a role in the immunological action of SLIT.
Mucosal Langerhans cells, a specialized subset of dendritic cells present in the mucosa, epidermis and lymph nodes are also suggested to have a distinct effect contributing to tolerance. The strategic location of Langerhans cells at the suprabasal epithelial layer may facilitate binding and processing of allergens in SLIT. Moreover oral Langerhans cells express the high affinity IgE receptor and seem to lack upregulation of CD83 and CCR7 upon allergen uptake.Citation19 This in turn may leave them in a more immature state and reduce their ability to migrate to the lymph nodes. Thus, direct contact with T-cells in the tissue instead of conventional contact in the lymph nodes may take place.Citation19,Citation20 Taken together, oral Langerhans cells might also play a role in sublingual SIT by the above described mechanism of tolerance induction via FcεRI dependent allergen binding and production of IL-10 and TGF-βCitation21. However, in vivo data monitoring functional changes of local DC subsets at the side of application over time of SIT are missing.
Allergen-Specific Regulatory Cells
The generation of allergen-specific suppressor T cells in the immunomodulatory process induced by SIT was first described by Rocklin et al. in 1980Citation22 and appears to the most decisive step in the successful development of tolerance. CD4+CD25+ cells are a subgroup of T cells that inhibit polyclonal and antigenic activation of CD4+CD25- cells in vitro.Citation23 These mechanisms are involved in the regulation of T-cell mediated immune responses, such as reactions to infectious agents, autoimmune diseases, allergic diseases and allograft rejections.Citation24
Various studies demonstrated the role of Treg cells in the prevention of allergic diseases. Adoptive transfer of Treg cells reversed asthmatic lung inflammation in a mouse model.Citation25 In humans, peripheral CD4+CD25+ Tregs from atopic donors seem to have reduced capacity to suppress allergen-induced T-cell proliferation and cytokine production.Citation26 Also, CD4+CD25+ cells are reduced in number and function in the lesional skin of patients with atopic dermatitis.Citation27 A negative correlation of peripheral CD4+FoxP3+ number to IgE levels and eosinophilia was demonstrated in symptomatic atopic individuals.Citation28 On the other hand, children who outgrow their cow’s milk allergy have higher frequency of circulating CD4+CD25+ T cells and decreased allergen-induced proliferative T cell response compared with children that remain allergic.Citation29
Basically, Treg cells can be divided into two main subsets, naturally occurring and inducible Tregs. Naturally occurring forkhead box P3 (FoxP3) expressing CD4+CD25+ regulatory T cells, which develop in the thymus by positive selection. The transcription factor FOXP3 is associated with the suppressive phenotype and is known to be a master regulator for Treg cell differentiation and function.Citation24
Inducible regulatory T cells are generated in the periphery in several tolerogenic settings. There are various different subsets, of which IL10-producing T regulatory type 1 (Tr1) cells play the most important role in maintenance of tolerance toward non-pathogenic environmental antigens, such as house dust mite, cat dander or birch pollens, in healthy individuals.Citation30 Moreover, they are decisive for the development of tolerance to allergens under SIT. Tr1 cells operate by multiple suppressive mechanisms, including the soluble mediators IL-10 and TGF-β and the surface molecules cytotoxic T lymphocyte antigen 4 (CTLA-4), glucocorticoid-induced TNF receptor and programmed cell death1.Citation24,Citation31
Both subsets, thymic selected CD4+CD25+Tregs and inducible Tr1 cells, seem to play together in the development of tolerance under subcutaneous and sublingual SITCitation32-Citation34: Studies demonstrated an increase of CD4+CD25+FOXP3+ and IL-10-producing Tr1 cells in nasal and oral mucosa and in the periphery during SIT as well as an association of CD4+CD25+ with clinical efficiency.Citation35-Citation38 Hypo-proliferation of PBMCs to allergens after SIT has been mainly attributed to the induction of T-regulatory cells.
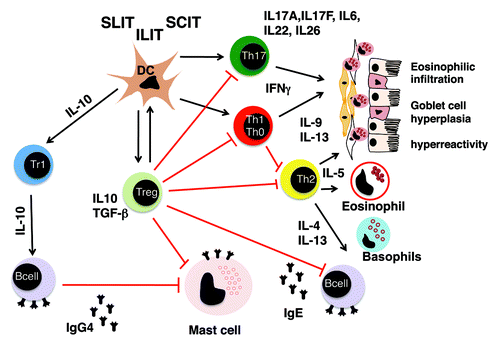
Treg cells exert their beneficial effects in the prevention of allergen-specific immune response and in tolerance development under SIT via interference with several major pathwaysCitation39,Citation40 (). They antagonize the inflammatory properties of effector Th1 cells (epithelial cell activation and apoptosis), Th2 cells (epithelial inflammation, mucous secretion, mast cell, eosinophil and basophil activation, IgE production) and Th17 cells (epithelial inflammation, chronic neutrophilic inflammation).Citation26 Moreover, they contribute to the generation of IgG4 and the suppression of specific IgE,Citation35,Citation41 induce tolerogenic DCs, while suppressing inflammatory DCsCitation42,Citation43 and display, directly and indirectly, suppressive effects on mast cells, eosinophils, basophilsCitation44 and resident tissue cells which mediate chronic tissue remodelingCitation25,Citation45
Figure 1. Mechanisms of allergen-specific immunotherapy: Subcutaneus and sublingual immunotherapy initially affects antigen-presenting cells at the side of administration and in the draining lymph nodes. This leads to the induction of Treg cells via so far not completely understood mechanisms. Treg cells in turn exert potent inhibitory effects by using multiple mechanisms. They antagonize the inflammatory properties of effector Th1cells (epithelial cell activation and apoptosis), Th2 cells (epithelial inflammation, mucous secretion, mast cell, eosinophil and basophil activation, IgE production) and Th17 cells (epithelial inflammation, chronic neutrophilic inflammation). Moreover, they contribute to the generation of IgG4 and the suppression of specific IgE, induce tolerogenic DCs while suppressing inflammatory DCs and display, directly and indirectly, suppressive effects on mast cells, eosinophils, basophils and resident tissue cells mediating chronic tissue remodeling.
The key player in suppression of allergic reactions seems to be IL-10, produced by Tr1 as well as by DC and antigen-presenting B cells. IL-10 production is observed under venom immunotherapy in vitro and in vivo,Citation35,Citation46 under house dust mite immunotherapy,Citation33 under grass pollen immunotherapyCitation47 as well as in healthy non-atopic patients upon allergen stimulation.Citation33,Citation35 Thus, the increase of IL10 by SIT seems to restore a tolerance that is constitutionally present in healthy individuals.
IL-10 inhibits T cell activation by blocking the co-stimulatory molecules CD2, CD28 and inducible T cell costimulator (ICOS) through dephosphorylation by Src homology 2 domain-containing tyrosine phosphatase (SHP-1).Citation32,Citation48 In monocytes, it inhibits cytokine synthesis and antigen-presenting capacity,Citation49 in mast cells, it decreases IgE-dependent degranulation.Citation50 Moreover, IL10 suppresses total and allergen-specific IgE while increasing IgG4 production.Citation35 The other cytokine produced by T-regulatory cells, TGF-β induces the conversion of CD4+CD25- into CD4+CD25+ cells via the expression of FOXP3 and is necessary for the expansion and function of CD4+CD25+ Treg cells.Citation51 In conclusion T-regulatory cells are key players in the network of events that are observed in case of tolerance induction during SIT.
B-cells are the central cell population to mount humoral immune responses. Aside from this function they a paramount of properties ranging from antigen presentation and initiation of immune responses to suppression of immune responses.Citation52 B-cells express co-stimulatory molecules and consequently are able to induce allergen specific T-cell responses. In contrast to these immunostimulatory properties, B-cells produce significant amounts of IL-10. These, so called regulatory B-cells or Bregs, have the ability to suppress the development, expansion and cytokine production of effector T-cell responsesCitation53 and monocyte cytokine production in an IL-10 dependent manner.Citation54 Several regulatory B-cell subsets have been described in humans (reviewed by Hussaarts et al.Citation55).
Regulation of Allergen-Specific IgE and IgG4
In contrast to T-cell tolerance, which occurs relatively at an early stage in the course of SIT, B-cell changes seem to develop at relatively later phase. Allergen-specific IgE transiently increases within the first weeks of SIT,Citation56 whereas later on changes seem to be modest. A mitigation of the season-associated rise of pollen-specific IgE was demonstrated in patients with seasonal rhinoconjunctivitis under SIT.Citation57 Very late in the course and after termination of SIT, some authors describe a decrease of allergen-specific IgE, occurring 1 to 3 y after implementation of therapy.Citation58,Citation59 The decline of specific IgE does not correlate with clinical improvementCitation57 and rather occurs at a later time point. Consequently, it is not an appropriate marker to assess SIT efficacy. The reason for the persistence of IgE despite clinical improvement may relate to long-lived bone-marrow-resident IgE-producing plasma cells.Citation60
Relatively early in SIT (weeks to months after start of treatment) an allergen dose dependent (10- to 100-fold) increase of IgG4Citation58,Citation61 is observed. This increase occurs relatively early and is more pronounced compared with the small rise in allergen-specific IgE.Citation56 Some studies also show an elevation of IgG1 and IgA.Citation33,Citation38 The mechanism of IgG4 in SIT as a ‘blocking effect’ was first described in 1982.Citation62 Allergen-specific IgG4 is thought to bind the allergen before it is linked to cell-bound IgE and therefore competes with specific IgE. However, this theory has some drawbacks. IgG4 is a marker of applied allergen dose,Citation56 but studies show contradictory correlation of IgG4 with clinical improvement.Citation63,Citation64 Moreover, clinical effects of SIT seem to precede the increase of IgG, especially in rush protocols.Citation65,Citation66 On the other hand, changes in the fine specificity in the epitope-binding regions of the T cell receptor have been documented by some authors within the first hours of rush immunotherapy.Citation67 In addition to the increase of specific IgG4 levels, SIT increases the blocking activity of IgG on the allergen capture and cross-linking of mast cell and basophil Fcε-receptor bound IgE.Citation68
Moreover, IgG4 does not activate the complement system, has a lower affinity for Fcγ receptors, interferes with basophil histamine release and reduces antigen-specific T cell proliferation by prevention of IgE-facilitated allergen binding to B cells and subsequent antigen-presentation.Citation65,Citation68 Formation of multivalent IgE-IgG-allergen complexes has been suggested to result in mast cell FcεRI autoinhibition. After binding of the allergen-IgG4 complex to the inhibitory FcγRIIb, this is phosphorylated by FcεRI-associated kinases. Phosphorylated FcγIIb then mediates a reciprocal inhibition of FcεRI signaling.Citation69,Citation70
The relevance of newly developing antigen-specific IgA in the development of allergen tolerance under SIT has to be elucidated. Allergen-specific secretory IgA was found to protect sensitized children from allergic symptoms in the early years of life, suggesting a possible protective role of IgA.
Several studies show that IgG4 is a better marker than IgE. However, instead of being an independent tolerance inducing factor, the increase of allergen-specific IgG4 during successful SIT might only be a surrogate marker for Treg activity since Tr1-produced IL-10 was shown to decrease total and allergen-specific IgE and to increase IgG production.Citation41 Moreover, it might be more important to measure the blocking activity via in vitro assays at an allergen-specific level.Citation65,Citation68
Shift of the Th1 / Th2 Cytokine Ratio
The balance between distinct T-cell subsets such as Th1, Th2, Th9, Th17, Th22, Tr1, CD4+CD25highFoxP3+ T cells, and their cytokines plays a pivotal role in directing the immune response to different kinds of antigens. The pro-allergenic T helper 2 (Th2) cells produce IL-4, IL-5 and IL-13, whereas the counter-regulatory Th1 cells produce IFN-γ, which are important in the defense against microbes.
Measurement of Th1/Th2 ratio in peripheral blood of patients undergoing SIT showed controversial results. Both, a shift toward Th1,Citation71,Citation72 as well as an unchanged Th1/Th2-ratio,Citation73-Citation75 was observed. However, cultures of PBMCs do not provide accurate insights into immune responses of affected organs and associated lymphoid tissue. Indeed, in tissue, results on the effect of SIT on cytokine expressions are more consistent. An increased Th1/Th2 ratio, with mostly unchanged Th2 but increased Th1 cytokines (IL-2, IFN-g and IL-12) was demonstrated in skin biopsy upon intradermal challenge after grass pollen SIT,Citation76 in mucosal biopsy upon intranasal provocation after pollen SIT,Citation77 and in nasal biopsies and nasal fluids in response to natural pollen exposure in patients after pollen SIT.Citation73,Citation75 However, these studies demonstrated the change of whole T cells in the tissues and due to technical problems could not focus on allergen-specific T cell subsets.
The mechanism responsible for this shift is not yet defined. IL10 might again be involved through its inhibitory effect on CD28 co-stimulatory signals since IL-5, but not IFN-γ production upon allergic stimulation, seems to be CD28 dependent.Citation78,Citation79 However, cytokine changes might also occur unrelated to IL-10. For example, different DC cell subsets are known to elicit different T-helper cytokine responses according to their maturation state.Citation31
Role of Allergen Specific Immunotherapy on Mast Cells, Basophils, Eosinophils and Neutrophils
Effects of SIT on inflammatory cells, including mast cells, basophils, eosinophils and neutrophils, have been described both at very early stages and in the late phase, occurring after a few months.
Specific immunotherapy represents the only causative treatment of allergic reactions and confers protection based on typical kinetics. Protection against systemic anaphylactic response to bee stings and inhibition of late phase reactions upon cutaneous provocation is observed already in the first hours of repetitive insect venom or drug rush desensitization protocols.Citation66 There is limited information on these early desensitization effects but gradual mediator release might be responsible for the early protection against anaphylaxis. Release of histamine and leukotrienes upon allergen challenge is not abolished during SIT, however, it does not induce a systemic anaphylactic reaction.Citation80 The proportion of released to total histamine was shown to be unchanged after allergen stimulation during desensitization, however, there is a decreased absolute amount of histamine release. Thus, it seems that piecemeal degranulation with release of histamine and leukotrienes below the threshold of anaphylaxis protects from systemic reactions by decreasing the granule contents and increasing the threshold of activation of mast cells and basophils.Citation80,Citation81
SIT reduces mast cell, basophil and neutrophil activation and IgE-mediated release of preformed and newly generated mediators by mast cells and basophils.Citation80-Citation83 Moreover, SIT inhibits the number and function of eosinophils,Citation84 mitigates eosinophil and neutrophil migration to the site of allergic reaction,Citation84 and reduces priming of eosinophil adhesion during allergen season.Citation85 IL-10 seems to plays a key in the anti-inflammatory actions on tissue cells, as it was shown to reduce degranulation of mast cellsCitation83 and a reduction of eosinophil chemotactic IL-5 production by Th2 cells.Citation79 Moreover, Treg cells were shown to directly inhibit FcεRI-dependent mast cell degranulation through cell to cell contact.Citation44
Recent data demonstrated that early mast cell and basophil suppression might be mediated via IgG binding to FcgRIIb on mast cells.Citation86 Changes in histamin receptorCitation31 expression, enhanced tryptophan degradation and upregulation of human IgG receptor inhibitory transcript expression in monocytes are also discussed to occur at a very early stage of SIT.Citation87
In late stages of SIT, the effect of SIT on tissue cells and inflammatory response leads to a suppression of late phase response in nasal mucosa and of skin test reactivity.Citation76,Citation88 It was shown, that it also decreases mucosal response to non-specific stimuli, which demonstrates a general reduction of underlying mucosal inflammation.Citation89
Conclusions
Allergen-specific immunotherapy is the only available causative treatment of allergic diseases that induces a number of immunological changes at an allergen specific level. In brief, it leads to the generation of allergen-specific regulatory T-cells and blocking antibodies and thereby counter-regulates in a direct or indirect way pathogenic effector cell populations that contribute the clinical features of allergic diseases.
However, established allergy is extremely difficult to treat and even systemic immunosuppressive therapy does not affect clinically manifest allergy.Citation90-Citation92 Despite of the benefits of SIT for most of the treated individuals, not everyone improves, and in some patients, changes are not permanent. Also, the duration of SIT is a controversial issue, with no consistent guideline. Therefore, reliable markers of successful and durable SIT are still a clinical unmet need.
On the other hand, therapeutic approaches that use new routes of application and increase the efficacy of antigen presentation leading to a decrease the number of allergen application are needed.Citation93,Citation94 Moreover future immunotherapeutic approaches may combine biological immune response modifiers with allergen-specific immunotherapy to induce long lasting immune tolerance.Citation95 Finally, there is also an eminent need for preventive strategies that favor tolerance induction early in life prior to sensitization or in sensitized individuals prior to allergy development.
References
- Noon L. Prophylactic inoculation against hayfever. Lancet 1911; http://dx.doi.org/10.1016/S0140-6736(00)78276-6
- Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, et al. Mechanisms of IFN-γ-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol 2012; 129:1297 - 306; http://dx.doi.org/10.1016/j.jaci.2012.02.020; PMID: 22445417
- Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2011; 127:701 - 21, e1-70; http://dx.doi.org/10.1016/j.jaci.2010.11.050; PMID: 21377040
- Akkoc T, de Koning PJ, Rückert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol 2008; 121:652 - 8, e1; http://dx.doi.org/10.1016/j.jaci.2007.12.1171; PMID: 18328893
- Basinski TM, Holzmann D, Eiwegger T, Zimmermann M, Klunker S, Meyer N, et al. Dual nature of T cell-epithelium interaction in chronic rhinosinusitis. J Allergy Clin Immunol 2009; 124:74 - 80, e1-8; http://dx.doi.org/10.1016/j.jaci.2009.04.019; PMID: 19523671
- Soyka MB, Treis A, Eiwegger T, Menz G, Zhang S, Holzmann D, et al. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy 2012; 67:790 - 8; http://dx.doi.org/10.1111/j.1398-9995.2012.02820.x; PMID: 22486709
- Meyer N, Zimmermann M, Bürgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitis. J Allergy Clin Immunol 2010; 125:858 - 65, e10; http://dx.doi.org/10.1016/j.jaci.2010.01.016; PMID: 20227751
- Meyer N, Christoph J, Makrinioti H, Indermitte P, Rhyner C, Soyka M, et al. Inhibition of angiogenesis by IL-32: possible role in asthma. J Allergy Clin Immunol 2012; 129:964 - 73, e7; http://dx.doi.org/10.1016/j.jaci.2011.12.1002; PMID: 22336080
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF Jr., Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 2010; 464:1362 - 6; http://dx.doi.org/10.1038/nature08901; PMID: 20200520
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010; 464:1367 - 70; http://dx.doi.org/10.1038/nature08900; PMID: 20200518
- Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol 2011; 41:1535 - 8; http://dx.doi.org/10.1002/eji.201141668; PMID: 21618506
- McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med 2002; 195:221 - 31; http://dx.doi.org/10.1084/jem.20011288; PMID: 11805149
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007; 317:256 - 60; http://dx.doi.org/10.1126/science.1145697; PMID: 17569825
- Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest 2009; 119:387 - 98; PMID: 19139565
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001; 2:725 - 31; http://dx.doi.org/10.1038/90667; PMID: 11477409
- de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004; 200:89 - 98; http://dx.doi.org/10.1084/jem.20040035; PMID: 15238608
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 2007; 204:105 - 15; http://dx.doi.org/10.1084/jem.20061660; PMID: 17200410
- Palomares O, Rückert B, Jartti T, Kücüksezer UC, Puhakka T, Gomez E, et al. Induction and maintenance of allergen-specific FOXP3+ Treg cells in human tonsils as potential first-line organs of oral tolerance. J Allergy Clin Immunol 2012; 129:510 - 20, 520, e1-9; http://dx.doi.org/10.1016/j.jaci.2011.09.031; PMID: 22051696
- Allam JP, Würtzen PA, Reinartz M, Winter J, Vrtala S, Chen KW, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol 2010; 126:638 - 45, e1; http://dx.doi.org/10.1016/j.jaci.2010.04.039; PMID: 20584546
- Novak N, Bieber T, Allam JP. Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy 2011; 66:733 - 9; http://dx.doi.org/10.1111/j.1398-9995.2010.02535.x; PMID: 21251016
- Allam JP, Bieber T, Novak N. Dendritic cells as potential targets for mucosal immunotherapy. Curr Opin Allergy Clin Immunol 2009; 9:554 - 7; http://dx.doi.org/10.1097/ACI.0b013e32833239a9; PMID: 19745726
- Rocklin RE, Sheffer AL, Greineder DK, Melmon KL. Generation of antigen-specific suppressor cells during allergy desensitization. N Engl J Med 1980; 302:1213 - 9; http://dx.doi.org/10.1056/NEJM198005293022201; PMID: 6154241
- McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. J Allergy Clin Immunol 2002; 110:693 - 702; http://dx.doi.org/10.1067/mai.2002.129339; PMID: 12417876
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775 - 87; http://dx.doi.org/10.1016/j.cell.2008.05.009; PMID: 18510923
- Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol 2008; 122:617 - 24, e6; http://dx.doi.org/10.1016/j.jaci.2008.05.048; PMID: 18672278
- Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet 2004; 363:608 - 15; http://dx.doi.org/10.1016/S0140-6736(04)15592-X; PMID: 14987885
- Verhagen J, Akdis M, Traidl-Hoffmann C, Schmid-Grendelmeier P, Hijnen D, Knol EF, et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol 2006; 117:176 - 83; http://dx.doi.org/10.1016/j.jaci.2005.10.040; PMID: 16387603
- Orihara K, Narita M, Tobe T, Akasawa A, Ohya Y, Matsumoto K, et al. Circulating Foxp3+CD4+ cell numbers in atopic patients and healthy control subjects. J Allergy Clin Immunol 2007; 120:960 - 2; http://dx.doi.org/10.1016/j.jaci.2007.05.036; PMID: 17631953
- Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med 2004; 199:1679 - 88; http://dx.doi.org/10.1084/jem.20032121; PMID: 15197226
- Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol 2006; 18:738 - 44; http://dx.doi.org/10.1016/j.coi.2006.06.003; PMID: 17023149
- Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol 2005; 116:961 - 8, quiz 969; http://dx.doi.org/10.1016/j.jaci.2005.09.004; PMID: 16275361
- Zimmermann M, Koreck A, Meyer N, Basinski T, Meiler F, Simone B, et al. TNF-like weak inducer of apoptosis (TWEAK) and TNF-α cooperate in the induction of keratinocyte apoptosis. J Allergy Clin Immunol 2011; 127:200 - 7, 207, e1-10; http://dx.doi.org/10.1016/j.jaci.2010.11.005; PMID: 21211655
- Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003; 33:1205 - 14; http://dx.doi.org/10.1002/eji.200322919; PMID: 12731045
- Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010; 12:CD002893; PMID: 21154351
- Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest 1998; 102:98 - 106; http://dx.doi.org/10.1172/JCI2250; PMID: 9649562
- Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One 2010; 5:e11028; http://dx.doi.org/10.1371/journal.pone.0011028; PMID: 20543955
- Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol 2008; 121:1467 - 72, 1472, e1; http://dx.doi.org/10.1016/j.jaci.2008.03.013; PMID: 18423565
- Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy 2010; 40:598 - 606; PMID: 20184605
- Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res 2011; 3:11 - 20; http://dx.doi.org/10.4168/aair.2011.3.1.11; PMID: 21217920
- Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol 2010; 40:1232 - 40; http://dx.doi.org/10.1002/eji.200940045; PMID: 20148422
- Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 2008; 63:1455 - 63; http://dx.doi.org/10.1111/j.1398-9995.2008.01774.x; PMID: 18925882
- Bellinghausen I, König B, Böttcher I, Knop J, Saloga J. Inhibition of human allergic T-helper type 2 immune responses by induced regulatory T cells requires the combination of interleukin-10-treated dendritic cells and transforming growth factor-beta for their induction. Clin Exp Allergy 2006; 36:1546 - 55; http://dx.doi.org/10.1111/j.1365-2222.2006.02601.x; PMID: 17177678
- Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997; 159:4772 - 80; PMID: 9366401
- Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008; 29:771 - 81; http://dx.doi.org/10.1016/j.immuni.2008.08.018; PMID: 18993084
- Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol 2009; 296:L307 - 19; http://dx.doi.org/10.1152/ajplung.00521.2007; PMID: 19028981
- Bellinghausen I, Metz G, Enk AH, Christmann S, Knop J, Saloga J. Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects. Eur J Immunol 1997; 27:1131 - 9; http://dx.doi.org/10.1002/eji.1830270513; PMID: 9174602
- Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol 2003; 111:1255 - 61; http://dx.doi.org/10.1067/mai.2003.1570; PMID: 12789226
- Taylor A, Akdis M, Joss A, Akkoç T, Wenig R, Colonna M, et al. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol 2007; 120:76 - 83; http://dx.doi.org/10.1016/j.jaci.2007.04.004; PMID: 17531298
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174:1209 - 20; http://dx.doi.org/10.1084/jem.174.5.1209; PMID: 1940799
- Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy 2001; 31:694 - 704; http://dx.doi.org/10.1046/j.1365-2222.2001.01069.x; PMID: 11422128
- Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 2004; 173:6526 - 31; PMID: 15557141
- Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol 2008; 8:391 - 7; http://dx.doi.org/10.1038/nri2315; PMID: 18437156
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28:639 - 50; http://dx.doi.org/10.1016/j.immuni.2008.03.017; PMID: 18482568
- Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117:530 - 41; http://dx.doi.org/10.1182/blood-2010-07-294249; PMID: 20962324
- Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol 2011; 128:733 - 9; http://dx.doi.org/10.1016/j.jaci.2011.05.012; PMID: 21684587
- Creticos PS, Van Metre TE, Mardiney MR, Rosenberg GL, Norman PS, Adkinson NF Jr.. Dose response of IgE and IgG antibodies during ragweed immunotherapy. J Allergy Clin Immunol 1984; 73:94 - 104; http://dx.doi.org/10.1016/0091-6749(84)90490-1; PMID: 6607272
- Lichtenstein LM, Ishizaka K, Norman PS, Sobotka AK, Hill BM. IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest 1973; 52:472 - 82; http://dx.doi.org/10.1172/JCI107204; PMID: 4119162
- Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol 2005; 116:608 - 13; http://dx.doi.org/10.1016/j.jaci.2005.06.004; PMID: 16159631
- Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989; 44:412 - 8; http://dx.doi.org/10.1111/j.1398-9995.1989.tb04172.x; PMID: 2802114
- Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, et al. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol 2009; 124:819 - 26, e4; http://dx.doi.org/10.1016/j.jaci.2009.06.047; PMID: 19815119
- Piconi S, Trabattoni D, Rainone V, Borgonovo L, Passerini S, Rizzardini G, et al. Immunological effects of sublingual immunotherapy: clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. J Immunol 2010; 185:7723 - 30; http://dx.doi.org/10.4049/jimmunol.1002465; PMID: 21076061
- Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol 1982; 70:261 - 71; http://dx.doi.org/10.1016/0091-6749(82)90062-8; PMID: 6811645
- Djurup R, Malling HJ. High IgG4 antibody level is associated with failure of immunotherapy with inhalant allergens. Clin Allergy 1987; 17:459 - 68; http://dx.doi.org/10.1111/j.1365-2222.1987.tb02040.x; PMID: 3677372
- Ewan PW, Deighton J, Wilson AB, Lachmann PJ. Venom-specific IgG antibodies in bee and wasp allergy: lack of correlation with protection from stings. Clin Exp Allergy 1993; 23:647 - 60; http://dx.doi.org/10.1111/j.1365-2222.1993.tb01791.x; PMID: 8221268
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008; 121:1120 - 5, e2; http://dx.doi.org/10.1016/j.jaci.2008.01.072; PMID: 18374405
- Goldberg A, Confino-Cohen R. Bee venom immunotherapy - how early is it effective?. Allergy 2010; 65:391 - 5; http://dx.doi.org/10.1111/j.1398-9995.2009.02198.x; PMID: 19839973
- Michils A, Baldassarre S, Ledent C, Mairesse M, Gossart B, Duchateau J. Early effect of ultrarush venom immunotherapy on the IgG antibody response. Allergy 2000; 55:455 - 62; http://dx.doi.org/10.1034/j.1398-9995.2000.00412.x; PMID: 10843426
- Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol 2004; 4:313 - 8; http://dx.doi.org/10.1097/01.all.0000136753.35948.c0; PMID: 15238798
- Daëron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest 1995; 95:577 - 85; http://dx.doi.org/10.1172/JCI117701; PMID: 7860741
- Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol 2004; 113:1025 - 34, quiz 1035; http://dx.doi.org/10.1016/j.jaci.2004.03.024; PMID: 15208578
- Ebner C, Siemann U, Bohle B, Willheim M, Wiedermann U, Schenk S, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy 1997; 27:1007 - 15; http://dx.doi.org/10.1111/j.1365-2222.1997.tb01252.x; PMID: 9678832
- Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Müller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol 1995; 154:4187 - 94; PMID: 7706753
- Klimek L, Dormann D, Jarman ER, Cromwell O, Riechelmann H, Reske-Kunz AB. Short-term preseasonal birch pollen allergoid immunotherapy influences symptoms, specific nasal provocation and cytokine levels in nasal secretions, but not peripheral T-cell responses, in patients with allergic rhinitis. Clin Exp Allergy 1999; 29:1326 - 35; http://dx.doi.org/10.1046/j.1365-2222.1999.00651.x; PMID: 10520053
- Till S, Walker S, Dickason R, Huston D, O’Brien F, Lamb J, et al. IL-5 production by allergen-stimulated T cells following grass pollen immunotherapy for seasonal allergic rhinitis. Clin Exp Immunol 1997; 110:114 - 21; http://dx.doi.org/10.1111/j.1365-2249.1997.494-ce1392.x; PMID: 9353157
- Wachholz PA, Nouri-Aria KT, Wilson DR, Walker SM, Verhoef A, Till SJ, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1:Th2 cytokine ratios. Immunology 2002; 105:56 - 62; http://dx.doi.org/10.1046/j.1365-2567.2002.01338.x; PMID: 11849315
- Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, et al. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest 1993; 92:644 - 51; http://dx.doi.org/10.1172/JCI116633; PMID: 8349803
- Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol 1996; 97:1356 - 65; http://dx.doi.org/10.1016/S0091-6749(96)70205-1; PMID: 8648033
- Larché M. Update on the current status of peptide immunotherapy. J Allergy Clin Immunol 2007; 119:906 - 9; http://dx.doi.org/10.1016/j.jaci.2007.02.015; PMID: 17418662
- Schandené L, Alonso-Vega C, Willems F, Gérard C, Delvaux A, Velu T, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol 1994; 152:4368 - 74; PMID: 7512591
- Eberlein-König B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization. Clin Exp Allergy 1995; 25:704 - 12; http://dx.doi.org/10.1111/j.1365-2222.1995.tb00007.x; PMID: 7584681
- Jutel M, Müller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy 1996; 26:1112 - 8; http://dx.doi.org/10.1111/j.1365-2222.1996.tb00496.x; PMID: 8911695
- Makino Y, Noguchi E, Takahashi N, Matsumoto Y, Kubo S, Yamada T, et al. Apolipoprotein A-IV is a candidate target molecule for the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol 2010; 126:1163 - 9, e5; http://dx.doi.org/10.1016/j.jaci.2010.06.031; PMID: 20810159
- Pierkes M, Bellinghausen I, Hultsch T, Metz G, Knop J, Saloga J. Decreased release of histamine and sulfidoleukotrienes by human peripheral blood leukocytes after wasp venom immunotherapy is partially due to induction of IL-10 and IFN-gamma production of T cells. J Allergy Clin Immunol 1999; 103:326 - 32; http://dx.doi.org/10.1016/S0091-6749(99)70509-9; PMID: 9949326
- Rak S, Björnson A, Håkanson L, Sörenson S, Venge P. The effect of immunotherapy on eosinophil accumulation and production of eosinophil chemotactic activity in the lung of subjects with asthma during natural pollen exposure. J Allergy Clin Immunol 1991; 88:878 - 88; http://dx.doi.org/10.1016/0091-6749(91)90244-I; PMID: 1744358
- Håkansson L, Heinrich C, Rak S, Venge P. Priming of eosinophil adhesion in patients with birch pollen allergy during pollen season: effect of immunotherapy. J Allergy Clin Immunol 1997; 99:551 - 62; http://dx.doi.org/10.1016/S0091-6749(97)70084-8; PMID: 9111502
- Uermösi C, Beerli RR, Bauer M, Manolova V, Dietmeier K, Buser RB, et al. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol 2010; 126:375 - 83; http://dx.doi.org/10.1016/j.jaci.2010.05.040; PMID: 20624641
- Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy 2010; 65:1558 - 65; http://dx.doi.org/10.1111/j.1398-9995.2010.02430.x; PMID: 20584008
- Iliopoulos O, Proud D, Adkinson NF Jr., Creticos PS, Norman PS, Kagey-Sobotka A, et al. Effects of immunotherapy on the early, late, and rechallenge nasal reaction to provocation with allergen: changes in inflammatory mediators and cells. J Allergy Clin Immunol 1991; 87:855 - 66; http://dx.doi.org/10.1016/0091-6749(91)90134-A; PMID: 2013680
- Rak S, Löwhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol 1988; 82:470 - 80; http://dx.doi.org/10.1016/0091-6749(88)90021-8; PMID: 3170995
- Dehlink E, Gruber S, Eiwegger T, Gruber D, Mueller T, Huber WD, et al. Immunosuppressive therapy does not prevent the occurrence of immunoglobulin E-mediated allergies in children and adolescents with organ transplants. Pediatrics 2006; 118:e764 - 70; http://dx.doi.org/10.1542/peds.2006-0370; PMID: 16950967
- Eiwegger T, Gruber S, Geiger C, Mayer E, Dehlink E, Bannert C, et al. Impact of systemic immuno-suppression after solid organ transplantation on allergen-specific responses. Allergy 2011; 66:271 - 8; http://dx.doi.org/10.1111/j.1398-9995.2010.02475.x; PMID: 21208218
- Gruber S, Dehlink E, Eiwegger T, Gut S, Jaksch P, Klepetko W, et al. Immunoglobulin e-mediated allergies in lung-transplanted adults. Transplantation 2007; 84:275 - 9; http://dx.doi.org/10.1097/01.tp.0000268075.82161.9d; PMID: 17667823
- Crameri R, Flückiger S, Daigle I, Kündig T, Rhyner C. Design, engineering and in vitro evaluation of MHC class-II targeting allergy vaccines. Allergy 2007; 62:197 - 206; http://dx.doi.org/10.1111/j.1398-9995.2006.01292.x; PMID: 17298430
- Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol 2012; 129:1290 - 6; http://dx.doi.org/10.1016/j.jaci.2012.02.026; PMID: 22464647
- Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol 2012; 129:1290 - 6; http://dx.doi.org/10.1016/j.jaci.2012.02.026; PMID: 22464647