Abstract
This was a multicenter, blinded, Phase II study (NCT00880893) conducted in Singapore. The primary objectives were to evaluate the safety of a tetravalent dengue vaccine (TDV) comprising four recombinant, live, attenuated viruses (CYD-TDV) and the dengue virus serotype-specific antibody responses before and 28 d after each vaccination. Participants were randomized 3:1 to receive three doses of CYD-TDV or a control vaccine at 0, 6 and 12 mo. Control vaccine was placebo for the first dose (all ages) and for subsequent doses, licensed hepatitis-A for children (aged 2–11 years) or influenza vaccine for adolescents (12–17 years) and adults (18–45 years). Between April and October 2009, 317 children, 187 adolescents and 696 adults were enrolled. In all age groups, reactogenicity was higher after the first injection of CYD-TDV than after placebo control. Reactogenicity after subsequent CYD-TDV doses was no higher than after the first dose, and tended to be lower or similar to that seen after active control vaccination. Seropositivity rates and geometric mean neutralizing antibody titers (GMTs; 1/dil) against all four dengue virus serotypes increased in all age groups after each of the three CYD-TDV doses. Post-dose 3, 66.5% of all participants were seropositive to all four serotypes, and 87.2% were seropositive to ≥ 3 serotypes; GMTs for all participants ranged from 43.0 against dengue virus serotype 1 to 100 against dengue virus serotype 4. GMTs were higher in children than in adolescents. These results support the continued development of CYD-TDV for the prevention of dengue disease.
Introduction
Dengue virus is a member of the Flavivirus genus, which includes yellow fever, Japanese encephalitis, and other arthropod-borne viruses that cause human disease.Citation1 Dengue disease is transmitted to humans by several species of mosquito within the genus Aedes, principally A. aegypti. Dengue disease represents a major and growing medical problem.Citation2 The World Health Organization (WHO) 2009 classification divides dengue fever into two groups: uncomplicated and severe.Citation3 All four serotypes of dengue virus can cause clinical manifestations, ranging from a mild febrile illness to a life-threatening shock syndrome. Infection with one serotype is believed to confer immunity to subsequent infection with the same serotype, but it does not provide durable protection against infection with other serotypes.Citation4 Epidemics of different serotypes can circulate simultaneously and, therefore, an individual can suffer secondary and tertiary dengue infections. More severe disease is thought to be associated with secondary infection with another serotype.Citation5
The incidence of dengue infections has continued to increase over the past two decades. The WHO estimates there may be 50–100 million dengue infections annually worldwideCitation6 resulting in ~22,000 deaths, mainly among children.Citation7 More than three billion people are at risk of dengue infection in over 100 countries,Citation8 many of whom live in urban areas of tropical and sub-tropical countries in the Americas, Southeast Asia and the Western Pacific.Citation9 The latter two regions account for 75% of the global burden of dengue disease.Citation10 In addition, disease incidence varies by age. In Southeast Asia, the incidence of dengue fever and dengue hemorrhagic fever is highest among children.Citation11,Citation12
In the absence of an approved, effective, specific treatment for dengue infection, control of the disease is reliant upon suppression of Aedes aegypti, or the development of appropriate vaccines. A dengue vaccine is not currently available. However, given the global scale of dengue disease and the expense of mosquito-prevention measures, vaccine development against the four serotypes of dengue virus responsible for the disease has become a global public health priority.Citation13
Dengue vaccine candidates include a recombinant live attenuated tetravalent dengue vaccine (TDV).Citation14,Citation15 This candidate vaccine (CYD-TDV, Sanofi Pasteur) contains four recombinant viruses (CYD-1–4), each of which has the genes encoding dengue pre-membrane and envelope proteins of one of the four dengue serotypes, as well as genes encoding the non-structural and capsid proteins of the attenuated yellow fever 17D vaccine virus.Citation16-Citation18 These viruses possess the antigenicity of the parental dengue virus and the well-characterized replication ability of the yellow fever 17D vaccine strain. Previous studies of the CYD-TDV candidate have established that a three-dose, 0–6–12-mo regimen induces robust and balanced neutralizing antibody responses with a favorable short-term safety profile, in different populations and age ranges, in flavivirus-endemic and non-endemic countries.Citation19-Citation23
Dengue disease is endemic in Singapore, a city-state in Asia.Citation24 After two decades of successful management in Singapore that relied primarily on an integrated vector-control program,Citation25,Citation26 there has been a resurgence of dengue disease.Citation27 The recent epidemiology of the disease in Singapore is characterized by a 5–6-y cycle; incidence rates increase within each cycle before declining for 1 or 2 y.Citation28 During 2010, 5,364 dengue infections were reported to the Ministry of Health in Singapore.Citation29 All four dengue serotypes have been detected by the Singapore dengue surveillance program, although the relative circulation frequencies of dengue virus serotypes change over time; for example, DEN-2 was the predominant circulating serotype at the time of the current study.Citation30 Seroprevalence surveys confirm a decline in the immunity level of the general population, providing ideal conditions for dengue transmission. Cases in adults predominate in Singapore. There has been a steady decline in the proportion of cases in patients aged < 15 y, whereas the proportion in patients aged ≥ 25 y has increased in recent years;Citation31 in 2010, 92.8% of cases were in patients aged ≥ 15 y (76.4% in those aged ≥ 25 y).
The present study is part of a global dengue clinical trial program sponsored by Sanofi Pasteur. A multicenter, observer-blinded (for the first vaccination), single-blinded (for the second and third vaccinations), Phase II study was conducted in Singapore to assess safety and immunogenicity data from a large-scale population (n = 1200) across a broad age range (2–45 y old). The primary objectives were to evaluate the safety of CYD-TDV and to assess its immunogenicity in a subset of individuals by evaluating dengue virus serotype-specific antibody responses pre- and 28 d post-vaccination. The present study has a 4-y follow-up period to assess the persistence of vaccine immunity. This contribution presents the safety and humoral immunogenicity results for up to 6 mo after the last vaccination. Cellular immune responses and dengue virus cross-neutralization data observed during the study will be reported separately.
Results
Participants
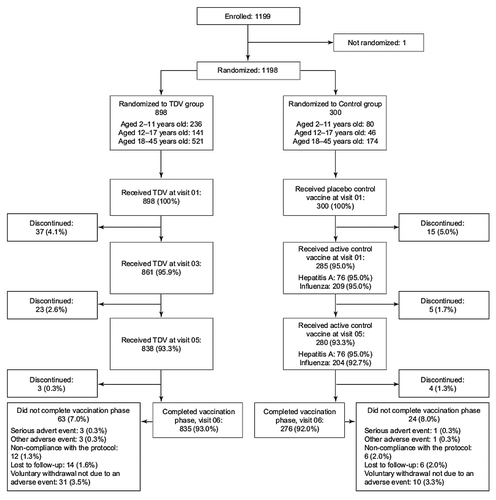
During a 6-mo period (7 April 2009 to 8 October 2009), 1,199 participants were enrolled: 316 children (aged 2–11 y), 187 adolescents (aged 12–17 y), and 696 adults (aged 18–45 y). Of these, 1198 participants were vaccinated; 898 received CYD-TDV and 300 received a placebo control vaccine. The vaccination phase of the study was completed by 835 (93.0%) individuals in the CYD-TDV group and by 276 (92%) individuals in the control group ().
A total of 63 (7.0%) participants randomized to the CYD-TDV group and 24 (8.0%) from the control group did not complete the vaccination phase of the study. Forty-one patients (3.4%) voluntarily withdrew due to relocation or job commitments; 20 individuals (1.7%) were lost to follow-up, and 18 (1.5%) were withdrawn by the investigator due to protocol violations (mostly related to pregnancy). There were four discontinuations following serious adverse events (SAEs), three (0.3%) in the CYD-TDV group (acute leukemia of ambiguous lineage, tuberculosis lymphadenitis, and tension headache secondary to allergic rhinitis) and one (0.3%) in the control group (papillary thyroid carcinoma). There was one discontinuation after an adverse event (AE) in the control group and three for AEs considered related to vaccination in the CYD-TDV group: fever, rash, and worsening cervical spondylosis.
Baseline demographic characteristics are presented in . There were fewer males enrolled in the CYD-TDV group compared with the control group, especially in the child and adult groups.
Table 1. Baseline demographic characteristics of participants who provided serum for immunogenicity analysis, by age and vaccine group (full analysis set for immunogenicity)
Safety and reactogenicity
Reactogenicity was higher in all age groups after the first injection of CYD-TDV compared with placebo control, but tended to be lower or similar after the second or third injection of CYD-TDV than after administration of hepatitis-A or influenza vaccines ().
Figure 2. Age-specific reactogenicity of CYD-TDV: proportion of participants by age and vaccine group with different categories of adverse events after each vaccination. Control group participants aged < 12 y at inclusion received intramuscular doses of hepatitis A vaccine and those aged ≥ 12 y received subcutaneous inactivated influenza vaccine.
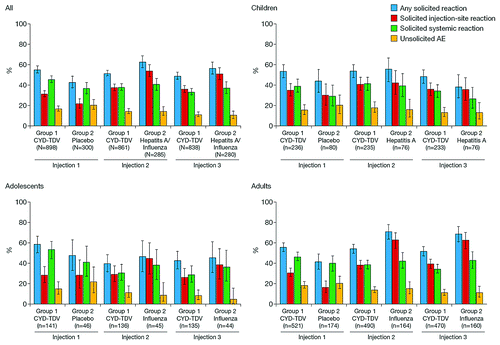
Solicited injection-site reactions after any vaccination were reported for 55% of participants in the CYD-TDV group and 67% of those in the control group (). Pain was most frequently reported after each of the three vaccinations, irrespective of vaccine received and the age group of the recipients. In the CYD-TDV group, the incidence of injection-site reactions was comparable after each vaccination (post-dose 1: 31.6%; post-dose 2: 37.8% post-dose 3: 36.2%) ().
Table 2. Percentage of individuals (by age and vaccine group) reporting at least one injection site and/or systemic reaction after any vaccination (safety analysis set)
In the adult sub-group, injection-site reactions were more frequently reported after influenza vaccination than after CYD-TDV vaccination. Most injection-site reactions were considered to be grade 1–grade 2 in intensity, and most were present for a maximum of 3 d. The only grade 3 injection-site reactions that occurred during the study were pain in nine (1.0%) participants in the CYD-TDV group and six (2.0%) participants in the control group; pain was more frequently reported in adults than in children ().
More solicited systemic reactions were reported after the first CYD-TDV vaccination (45.9% [95% CI: 42.5, 49.2]) than after the placebo control (37.0% [31.5, 42.8]); reporting rates were similar after the second and third vaccinations (). Systemic events were more frequent in adolescents than in children or adults after the first vaccination with CYD-TDV or placebo. Solicited systemic reactions tended to decrease in adolescents (post-dose 1: 53.2% [44.6, 61.6]; post-dose 2: 30.9% [23.2, 39.4]; post-dose 3: 28.9% [21.4, 37.3]) but did not decrease after second and third vaccinations with CYD-TDV in adults and children (). Headache, myalgia, and malaise were the most frequently reported solicited systemic reactions after each of the three vaccinations, whatever the vaccine received and the age group, followed by fever and asthenia (). Fever was more frequent in children compared with adolescents and adults after CYD-TDV or control vaccines. Most episodes of solicited systemic reactions occurred between Day 0 and Day 3 after vaccination, were usually grade 1–grade 2 in intensity, and were present for a maximum of 3 d. Of the few grade 3 or long-lasting systemic reactions reported, most cases occurred concomitantly with diseases such as upper respiratory tract infections or other infections.
There were 51 SAEs during the study, that were equally distributed both between the two groups (4.3% in each group) and throughout the 28-d post-vaccination period. Two SAEs may be of note. A 9-y-old boy in the TDV group was diagnosed with a tension headache secondary to untreated allergic rhinitis 17 d after the second CYD-TDV injection. Although the specific etiology could not be established, the timing of this SAE led the investigator to consider it to be “possibly related” to the study vaccine and the child was therefore withdrawn from the study. An independent data monitoring committee (IDMC) subsequently considered this SAE to be “not related.” The second SAE of interest occurred in a 42-y-old woman who was hospitalized for 3 d with suspected dengue fever 152 d after the third CYD-TDV vaccination even though etiology was unconfirmed (see below). All other SAEs reported were assessed to be unrelated to vaccination; none were life-threatening and no deaths occurred.
Unsolicited AEs were reported by 17.0% (95% CI: 14.6, 19.7) of participants in the CYD-TDV group and 20.7% (95% CI: 16.2, 25.7) in the control group after the first vaccination. These proportions were similar after the second and third vaccinations (14.5% and 14.4%, and 11.2% and 10.7%, respectively). The most frequently occurring unsolicited AEs were “infections and infestations” (mostly upper respiratory tract infections) and “respiratory, thoracic, and mediastinal disorders” (mostly cough and rhinorrhoea), most of which were considered to be “not related” to vaccination.
Five AEs were rashes, all reported by participants in the CYD-TDV group. One adult participant with a history of childhood asthma, food allergy and G6PD deficiency, experienced a first rash lasting 8 d (grade-1 intensity) on the right side of neck one day after the first CYD-TDV vaccination, and was considered to be unrelated to vaccination. Eight days after the second vaccination this participant reported generalized rash over the neck, chest, abdomen, thigh and upper arm. Seven days later the event resolved after treatment. This case of generalized rash was considered as vaccine-related and the participant was withdrawn from further vaccination in the study. The other three rashes were grade 1 macular, maculo-papular or non-specific rashes that were considered unrelated to vaccination.
Post-vaccination viremia and biological investigations for febrile episodes
No vaccine viremia was detected by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) methods in the 28 participants (2.3%) who reported febrile episodes within 28 d post-vaccination. However, as serum for these analyses were collected up to 5 d after the fever onset, it is possible that viremia was missed. Most changes in biochemical and hematological parameters concomitant with febrile episodes were minor and transient, and were not considered clinically significant by the investigator. In one adult in the control group, fever developed 26 d after the third vaccination, and dengue infection was confirmed (dengue NS1 Ag-positive, dengue IgM-positive).
Pregnancy follow-up
Of the 13 pregnancies reported, three were in women who were possibly vaccinated soon after conception (last menstrual period [LMP] close to vaccination (< 15 d) and resulted in two normal births; one woman had an elective abortion because the pregnancy was unplanned. Of the 10 pregnancies in women vaccinated before conception, there was one spontaneous abortion at 4 weeks’ gestation in a 23-y-old woman without risk factors (LMP > 4 mo post-vaccination) and one ruptured ectopic pregnancy at 3 weeks’ gestation in a 30-y-old woman with a history of a previous miscarriage and two live births (LMP > 3 mo post-vaccination). Two women had elective abortions (unplanned pregnancies) and the remainder (n = 6) had normal births.
Hospitalization for suspected dengue disease
One 42-y-old woman was hospitalized for 3 d with clinically suspected dengue fever, 5 mo (i.e., 152 d) after the third CYD-TDV vaccination. Fever (maximum body temperature: 39.2°C) did not subside after taking paracetamol and was accompanied by “whole body ache,” headache, fatigue, malaise, nausea, and arthralgia in the knee and elbow. She was admitted to hospital for medical surveillance due to low platelet count (68 × 103/µL) at which point she contacted the study site to inform them of the febrile episode, in accordance with the protocol. The case was classified as “probable” dengue disease using serological criteria but was not virologically confirmed according to the protocol-defined endpoint assays. Dengue screen qRT-PCR was positive but the endpoint assays (i.e., dengue serotype-specific qRT-PCRs and NS1 antigen) were negative. Dengue IgM and IgG were positive in acute (collected on Day 7 of the episode) and convalescent samples, but did not increase between the two. Serotype-specific 50% plaque reduction neutralization test (PRNT50) antibody titers measured 1 mo after the third dengue vaccination (i.e., 4 mo before this episode) were 209, 152, 155 and 403 for serotypes 1 to 4, respectively. PRNT50 titers in the acute and convalescent samples were more than 10-fold higher than these values (range: 3904–10703). This case was reviewed by the IDMC and considered to be possible dengue or chikungunya infection but was not considered to be severe dengue disease. Diagnostic tests for chikungunya and hepatitis A and C (i.e., anti-HAV IgG and anti-HCV IgG) were negative. This SAE was assessed to be not related to CYD-TDV by the investigator and IDMC, and the participant was allowed to continue in the study.
Immunogenicity
Baseline (pre-dose 1) and post-dose 3 (28 d after the third dose) data were available from 600 participants. Twenty-eight days after the third dose of CYD-TDV, in all age groups combined, seropositivity rates ranged from 77.1% to 94.1% for serotype 1 though serotype 4 (), and geometric mean titers (GMTs; 1/dil) ranged from 43.0 to 100 for serotype 1 through 4 (). Seropositivity rates and GMTs against each serotype increased in each age group, after each CYD-TDV vaccination (data not shown). Seropositivity rates against serotypes 1–4 were higher in children than in older participants: 91.0–97.9% in children, 67.4–92.9% in adolescents, and 71.7–93.7% in adults (). Similarly, serotype-specific GMTs were higher in children than adolescents, despite similar baseline GMTs in the two age groups (). Baseline seropositivity (PRNT50 titer ≥ 10 1/dil) to at least one dengue virus serotype was detected in 26.5% of participants (19.6% of children, 13.5% of adolescents, and 46.5% of adults) (). After the first vaccination, GMTs were higher against serotypes 4 and 3 (). After the second and third vaccinations immune responses increased against all serotypes so that after the third CYD-TDV vaccination, 66.5% of all participants were seropositive to all four dengue virus serotypes, 87.2% were seropositive to at least three serotypes, and 97.3% were seropositive to two or more serotypes ().
Figure 3. Seropositivity rates (percentage of participants PRNT50 titer ≥ 10 1/dil) against each of the four dengue virus serotypes (1, 2, 3 and 4) at baseline and 28 d after the third vaccination in all participants and in each of the three age groups.
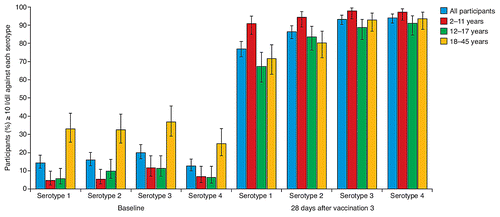
Table 3. GMTs for each of the four dengue virus serotypes at baseline and 28 d after the third vaccination by age and vaccine group (full analysis set: CYD-TDV n = 438; Control n = 147; using participants available for each endpoint)
Table 4. Seropositivity against at least one, two, three or four dengue virus serotypes: percentage [95% CI] of participants available for each endpoint with titers ≥ 10 1/dil) 28 d after the third vaccination by age and vaccine group (full analysis set: CYD-TDV n = 438; Control n = 147; using participants available for each endpoint)
Table 5. PRNT50 antibody responses for each of the four dengue virus serotypes (95% CI) before and 28 d after the first and second CYD-TDV vaccinations in two separate cohorts of the CYD-TDV group
In the control group, serotype-specific dengue antibody responses remained largely similar to baseline. The proportion who were seropositive against at least one serotype increased from 32.4% (95% CI: 24.9, 40.7) to 43.5% (95% CI: 34.9, 52.4); 10 children and four adolescents seroconverted for at least one serotype during the vaccination period.
Discussion
The WHO estimates that 2.5 billion people are at risk for dengue infection worldwide,Citation6 although a more recent estimate suggests that the at-risk population could comprise more than 3 billion people.Citation8,Citation32 Consequently, the entry of a dengue vaccine candidate into the latter stages of a clinical trial program is a long-awaited and exciting development. The present study of CYD-TDV in children, adolescents, and adults undertaken in Singapore, a country of fluctuating dengue endemicity, complements the findings from other studies.Citation19-Citation23,Citation33
This multicenter trial is a key element of a global, phase II clinical trial program designed to evaluate the safety and immunogenicity of the live, attenuated dengue vaccine candidate CYD-TDV across different settings in South America and Asia.Citation15 It is the largest Phase II safety and immunogenicity trial of a candidate dengue vaccine conducted to date. The strengths of this investigation include its basis in a large, randomized population, covering the various age ranges (adults, adolescents, and children as young as 2 y old) of individuals who could benefit from a vaccine that protected against dengue disease in countries with low dengue endemicity.
Our study showed that, in a predominantly dengue-naïve population, three doses of CYD-TDV elicited a balanced, neutralizing antibody response against all four dengue serotypes and with a satisfactory safety profile compared with two licensed control vaccines. Overall, the results were consistent with those of a randomized, controlled phase I trial of the safety and immunogenicity of CYD-TDV in dengue-naïve children, adolescents, and adults in Mexico City—a non-endemic area for dengue—with a similar protocol to that used in the present study.Citation33
In our study, participants of all ages demonstrated a robust immune response to CYD-TDV, though a stronger response was observed in children and adults than in the adolescent group. CYD-TDV has been shown to elicit a strong immune response in a dengue-immune population including adolescents and adultsCitation34 and in flavivirus-vaccinated adult individuals,Citation19 highlighting the benefit of pre-existing flavivirus immunity on the immunogenicity of CYD-TDV. In addition, as found in Mexico City,Citation33 there was a somewhat lower response to CYD-TDV vaccination in adolescents and adults in dengue-naïve populations. The apparent difference in response between adolescents and adults may be explained by the low prevalence of dengue-immunity among adolescents in our study (13.5%). No published data are available for baseline seroprevalence in this age group in the general population in Singapore, although in 2007 baseline seroprevalence in outbreak areas was 16.8%, which is likely to be higher than that in the general population (Lee Ching NG, National Environment Agency, Singapore; personal communication). We suspect that the low rate of dengue-immune adolescent participants may reflect the unique epidemiological profile of dengue disease within Singapore, a country with fluctuating endemicity levels that lie between the high endemicity seen in countries such as Vietnam and the lower levels found, for example, in the USA. Another factor that may explain the lower response to CYD-TDV in adolescents compared with that observed in children and adults might be the balanced sex distribution in the adolescent age group (male:female ratio, 1.014), compared with a slight predominance of females in the two other age groups (male:female ratios in children and adults were 0.795 and 0.609, respectively). Several studies report greater humoral and cell-mediated immune responses to vaccination in females than in males, possibly due to a negative effect of androgens on the immune response.Citation35
CYD-TDV had a favorable safety profile in the different age cohorts employed in the present study. It is most likely that the one reported case of tension headache secondary to untreated allergic rhinitis in a 9-y-old boy receiving TDV and considered to be possibly related to the study vaccine by the investigator was coincidental. The 42-y-old woman with clinically suspected dengue fever is intriguing, and further investigations conducted to assess alternative etiologies were negative. When tested by dengue PRNT50, the acute serum sample revealed titers more than 10-fold higher than those seen post-dose 3, suggesting that a dengue infection had been contracted between these two time points. There was no further increase in titer between the acute and convalescent samples, although it should be noted that the acute sample was collected late (Day 7) after fever onset. The late collection of the acute sample after the onset of fever may also explain the variance among dengue virological assay results.
The participants enrolled in this study are being followed for 4 y after the third vaccination with the objective of documenting the long-term safety of CYD-TDV and the persistence of humoral and cellular immunity. The present study showed good retention of the participants to secure the 4 y’ follow-up, with only 7.3% of the enrolled population (equally distributed between CYD-TDV and control groups) not completing the vaccination phase of the study. Many withdrawals were not vaccine-related, most being due to employment commitments, overseas relocation or non-compliance with the protocol as a result of pregnancy.
In conclusion, this large phase II study of a live-attenuated, tetravalent, recombinant dengue vaccine in a broad age range of participants confirms that the overall safety profile of CYD-TDV is satisfactory in terms of reactogenicity and AEs. Furthermore it supports the use of a three-dose CYD-TDV regimen to elicit neutralizing antibody responses against all four dengue virus serotypes, particularly for populations that are mainly dengue-naïve before vaccination. Variability in the immune responses according to serotype and age group, observed particularly before the third vaccination, are consistent with observations from other phase I and phase II trials. Our results support the continued development of CYD-TDV for the prevention of dengue disease, and herald the promise of a commercially available vaccine in the coming years.
Methods
Participants and ethical conduct
The study was conducted in pediatric and medical departments of five hospitals in Singapore. The vaccination period was April 2009 to October 2010. Individuals aged 2–45 y and in good health based on medical history and physical examination were eligible. Participants were ineligible if they: had febrile illness (temperature ≥ 37.5°C) or moderate or severe acute illness/infection on the day of the first vaccination; had any immunodeficiency or chronic illness that could interfere with the trial results; were in receipt of blood or blood-derived products in the previous 3 mo that might interfere with the assessment of immune responses; were in receipt (or planned receipt) of any vaccine in the 4 weeks preceding and following the first trial vaccination; had a history of thymic disease or myasthenia; had a previous hepatitis-A vaccination (for children aged < 12 y); or planned to move to another country or participate in another clinical trial within 18 mo of enrolment in the present study.
Contraindications to receiving the second or third CYD-TDV dose included evidence of systemic hypersensitivity (especially to egg proteins or neomycin) to the previous study vaccination, an ongoing clinical AE or biological abnormality related to the previous study vaccination or SAE related to the trial vaccine after the previous study vaccination.
Pregnant or breast-feeding women were also excluded from all vaccinations. In addition, all enrolled post-pubertal women were required to have a negative urine pregnancy test and to use an effective method of contraception (or abstinence from sexual intercourse) for ≥ 4 weeks before the first vaccination and until ≥ 4 weeks after the last vaccination. Women who became pregnant during the course of the study were not re-vaccinated.
The trial was conducted in accordance with the Declaration of Helsinki, International Conference of Harmonization guidelines for Good Clinical Practice, applicable national and local requirements, and was registered with ClinicalTrials.gov (NCT00880893).
Participants aged 21 y or older, and the parents or legal representative of participants younger than 21 y old, provided written informed consent. Furthermore, participants aged 6–12 y were also asked to provide their written assent. If a participant or his/her parent/legal representative were unable to read and sign the informed consent or assent form, it was signed by an impartial witness.
The study protocol and consent forms were approved by two local Independent Ethics Committees (Domain E, Domain Specific Review Board and Singhealth Centralised Institutional Review Board). The study sites fulfilled criteria set out in WHO guidelines for clinical evaluation of dengue vaccines in endemic areas and the study was conducted in accordance with the draft guidelines of the WHO Expert Committee on Biological Standardization, which were adopted in October 2011.Citation36-Citation38
Trial design
This was a randomized, controlled, multicenter, Phase II trial. Participants were divided into three age groups: children (aged 2–11 y), adolescents (aged 12–17 y) and adults (aged 18–45 y). Recruitment tools included direct mailing, poster and flyer campaigns at hospitals, clinics, and on trains and buses, as well as magazine and radio advertisements. Children and adolescents were also recruited from campaigns within kindergartens, primary schools, and secondary schools.
At enrolment, participants were randomized 3:1 (CYD-TDV group: control group), to receive subcutaneous doses of CYD-TDV (Sanofi Pasteur) containing approximately 5 log10 cell culture infectious dose 50% of each of the four CYD dengue vaccine viruses per 0.5-mL dose or a control vaccine. A randomization list assigning inclusion numbers to one of the vaccine groups was generated by block randomization, with stratification by age group and trial center, using the Proc Plan procedure (SAS version 8.2). Enrolled participants were sequentially attributed inclusion numbers using an interactive voice-recognition system (IVRS) before vaccination. An IVRS was then used a second time together with the attributed inclusion number by separate trial personnel who were not involved in the safety assessments to be informed which product to administer. The investigator remained blind for this first, 0.9% saline placebo-controlled injection. For the second and third vaccinations, participants allocated to the CYD-TDV group received two additional subcutaneous doses of CYD-TDV, whereas in the control group, commercial batches of vaccines licensed in Singapore were used. Participants aged < 12 y at inclusion received intramuscular doses of hepatitis-A vaccine (inactivated virus; Havrix® pediatric formulation 720, GSK; 2 × 0.5-mL doses) and those aged ≥ 12 y received subcutaneous inactivated influenza vaccine (Vaxigrip®, Sanofi Pasteur; 2 × 0.5-mL doses) compliant with the WHO recommendations for Northern and Southern hemisphere formulations for 2009. Investigators knew which vaccine was administered due to the different vaccination route for children. However participants were not informed of which vaccine was given, and assuming that children cannot distinguish between an intramuscular and a subcuteanous injection, we considered that the administration of second and third vaccinations was single-blinded.
Vaccines were administered at visits 0, 6 and 12 mo. At these visits, participants underwent physical examination and pregnancy tests before vaccination and blood samples were taken. The first 600 participants (CYD-TDV group n = 450, control group n = 150; 200 from each of the three age groups) were included in the assessment of the humoral immune response to the parental dengue strains used to engineer the CYD-TDV recombinant vaccine viruses. These participants were randomly allocated to cohort 1 (n = 300) or cohort 2 (n = 300) with different time points for blood sampling. For participants randomized to the immunogenicity subset, blood samples were taken before and 28 d after the first and third vaccinations (cohort 1), and before the first and second vaccinations and 28 d after the second and third vaccinations (cohort 2). Participants also received four follow-up telephone calls (8 d after the first and second vaccinations, and 3 mo and 6 mo after the last vaccination).
Immunogenicity, virological and serological analyses were undertaken at Global Clinical Immunology (GCI) Sanofi Pasteur, (Swiftwater) with diagnostic enzyme immunosorbent assay (ELISA) testing performed at the Center for Vaccine Development, (University of Mahidol), a WHO Reference laboratory on serology and virology for dengue and JE viruses. All testing was done by personnel blinded to the treatment groups.
Endpoint assessments
Safety and reactogenicity
The analysis of safety included all trial participants. After each vaccination, participants were observed for 30 min and any AEs during this period were recorded. Each participant or parent was provided with a thermometer, a ruler, and a diary card to record AEs in a similar manner to that used in phase I studies with a TDV.Citation21,Citation33,Citation34 Solicited injection-site and systemic reactions were monitored 7 d and 14 d post-vaccination, respectively, and rated on a three-grade intensity scale (). Participants and parents were also asked to record unsolicited (spontaneously reported) AEs occurring within 28 d after injection on the diary cards. SAEs were monitored throughout the study and for up to 6 mo after the last vaccination. Investigators assessed any potential relationship to the vaccine for all reported AEs, reviewed laboratory results, and assessed the clinical significance of any abnormal values. An IDMC reviewed SAEs from this study, together with SAEs from other studies from the sponsor’s global dengue clinical trial program every 2 weeks (monthly from January 2010), AEs quarterly, and undertook prompt review of related or possibly related SAEs.
Table 6. Definitions of solicited injection-site and systemic reactions
Post-vaccination viremia and biological investigations for febrile episodes
Participants who reported febrile episodes (defined as fever ≥ 38°C on at least 2 consecutive days since the last visit) occurring within 28 d after each vaccination were assessed for dengue vaccine viremia and wild-type dengue viremia by qRT-PCR as well as biochemical and hematological parameters. In these cases, an additional blood sample was taken within 5 d after the onset of fever (acute-phase serum). Vaccine viremia qRT-PCR assays consisted of a screening assay to detect the YF component of the vaccine and four subsequent CYD serotype-specific assays.Citation38 Virological detection of wild-type dengue was undertaken using qRT-PCR and dengue NS1 Antigen ELISA (Platelia™, Biorad Laboratories).Citation39 For the qRT-PCR, a screening assay to detect any wild-type dengue (developed based upon TaqMan chemistry)Citation40 and four subsequent serotype-specific wild-type dengue qRT-PCR assays were applied.
Pregnancy follow-up
Women withdrawn from the study because of pregnancy were followed up to report on any complications and the outcome of the pregnancy.
Hospitalization for suspected dengue disease
The occurrence of hospitalized suspected dengue cases was reported throughout the trial. Dengue cases were identified by passive surveillance during planned visits or telephone calls at least every 4 mo. Subjects were recalled to the study center as soon as possible in case of dengue suspicion. Definition of a hospitalized dengue case was acute febrile illness (temperature ≥ 38°C) on at least 2 consecutive days, without evidence of localized infection and with signs indicating severity necessitating hospitalization. Dengue case confirmation was based on the dengue diagnostic testing results performed on paired acute and convalescent samples (i.e., NS1 antigen ELISA and/or dengue wild-type serotype-specific qRT-PCR as well as dengue IgM/IgG ELISA in acute sample taken within 5 d after the onset of fever and dengue IgM/IgG ELISA in convalescent sample taken 7–14 d after). In addition, biological parameters (i.e., hematology and liver function test) and routine dengue diagnostic tests (i.e., dengue IgM/IgG) were performed locally for dengue case management purpose.
Virological testing and serological testing were performed using commercially available dengue NS1 ELISA (PlateliaTM dengue NS1 Antigen from Bio-Rad) and dengue ELISA-specific IgM and IgG kits (EL1500G and EL1500M; Focus Diagnostics Inc.). Cases were classified as virologically “confirmed” (positive dengue NS1 antigen ELISA and/or dengue wild-type serotype-specific qRT-PCR) or “probable” based on serological criteria (positive for IgM and/or 4-fold rise of IgG antibody titers between acute and convalescent samples). IDMC undertook prompt review of hospitalized dengue cases.
Immunogenicity
Neutralizing antibody levels against the four dengue serotype parental strains of TDV were assessed using a PRNT50 compliant with WHO guidelines.Citation41,Citation42 Serial, 2-fold dilutions of heat-inactivated serum were mixed with a constant challenge dose of each of the four CYD-TDV dengue serotypes. The mixtures were inoculated into wells of a 24-well microplate of confluent Vero cells. After adsorption, cell monolayers were overlaid with carboxymethylcellulose and incubated for several days. Plaques were then fixed, immunostained, and numerated in each well. The formation of infected foci indicated the presence of cells infected with dengue virus. The neutralizing antibody GMT were calculated and expressed as the highest reciprocal serum dilution at which the mean count of infective foci was reduced by 50% compared with the mean viral focus count obtained from the control wells. The lower limit of quantitation of the assay was 10 [1/dil]. Samples with lower limit of quantitation titers ≥ 10 were considered to be seropositive. The percentage of participants with seropositive samples was used to provide the seropositivity rate.
Sample size and study populations
The planned study sample size of 1,200 volunteers, comprising 400 participants in each age group (900 CYD-TDV group; 300 control group), was set arbitrarily to provide a 95% probability of observing a safety event that had a true incidence of 0.3% for the CYD-TDV Group (1% in each age stratum by the rule of three). This sample size also had > 80% power to detect a difference of 10% between the CYD-TDV and control groups.
All planned analyses were descriptive with no hypothesis testing. Analyses were performed on all available data, with no replacement of missing data. The full analysis set (FAS) for immunogenicity comprised participants who received at least one dose of CYD-TDV or control vaccine, had a blood sample taken, and had a result available after vaccination. The safety analysis set (SAS) comprised participants who received at least one dose of CYD-TDV or control vaccine.
| Abbreviations: | ||
| AE | = | adverse reaction |
| ELISA | = | enzyme immunosorbent assay |
| FAS | = | full analysis set |
| GMT | = | geometric mean titer |
| IDMC | = | Independent Data Monitoring Committee |
| LMP | = | last menstrual period |
| PRNT50 | = | 50% plaque reduction neutralization test |
| qRT-PCR | = | quantitative reverse transcription-polymerase chain reaction |
| RT-PCR | = | reverse transcription-polymerase chain reaction |
| SAE | = | serious adverse event |
| SAS | = | safety analysis set |
| TDV | = | tetravalent dengue vaccine |
| WHO | = | World Health Organization |
Acknowledgments
The authors would like to thank all of the volunteers and their parent/guardians who participated in the trial, Dr Sutee Yoksan at the Center for Vaccine Development, Mahidol University, Bangkok Thailand, and also their fellow investigators and the study-site personnel in the pediatric and medical departments at the National University Hospital, KK Women’s and Children’s Hospital, Singapore General Hospital and Changi General Hospital, Singapore, for their valuable contributions to the study, and at Sanofi Pasteur, Mark Boaz and Grenville Marsh for comments and suggestions on the drafts of this manuscript.
Trial registration
National Clinical Trials Identifier (NCT ID): NCT00880893
Disclosure of Potential Conflicts of Interest
Y.S.L. provided scientific advice throughout the study design and conduct phases, was responsible for communication with the ethics committees, and contributed to interpreting the results; A.W.S., S.A., L.P.S., C.Y.C., H.N.L. and M-L.H.O. were the study principal investigators and C.Y.L. an investigator. They received no direct payment from the study sponsor for their contributions. Y.S.L. received support from Sanofi Pasteur for travel to present this study at the American Society for Travel Medicine and Hygiene’s 61st annual meeting. A.B., A.W.T. and D.C. designed the study and interpreted the results and are employed by Sanofi Pasteur. All authors reviewed the manuscript drafts, and approved the final version. Sanofi Pasteur provided financial support and was involved in the protocol design, data analysis and the decision to submit this manuscript. The authors take full responsibility for the content of this paper and thank Graham Joint (supported by Sanofi Pasteur) for preparing the drafts.
References
- Schweitzer BK, Chapman NM, Iwen PC. Overview of the Flaviviridae with an emphasis on the Japanese Encephalitis group viruses. Lab Med 2009; 40:493 - 9; http://labmed.ascpjournals.org/content/40/8/493.full.pdf http://dx.doi.org/10.1309/LM5YWS85NJPCWESW
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10:Suppl S98 - 109; http://dx.doi.org/10.1038/nm1144; PMID: 15577938
- World Health Organization. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 2nd Edn. Geneva: World Health Organization. 2009. Available at: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf.
- Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis 2006; 19:429 - 36; http://dx.doi.org/10.1097/01.qco.0000244047.31135.fa; PMID: 16940865
- Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007; 30:329 - 40; http://dx.doi.org/10.1016/j.cimid.2007.05.010; PMID: 17645944
- World Health Organization. Dengue and dengue haemorrhagic fever, Factsheet number 117 (Revised March 2009). 2009a. Available at: http://www.who.int/mediacentre/factsheets/fs117/en/index.html.
- World Health Organization. Global Alert and Response (GAR): Impact of Dengue. 2012. Available at: http://www.who.int/csr/disease/dengue/impact/en/.
- Halstead SB. Dengue. Lancet 2007; 370:1644 - 52; http://dx.doi.org/10.1016/S0140-6736(07)61687-0; PMID: 17993365
- Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol 2010; 8:Suppl S7 - 16; http://dx.doi.org/10.1038/nrmicro2460; PMID: 21079655
- SEARO. Situation update of dengue in the SEA Region, 2010. Available at: http://www.searo.who.int/LinkFiles/Dengue_Dengue_update_SEA_2010.pdf.
- Halstead SB. Dengue in the Americas and Southeast Asia: do they differ?. Rev Panam Salud Publica 2006; 20:407 - 15; http://dx.doi.org/10.1590/S1020-49892006001100007; PMID: 17341332
- Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. [x;; .] Med Clin North Am 2008; 92:1377 - 90; http://dx.doi.org/10.1016/j.mcna.2008.07.002; PMID: 19061757
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol 2007; 5:518 - 28; http://dx.doi.org/10.1038/nrmicro1690; PMID: 17558424
- Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin 2010; 6:696 - 705; http://dx.doi.org/10.4161/hv.6.9.12739; PMID: 20861669
- Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011; 29:7229 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.06.094; PMID: 21745521
- Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M, et al. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol 2000; 74:5477 - 85; http://jvi.asm.org/content/74/12/5477.full.pdf http://dx.doi.org/10.1128/JVI.74.12.5477-5485.2000; PMID: 10823852
- Guirakhoo F, Arroyo J, Pugachev KV, Miller C, Zhang ZX, Weltzin R, et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol 2001; 75:7290 - 304; http://jvi.asm.org/content/75/16/7290.full.pdf http://dx.doi.org/10.1128/JVI.75.16.7290-7304.2001; PMID: 11462001
- Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010; 28:632 - 49; http://dx.doi.org/10.1016/j.vaccine.2009.09.098; PMID: 19808029
- Qiao M, Shaw D, Forrat R, Wartel-Tram A, Lang J. Priming effect of dengue and yellow fever vaccination on the immunogenicity, infectivity, and safety of a tetravalent dengue vaccine in humans. Am J Trop Med Hyg 2011; 85:724 - 31; http://www.ajtmh.org/content/85/4/724.full.pdf http://dx.doi.org/10.4269/ajtmh.2011.10-0436; PMID: 21976579
- Guirakhoo F, Kitchener S, Morrison D, Forrat R, McCarthy K, Nichols R, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vacc 2006; 2:60-7. Available at: http://www.landesbioscience.com/journals/vaccines/guirakhooHV2-2.pdf.
- Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis 2010; 201:370 - 7; http://jid.oxfordjournals.org/content/201/3/370.full.pdf http://dx.doi.org/10.1086/649916; PMID: 20059357
- Crevat D, Reynolds D, Langevin E, Capeding MR. Safety and immunogenicity of a tetravalent dengue vaccine in flavivirus-naïve and immune paediatric populations with two vaccination regimens. [Abstract 395] Am J Trop Med Hyg 2009; 81:Suppl 1 113
- Forrat R, Poo JL, Galán Herrera JF. Immune response to tetravalent dengue vaccination in Mexican subjects: the effects of yellow fever vaccination. [Abstract 387] Am J Trop Med Hyg 2008; 79:Suppl 6 114
- Wilder-Smith A, Foo W, Earnest A, Sremulanathan S, Paton NI. Seroepidemiology of dengue in the adult population of Singapore. Trop Med Int Health 2004; 9:305 - 8; http://onlinelibrary.wiley.com/doi/10.1046/j.1365-3156.2003.01177.x/pdf http://dx.doi.org/10.1046/j.1365-3156.2003.01177.x; PMID: 15040570
- Burattini MN, Chen M, Chow A, Coutinho FA, Goh KT, Lopez LF, et al. Modelling the control strategies against dengue in Singapore Epidemiol Infect 2008; 136:309-19. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2870819/pdf/S0950268807008667a.pdf.
- Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis 2006; 12:887 - 93; http://dx.doi.org/10.3201/10.3201/eid1206.051210; PMID: 16707042
- Goh KT. Dengue--a re-emerging infectious disease in Singapore. Ann Acad Med Singapore 1997; 26:664 - 70; PMID: 9494676
- Lee KS, Lai YL, Lo S, Barkham T, Aw P, Ooi PL, et al. Dengue virus surveillance for early warning, Singapore. Emerg Infect Dis 2010; 16:847 - 9; http://wwwnc.cdc.gov/eid/article/16/5/09-1006_article.htm PMID: 20409381
- World Health Organization Western Pacific Region. Cases of dengue, including imported cases, and dengue-attributed deaths in the Western Pacific Region for 2010. Available at: http://www2.wpro.who.int/NR/rdonlyres/F05F1AFF-8D7B-4251-A64F-85809938DEE1/0/Table1201122005_RA_Denguesurv_WPR.jpg.
- Singapore Ministry of Health. Communicable Diseases Surveillance in Singapore 2010: Annual Report. Chapter II. Vector-Borne Diseases. Surveillance of dengue virus serotypes, 1992–2010; p33. Available at: http://www.moh.gov.sg/content/dam/moh_web/Publications/Reports/2011/Communicable%20Diseases%20Surveillance%20in%20Singapore%202010/Vector-borne%202010.pdf.
- Singapore Ministry of Health. Review of Singapore’s dengue prevention and control programme. Epidemiol News Bull (October–December) 2006; 32. Available at: http://www.moh.gov.sg/content/dam/moh_web/Statistics/Epidemiological_News_Bulletin/2006/ENB04Q_06.pdf.
- Beatty M, Letson W, Edgil D, Margolis H. Estimating the total world population at risk for locally acquired dengue infection. Abstract presented at the 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene. ASTMH Abstract Book 2007; Abstract 768: 221. Available at: http://www.astmh.org/AM/Template.cfm?Section=Meeting_Archives&Template=/CM/ContentDisplay.cfm&ContentID=1514
- Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated tetravalent dengue vaccine in dengue-naïve children, adolescents, and adults in Mexico City: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J 2010; 30:e9 - 17; http://dx.doi.org/10.1097/INF.0b013e3181fe05af; PMID: 21042231
- Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine 2011; 29:3863 - 72; http://dx.doi.org/10.1016/j.vaccine.2011.03.057; PMID: 21477675
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. [Erratum in: Lancet Infect Dis. 2010;10:740] Lancet Infect Dis 2010; 10:338 - 49; http://dx.doi.org/10.1016/S1473-3099(10)70049-9; PMID: 20417416
- Edelman R, Hombach J. “Guidelines for the clinical evaluation of dengue vaccines in endemic areas”: summary of a World Health Organization Technical Consultation. Vaccine 2008; 26:4113 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.05.058; PMID: 18597906
- World Health Organization. Guidelines for the clinical evaluation of dengue vaccine in endemic areas, WHO/IVB/08.12. 2008. Available at: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.12_eng.pdf.
- World Health Organization. Guidelines on the Quality, Safety and Efficacy of Dengue Tetravalent Vaccines (Live, Attenuated). WHO/BS/11.2159. Geneva: WHO; 2011. Accessed at http://www.who.int/biologicals/BS2159_Dengue_WITH_LINE_NUMBERS_TRS_v47_18Jul2011_sub2.pdf.
- Mantel N, Aguirre M, Gulia S, Girerd-Chambaz Y, Colombani S, Moste C, et al. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever-dengue vaccines. J Virol Methods 2008; 151:40 - 6; http://dx.doi.org/10.1016/j.jviromet.2008.03.026; PMID: 18501437
- Huhtamo E, Hasu E, Uzcátegui NY, Erra E, Nikkari S, Kantele A, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol 2010; 47:49 - 53; http://dx.doi.org/10.1016/j.jcv.2009.11.001; PMID: 19963435
- Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99:285 - 90; PMID: 6031202
- Roehrig JT, Hombach J, Barrett AD. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol 2008; 21:123 - 32; http://dx.doi.org/10.1089/vim.2008.0007; PMID: 18476771