Abstract
Vaccinations are increasingly used to fight infectious disease, and DNA vaccines offer considerable advantages, including broader possibilities for vaccination and lack of need for cold storage. It has been amply demonstrated, that electroporation augments uptake of DNA in both skin and muscle, and it is foreseen that future DNA vaccination may to a large extent be coupled with and dependent upon electroporation based delivery. Understanding the basic science of electroporation and exploiting knowledge obtained on optimization of DNA electrotransfer to muscle and skin, may greatly augment efforts on vaccine development. The purpose of this review is to give a succinct but comprehensive overview of electroporation as a delivery modality including electrotransfer to skin and muscle. As well, this review will speculate and discuss future uses for this powerful electrotransfer technology.
Introduction
For nearly 20 years there has been a focus on using DNA as a tool for creating new efficient vaccines.Citation1 The idea of a DNA vaccine is in fact very simple: inject a plasmid encoding a relevant antigen into e.g., a muscle, ensure that the plasmid is transcribed and the protein/antigen is produced, with the initiation of an immune response. DNA vaccines could be, if working as effectively in the real world as in theory and preclinical studies, an efficient and novel opportunity to prevent infectious diseases.
The ease by with it is possible to produce GMP-DNA, the possibility of multi-potent vaccines, the high level of safety due to absence of viral elements in the manufacturing and the stability of the DNA molecule making a ‘cold-chain’ superfluous, makes it well worth exploring the effect of DNA vaccines.
On a global economy perspective DNA technology may enable cheaper vaccines, and thus greater availability and higher likelihood for successful vaccination programs. Different approaches for optimizing the immunological response after a DNA vaccination are under investigation, covering aspects such as conjugates or molecular adjuvants, often in combination with exploitation of physical methods to improve the cellular uptake of the plasmid.Citation2 A well-known physical technique is electroporation, which is a non-viral means for transferring genes and other non-permeant molecules across the cell membrane.
Studies with electroporation assisted DNA vaccinations have shown that it is possible to obtain both a cellular and a humoral immunological response, and in the preclinical setting the technique has shown promising results.Citation3 However, the effect in large animals and humans may still be improved.Citation4 The aim of this paper is to look at electroporation based DNA vaccines from a practical point of view. We have considerable experience in using electroporation, both in preclinical studiesCitation5-Citation7 and in the clinical settingCitation8,Citation9 (gene transfection to various tissues and electrochemotherapy for treatment of cutaneous metastases) and hope that the knowledge we have achieved can be of use to researchers working with electroporation for DNA vaccinations.
Electroporation: Basic concepts
The principle behind electroporation is strikingly simple; by applying an electric field that surpasses the electrical capacitance of the cell membrane, cells may be rendered transiently permeabilized due to entrance of water into the membrane and formation of hydrophilic permeation structures.Citation10,Citation11
shows how the cell membrane responds to an external electric field, and also how electrotransfer of drugs and genes work differently.Citation12 For drugs, and other small molecules, simple diffusion happens through the cell membrane after permeabilization. Diffusion will take place as long as the cell membrane is permeabilized, i.e., also after the pulses have been given. On the contrary, DNA is too large to enter through the hydrophilic pores by simple diffusion. DNA (and other nucleotides) are polyanions, with a plethora of negative charges, enabling the molecule to move in an electric field.Citation13 However, moving DNA is not sufficient; the cell membrane also needs to be in a permeabilized state in order to allow passage of the DNA molecule.
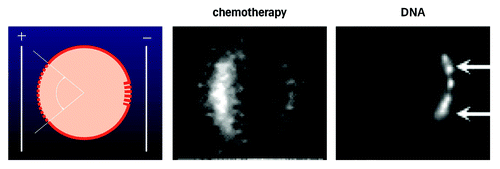
Figure 1. The figure in the left panel depicts a cell, upon which an electric field is exerted. As the cell interior is negative, the electric force will be largest upon the pole of the cell facing the positive electrode, and, therefore, dielectric breakdown will first, and foremostly, occur here. A smaller window, but with more extensive permeabilization will form on the pole facing the negative electrode. In the center panel, a cell permeabilised in the presence of a small fluorescent molecule is shown (from B. Gabriel, CNRS, Toulose, as also described in ref. Citation12). DNA transfer appears to work differently from transfer of small molecules. Thus, the DNA is too large to penetrate through small permeabilisations. Instead, the DNA may be ‘pushed’ through the membrane already rendered permeable, by electrophoretic forces exerted by the field. As DNA (and most other nucleotides) is a polyanion, with a massive presence of negative charges, the electrical forces relative to the molecule size means that DNA may be moved in the field. In the right panel, it is seen how fluorescently labeled DNA enters the cell on the pole of the cell facing the negative electrode (being attracted toward the positive electrode, and repulsed from the negative electrode) from M. Golzio at CNRS Toulouse, also described in ref. Citation50.
Pulses may be optimized to achieve either a greater degree of permeability of the cell membrane (for passive diffusion of drugs), or a greater degree of electrophoretic effect. As mentioned below, there are various ways to go about this, but generally a series of short high voltage pulses (e.g., 8 pulses of 0.1 ms at 1,000 V/cm voltage to electrode distance) is used for drug delivery,Citation14 and a combination involving long low voltage pulses is used for DNA transfer (e.g., 1 pulse of 0.1 ms, 800 V/cm and 1 long pulse of 400 ms 80 V/cm).Citation15
The permeabilization structures will start to form in a matter of microseconds during the first pulse, and will reseal in the order of minutes after the pulses have ended.Citation16-Citation20 Drugs may diffuse into cells as long as the permeabilized state exists, and may therefore also be added just after pulsation. However, DNA must be added prior to the electric pulse, in order to be subjected to the electrophoretic effect necessary to transfer the DNA across the cell membrane; indeed it has been shown that when DNA is added after the pulses, but while the cell is still permeabilized, there is no transfection.Citation21
Other nucleotides, which are similar to DNA (e.g., RNA), can be optimally transferred using similar pulsing sequences. For different nucleotides, e.g., oligonucleotides such as PNA (peptide nucleic acid) or LNA (locked nucleic acid), actually the ‘drug electrotransfer parameters’ may be more appropriate.Citation22
Pulses in every form and shape
The classical laboratory electroporator operates with exponentially decaying pulses.Citation23 This is the technologically least complicated, and allows for use as a reasonable priced and reasonable sized laboratory electroporator. In the exponentially decaying pulse, pulse duration will increase when amplitude is increased. This offers fewer possibilities for optimization – on the other hand for transfection of E-coli, it is the one transfected clone that matters, not the survival of the remaining E-coli.
For use in mammalian cell cultures, and in vivo work, it is a different story. Here, high cell viability together with good transfection rates are in demand. In square wave pulse generators, it is possible to independently control pulse amplitude and duration, enabling much better optimization. Finally, for electroporation in the clinical setting, a number of regulatory standards need to be met for the equipment.Citation23
The electric pulses may be voltage clamped,Citation23 where the current will vary, or current clamped with varying pulse amplitude or duration.Citation24 The number of possibilities for pulse combinations is really infinitely large. The number of pulses may be varied in numerous ways; the frequency, the amplitude, the duration—and then different pulse forms may be combined. Below some examples are mentioned for gene electrotransfer to respectively skin and muscle—the primary target tissues for DNA vaccinations. Furthermore, electrode geometry greatly influences the actual electric field and local factors in the tissue may alter field distribution, e.g., the presence of stratum corneum in the skin, muscle fiber direction.Citation13,Citation25
Electroporation nomenclature
As in all scientific fields, nomenclature evolves with the development of the field. Here are some definitions commonly used.
Electroporation or electropermeabilization; these terms are used interchangeably. From a scientific viewpoint electropermeabilization may be more correct, since what has been scientifically documented is the permeabilized state, whereas the term pore may lead the mind into thinking of more formal pore-like structures.
Electrotransfer: Describes the movement of molecules into cells, by either passive diffusion or electrophoresis, made possible by the use of electric pulses. From this term we derive DNA electrotransfer, RNA electrotransfer, PNA electrotransfer, etc.
Electrochemotherapy (ECT): The use of electroporation to enhance the uptake of chemotherapy in a tumor.
Gene Electrotransfer
Gene electrotransfer in general
Gene electrotransfer or electroporation assisted gene transfection is the combination of electric pulses with injection of a gene, often naked plasmid DNA. The response of the gene transfection is dependent on the plasmid injected and the purpose of the treatment; hence two main purposes for gene electrotransfer exist namely gene therapy and DNA vaccination.
In gene therapy the aim of gene transfection is to render a tissue, e.g., muscle, skin, or tumor, capable of producing a specific protein, which is encoded by the plasmid. This can either be a relevant protein the body needs, e.g., due to a protein deficiency disorder or it can be a therapeutic compound with e.g., antineoplastic effect on cancer cells.Citation26 Normal tissues such as muscle and skin are frequently investigated for production of therapeutic proteins with local or systemic effect after gene electrotransfer, but direct transfection of tumors with plasmids encoding antineopolastic molecules is a possibility as well.
Development of DNA vaccines represents the other therapeutic possibility of gene electrotransfer, where electroporation acts as an effective tool for transfecting plasmids encoding antigens against specific epitopes and thus enhancing an immune response.Citation3 DNA vaccines can furthermore be divided into two groups: (1) prophylactic vaccines, which serves at creating an immune response against a known infectious agent, and (2) therapeutic vaccines, which aims at using the body’s immune system to react adequately to a tumor antigen e.g., in order to achieve an anticancer effect.
In preclinical studies, rodents are the animals most often used for gene transfection.Citation26 The reasons are naturally the availability and the price opposed to the cost of larger animals. A number of studies have been performed with gene electrotransfer to various tissues, but mainly to muscle, skin and tumor.Citation27 There are however some issues, which must be taken into account concerning animal model when it comes to muscle, skin or tumor transfection. These will be discussed in the following sections.
There exist many types of electrodes for gene transfection, both to muscle and skin, but they can generally be divided in two groups: the non-invasive, which consist of plates, patches and wires that are placed on the skin or around the injected volume and the invasive electrodes, which consist of different needle arrays that are inserted into the tissue. The plate electrodes are primarily used in rodents and small animals, whereas the needle electrodes often are used in larger animals, such as the pig, and in clinical studies. There is, however constant research ongoing in order to minimize the discomfort connected with both injection of plasmid and penetration of tissue with the needles. To this end different types of patches and superficial electrodes are being explored.Citation28
The electric pulses chosen for gene transfection are numerous and different research groups have found their preferable electrical parameters and electrode configuration suitable for achieving a desired response.Citation26 As seen in , the electrical parameters look like a scatter plot when depicted on a graph, although there is a tendency toward the higher field strength the shorter pulse duration. That makes sense since the purpose for using electroporation for transfection with DNA, both for vaccination and for production of therapeutic proteins, is that the transfected cells must survive the procedure and restore their equilibrium after the delivery of the electrical pulses. Crudely, the pulse parameters are divided in high voltage pulses (HV) exceeding the 400 V/cm and with a duration in the µs range and the low voltage pulse (LV) below 400 V/cm with a duration in the millisecond range. The distinction between the HV and LV is not exact, but arbitrary, and definitions may vary.
Figure 2. Electric pulses for gene electrotransfer to skin. The graph depicts the different pulse combinations that have been used for gene electrotransfer to skin. There is no consensus regarding which electrical parameter is the most effective, but efficiency may be correlated with electrode type and electric field distribution
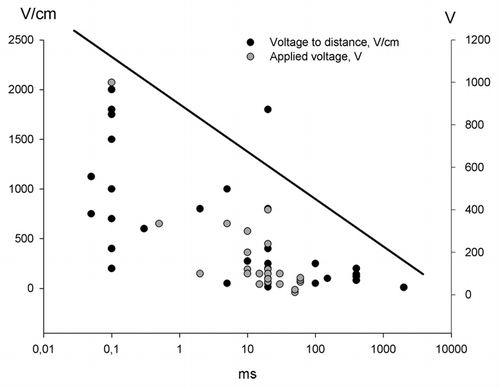
shows that many pulse combinations have been used for gene transfection. Some authors have succeeded in achieving gene expression after delivery of HV pulses only,Citation29 however there is a general acceptance that there has to be an element of LV pulses in order to attract the DNA to and through the cell membrane.Citation30
A convenient aspect of electroporation is that the technique itself is not immunogenic so it is thus possible to treat the same animal or patient several times.Citation31 Furthermore each spot of gene transfection is small and confined to the size of the electrodes and the procedure can if necessary be repeated on several locations at the same session.
Gene electrotransfer is a well-tolerated treatment. It is performed very quickly, and the amount of discomfort is tolerable. Minor adverse effects are mostly related to injection of the plasmid and insertion of the electrodes, which can be performed in combination with local anesthesia if necessary as well as the delivery of the electric pulses, which creates a short contraction of the muscle treated (muscle transfection) or the muscle below (skin and tumor treatment). The contraction is reported by patients to be a discomfort; however, healthy volunteers have tended to have no objections to repeated treatments.Citation32
Furthermore, gene electrotransfer in combination with DNA vaccines is a safe technique, since no viral components are necessary to create the immunological response. Naked plasmid DNA can easily be manufactured and distributed and the procedure is in fact very simple and easy to perform. The equipment needed is relatively inexpensive, and it can easily be transported and disseminated around the world. Furthermore it has been shown that it is possible, in a safe and non-toxic way, to turn off the expression of a transfected gene after gene electrotransfer, if the same tissue is re-electroporated in combination with local injection of calcium.Citation33
It is important to create the best conditions for gene transfection with any type of gene. The pulses must be delivered at the exact spot, where the plasmid has been injected. The lack of consistency and preciseness can be one of the reasons for a lack of electroporation mediated enhancement of responses.
Gene electrotransfer to muscle
The organ most often used for gene electrotransfer, apart from tumor, is the muscle.Citation31 The qualities the muscle cells possess render them an obvious target for gene transfection. The muscles are under normal conditions readily available and can be reached either through the skin or directly with minimal surgical intervention, both in the preclinical and the clinical setting. Muscle cells are post-mitotic and thus capable of creating a long-term expression after a gene transfection. The blood supply is abundant and they are indeed adequate for producing proteins and releasing them to act systemically. After gene transfection with the use of electroporation it is thought that the electric pulses, even though they are not immunogenic themselves, attract immune active cells, which can stimulate an immune response after e.g., transfection with a DNA vaccine. The muscle is thus a frequently used organ for clinical trials involving vaccination protocols.
An important issue regarding gene transfection to muscles is the question of delivering the electric pulses properly. The muscle consists of muscle fibers running throughout the length of the muscle and since the grade of permeabilization after electroporation is dependent on the shape of the cells,Citation34 there is a significant impact of the direction of the electric field on the muscle fibers. The damage to the cells can be minimized if the electric field is perpendicular to the direction of the muscle fibers.Citation35
As opposed to the skin where there is a tendency toward the higher dose of DNA transfected the higher expression, the muscle cells only need few µg of plasmid DNA to create a systemic relevant level of expression. There is on the contrary a risk of overdosing, since too high a dose of DNA seems to have a toxic effect on the muscle cells.Citation5,Citation36
A disadvantage with gene transfection to the muscle is that one has to overcome the barrier created by the skin in order to permeabilize the muscle fibers underneath. In the preclinical setting the plate electrodes are often used in small animals and the electric pulses are adjusted to reach a proper result, whereas the needles have shown to be effective in larger animals and in the clinical setting. The field strength can consequently be lower since the insulating properties of the skin then has been by-passed.
Gene electrotransfer to skin
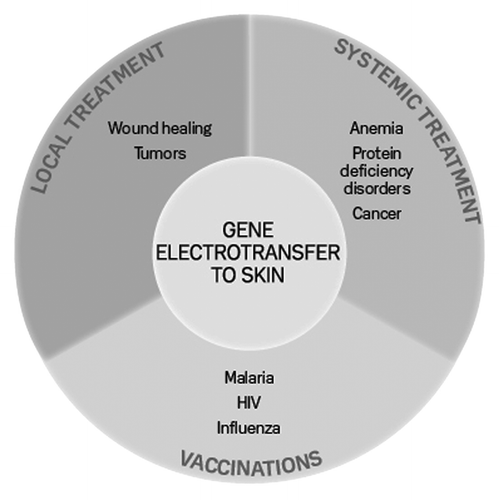
The skin is the largest organ in the body and there are many future possibilities for gene therapy delivered through that organ, summarized in .Citation37 Many of these are being explored both in the preclinical and the clinical setting. Due to the presence of antigen presenting cells, the skin is an obvious target for DNA vaccinations and electroporation is a safe and efficient means for improving the antigen response.
Figure 3. Therapeutic groups for gene electrotransfer to skin. Gene therapy to skin can be divided in three therapeutic groups: local treatment, systemic treatment and DNA vaccination. The borders between the groups are arbitrary and will depend on the transfected gene (from ref. Citation37)
Besides DNA vaccinations, the skin has the capability to produce cytokines and hormones, which can have a systemic effect, and can thus be a target for gene therapy where the purpose is protein production rather than immune reactions.Citation6,Citation38
The skin is divided in three layers, the epidermis, the dermis and the subcutaneous layer and each of them contains different cell types, which in theory can be targeted by either different electrical parameters or cell specific promoters.Citation39 This means that it could be a possibility to obtain differentiated responses after a gene transfection.
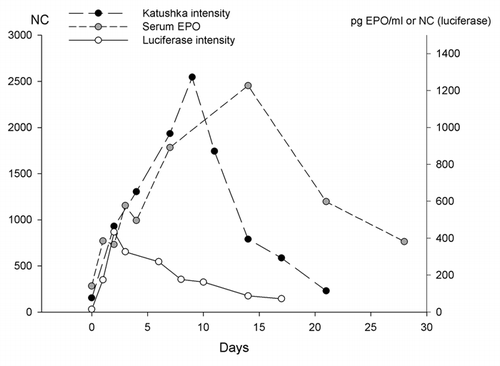
Compared with muscle cells, the skin is a more changeable organ, where cell renewal is constantly occurring. The most superficial layer, the epidermis, consists mainly of keratinocytes, which are created from stem cells on the basal layer but then grow more and more superficially and end up being flat keratin-rich, but empty membrane shells and create the cornified layer. It is thus envisioned that transfecting the keratinocytes may result in a short-term expression of only few weeks compared with the muscle, where an expression can be measured for several months, .Citation5,Citation6
Figure 4. Duration of expression after gene electrotransfer to skin. Reporter plasmids encoding luciferase are often used in gene transfection studies. In this graph the duration of luciferase expression is compared with transfection of two other compounds, pTagFP-635 encoding a red fluorescent protein, Katushka and a plasmid encoding the hormone erythropoietin, EPO. It is seen that the duration of expression peaks after two days (luciferase), nine days (Katushka) and two weeks (EPO). This is short compared with expression in muscles, which can be several months (modified from ref. Citation45)
In theory, a longer expression must be expected if other cell types, such as fibroblasts in the dermis, are transfected. Unfortunately there is still no clear evidence of which cells are in fact transfected and thus responsible for the expression seen after transfection. This is probably due to the existence of many different electroporation protocols and hence the targeting of different cell types. If the focus is protein production after gene therapy the level and the duration of the expression achieved is naturally important. However, if the purpose is DNA vaccination instead the concerns about level and duration of an expression may not be so crucial as long as the expected immune response is achieved.
The mouse is the animal most often used in the preclinical studies of gene electrotransfer to skin.Citation26 However when the skin is the focus of transfection, other animals, particular the pig, are better choices. There are two reasons for choosing the porcine model over the mouse: (1) Mouse skin contains many hair follicles and that may in theory have an impact on the expression after electroporation. One reason for the success of transfecting mice skin compared with human skin could be the fact that cells in the hair follicles are very suitable for gene transfection and hence production of the expected response.Citation40 (2) Porcine skin is more similar to human skin, both in texture and composition. As in human skin, which only has a subcutaneous muscle layer in the platysma under the chin and in the male scrotum, the porcine skin does not contain the panniculus carnosus, which is a subcutaneous muscle layer present in many types of animal skin, including rodents. Furthermore porcine skin is thicker and the risk of injecting the plasmid subcutaneously is smaller.
In the preclinical setting the plasmids most often transfected have been encoding reporter proteins such as luciferase, green fluorescent protein (GFP) and β-galactosidase. There are however drawbacks related to all three plasmids: (1) Luciferase is easily measured both in vitro and in vivo but it displays a rather high level of variance, often several logs, which makes it difficult to compare different studies.Citation41 (2) GFP is a fluorescent molecule in the green spectrum, but since the skin itself contains a significant level of autofluorescence, it can be very difficult to distinguish between transfected cells and background fluorescence.Citation25,Citation42 (3) Beta-galactosidase is an enzyme, which converts a substrate, X-gal, to a blue color. There have been reports of false positive staining in porcine skin,Citation25 boneCitation43 and neural tissue,Citation44 which must have an impact on interpreting the results.
The drawbacks of GFP and β-galactosidase are to our knowledge the main reasons for the lack of certainty about which cells are responsible for the expression after gene transfection to skin. Other new markers have to be developed, and studies using e.g., a plasmid encoding Katushka, a fluorescent molecule in the far red area, could be an option.Citation45,Citation46
Gene electrotransfer to tumors
Gene electrotransfer to tumors are mostly aimed at the production of proteins with anticancer effect such as e.g., the antiangiogenic plasmid AMEPCitation47 or transfection with immune-active products such as IL-12.Citation48 The differences, advantages and disadvantages between muscle and skin for gene transfection, are summarized in .
Table 1. Comparison of gene electrotransfer to muscle and skin, from a clinical point of view
Clinical trials with electroporation and DNA plasmids
The first clinical studies with gene electrotransfer to muscle and tumor have been published and more are underway.Citation48 The clinical setting mimics the preclinical and particularly the muscle is the object for DNA vaccinations. Different devices are used for application of the electrical pulses, but mutual for them all is the use of needle electrodes. The muscle is the organ most often used for gene transfection and the choice of this organ for clinical trials is based on robust data. The muscle is a natural ‘protein-factory’ and is able to produce a large amount of protein after transfection with small amount of plasmid.Citation5 The next few years will clarify many difficulties regarding DNA vaccination and help finding the optimal parameters for clinical trials. It is still too early to state, whether the muscle or the skin is the optimal organ for DNA vaccinations; it could be that a combined approach will prove to be the most efficient in eliciting an immune response. summarizes the clinical studies currently running with DNA transfection and electroporation.
Table 2. Clinical trials using electroporation and gene transfection
Pitfalls
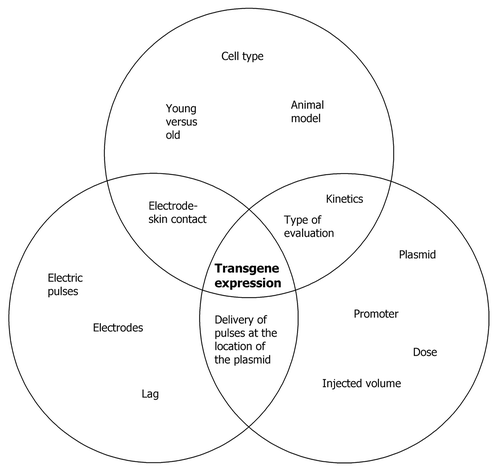
The gene electrotransfer procedure consists of several steps, each of which can result in issues that may decrease the efficiency of the method (summarized in ). The steps of the procedure can be divided in the following parts:
Figure 5. The importance of consistency in the gene electrotransfer procedure. Gene electrotransfer is simple and easy to perform, but is in many aspects a complicated process with many interactions. There are thus many aspects, which need to be evaluated when electroporative delivery fails to induce a response.
Preparation of the plasmid/gene
Present laboratory facilities and the capability to produce GMP DNA for clinical use secure a high level of consistence and stability in the production of the actual gene. It should thus be possible to produce uniform batches and minimize the risk of inter-batch-variability.
Injection of DNA
DNA is a highly viscous and hydrophilic molecule and to obtain an efficient expression it must be injected prior to the electric pulse. For gene transfection different issues exist in different organs. For gene electrotransfer to the skin, in rodents or where the skin is particularly thin, the injection must be performed with utmost care in order to prevent subcutaneous leakage. However, if the injection is made properly and is located in the skin, it is visible as an intradermal bleb and stays there for several minutes
In the muscle conditions are quite different. The muscle consists of muscle cells or fibers situated inside the muscle fascia. Once a liquid or DNA is injected into the muscle it has the possibility of dispersing in the muscle by the length of the fibers. In small animals such as mice it does not have a significant impact on the result, since the muscles are small and can be encompassed by the electrodes. In larger animals and humans the conditions are different with the muscle fibers being much longer. This is why it is suggested that the electrodes are inserted into the tissue initially, followed by injection of the plasmid with the subsequent delivery of the electric pulses.Citation31
There are several commercial products available where the substance, e.g., the plasmid for gene transfection, is transferred into the organ by air or a jet stream.
Injection of a plasmid into e.g., a cutaneous or subcutaneous tumor may present other challenges. Some tumors have a soft structure while others are very hard and difficult to penetrate. The important factor is to use the correct type of needle suitable for the tumor in question and not to inject with too much force, since the plasmid can leak out instead of being inside the tumor.
Electrodes for delivery of the electric pulses
As mentioned previously the electrodes for electroporation consist of different types. However three important issues must be taken into account in order to get optimal transfection: (1) The electric field must encompass the area of the injected plasmid, (2) a proper contact between electrodes and skin must be secured and (3) a homogenous electric field distribution, which ensures that all the cells in the intended area get reversibly electroporated with a low risk of induction of cell death.
The pulse parameters
The pulse parameters are closely related to the electrodes. There is, however, a wide range of possible pulse parameters which can be efficient for gene transfection. For DNA vaccination the main purpose is to transfect the cell and secure that the cell membrane is able to restore the equilibrium afterwards and the electric pulse parameters must hence be appropriate.
Level of expression and evaluation of the response
With respect to protein production it is crucial that the level of circulating protein is high enough to have the intended effect. Quite different is the situation with DNA vaccination. As long as a certain threshold for provoking an immune response has been achieved, there is no need for further production of expressed proteins/antigens. One question is; for how long time is the expression needed to last before a relevant immune reaction has been reached. Also can there be differences between the muscle and skin both in terms of cells transfected (e.g., antigen presenting cells) and duration of expression? Future studies will hopefully provide information on these important questions.
In the preclinical setting differences often exist in how expression is evaluated, whether in terms of protein production or in terms of measuring an immune response. As a consequence it can be difficult to make valid comparisons between studies. Instead of finding unusual ways of analyzing results it is more convenient to make use of solid methods, which make the results more reliable and comparable. The same is valid for the clinical setting, where international guidelines and recommendations for responses must be followed.
Future Perspectives
It has been stated, and reasonably so, that DNA vaccines are one of the future technologies for vaccination. As well electroporation will likely have an important role as a technology to boost DNA vaccine efficacy. Important progress has been made, but at the same time it is a reasonable assumption that further optimization of DNA injection, pulse configuration and electrode geometry may further improve efficacy and at the same time decrease variability.
Looking at vaccination coverage on a global scale, there is still much to be done in order to prevent preventable infectious diseases. In addition, it was recently estimated that one in six cancers worldwide is caused by preventable infectious disease, principally of viral etiology such as hepatitis B and C as well as human papilloma virus.Citation49
Electroporation delivered DNA vaccines may offer the possibility to vaccinate with multiple epitopes, while being relatively cost-effective and not requiring a cold chain. This may, in effect, suggest that one of the most economical and effective prophylactic and therapeutic weapons against a number of diseases may be DNA-based vaccines and therapeutics that are administered through delivery enhancement technologies.
References
- Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356:152 - 4; http://dx.doi.org/10.1038/356152a0; PMID: 1545867
- Rochard A, Scherman D, Bigey P. Genetic immunization with plasmid DNA mediated by electrotransfer. Hum Gene Ther 2011; 22:789 - 98; http://dx.doi.org/10.1089/hum.2011.092; PMID: 21631165
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time?. Nat Rev Genet 2008; 9:776 - 88; http://dx.doi.org/10.1038/nrg2432; PMID: 18781156
- Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 2009; 17:585 - 92; http://dx.doi.org/10.1038/mt.2009.5; PMID: 19223870
- Hojman P, Gissel H, Gehl J. Sensitive and precise regulation of haemoglobin after gene transfer of erythropoietin to muscle tissue using electroporation. Gene Ther 2007; 14:950 - 9; http://dx.doi.org/10.1038/sj.gt.3302951; PMID: 17410179
- Gothelf A, Hojman P, Gehl J. Therapeutic levels of erythropoietin (EPO) achieved after gene electrotransfer to skin in mice. Gene Ther 2010; 17:1077 - 84; http://dx.doi.org/10.1038/gt.2010.46; PMID: 20410932
- Agerholm-Larsen B, Iversen HK, Ibsen P, Moller JM, Mahmood F, Jensen KS, et al. Preclinical validation of electrochemotherapy as an effective treatment for brain tumors. Cancer Res 2011; 71:3753 - 62; http://dx.doi.org/10.1158/0008-5472.CAN-11-0451; PMID: 21507935
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer, Suppl 2006; 4:3 - 13; http://dx.doi.org/10.1016/j.ejcsup.2006.08.002
- Matthiessen LW, Muir T, Gehl J. Electrochemotherapy for larger malignant tumors. In: Kee S, Gehl J, Lee EW, editors. Clinical aspects of electroporation. 1st ed. Springer, 2011: p. 103-14.
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand 2003; 177:437 - 47; http://dx.doi.org/10.1046/j.1365-201X.2003.01093.x; PMID: 12648161
- Vernier PT, Ziegler MJ, Sun Y, Chang WV, Gundersen MA, Tieleman DP. Nanopore formation and phosphatidylserine externalization in a phospholipid bilayer at high transmembrane potential. J Am Chem Soc 2006; 128:6288 - 9; http://dx.doi.org/10.1021/ja0588306; PMID: 16683772
- Gabriel B, Teissié J. Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys J 1997; 73:2630 - 7; http://dx.doi.org/10.1016/S0006-3495(97)78292-4; PMID: 9370457
- Mahmood F. Understanding electric fields for clinical use. In: Kee S, Gehl J, Lee EW, editors. Clinical aspects of electroporation. 1st ed. Springer, 2011: p. 31-44.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev 2003; 29:371 - 87; http://dx.doi.org/10.1016/S0305-7372(03)00073-2; PMID: 12972356
- André FM, Gehl J, Sersa G, Préat V, Hojman P, Eriksen J, et al. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum Gene Ther 2008; 19:1261 - 71; http://dx.doi.org/10.1089/hum.2008.060; PMID: 19866490
- Kinosita K Jr., Tsong TY. Voltage-induced pore formation and hemolysis of human erythrocytes. Biochim Biophys Acta 1977; 471:227 - 42; http://dx.doi.org/10.1016/0005-2736(77)90252-8; PMID: 921980
- Rols MP, Teissié J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys J 1990; 58:1089 - 98; http://dx.doi.org/10.1016/S0006-3495(90)82451-6; PMID: 2291935
- Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci USA 1992; 89:4524 - 8; http://dx.doi.org/10.1073/pnas.89.10.4524; PMID: 1584787
- Saulis G. Pore disappearance in a cell after electroporation: theoretical simulation and comparison with experiments. Biophys J 1997; 73:1299 - 309; http://dx.doi.org/10.1016/S0006-3495(97)78163-3; PMID: 9284298
- Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta 2002; 1569:51 - 8; http://dx.doi.org/10.1016/S0304-4165(01)00233-1; PMID: 11853957
- Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 1999; 96:4262 - 7; http://dx.doi.org/10.1073/pnas.96.8.4262; PMID: 10200250
- Joergensen M, Agerholm-Larsen B, Nielsen PE, Gehl J. Efficiency of cellular delivery of antisense peptide nucleic acid by electroporation depends on charge and electroporation geometry. Oligonucleotides 2011; 21:29 - 37; http://dx.doi.org/10.1089/oli.2010.0266; PMID: 21235293
- Staal LG, Gilbert R. Generators and applicators; equipment for electroporation. In: Kee S, Gehl J, Lee EW, editors. Clinical aspects of electroporation. 1st ed. Springer, 2011: p. 66-202.
- Khan AS, Smith LC, Abruzzese RV, Cummings KK, Pope MA, Brown PA, et al. Optimization of electroporation parameters for the intramuscular delivery of plasmids in pigs. DNA Cell Biol 2003; 22:807 - 14; http://dx.doi.org/10.1089/104454903322625019; PMID: 14683591
- Gothelf A, Mahmood F, Dagnaes-Hansen F, Gehl J. Efficacy of transgene expression in porcine skin as a function of electrode choice. Bioelectrochemistry 2011; 82:95 - 102; http://dx.doi.org/10.1016/j.bioelechem.2011.06.001; PMID: 21724474
- Gothelf A, Gehl J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr Gene Ther 2010; 10:287 - 99; http://dx.doi.org/10.2174/156652310791823443; PMID: 20557284
- Mir LM, Moller PH, André F, Gehl J. Electric pulse-mediated gene delivery to various animal tissues. Adv Genet 2005; 54:83 - 114; http://dx.doi.org/10.1016/S0065-2660(05)54005-7; PMID: 16096009
- Donate A, Coppola D, Cruz Y, Heller R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS ONE 2011; 6:e19181; http://dx.doi.org/10.1371/journal.pone.0019181; PMID: 21559474
- Drabick JJ, Glasspool-Malone J, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther 2001; 3:249 - 55; http://dx.doi.org/10.1006/mthe.2000.0257; PMID: 11237682
- Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther 2007; 7:67 - 77; http://dx.doi.org/10.2174/156652307779940207; PMID: 17305529
- Hojman P. Basic principles and clinical advancements of muscle electrotransfer. Curr Gene Ther 2010; 10:128 - 38; http://dx.doi.org/10.2174/156652310791110994; PMID: 20222860
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS ONE 2011; 6:e19252; http://dx.doi.org/10.1371/journal.pone.0019252; PMID: 21603651
- Hojman P, Spanggaard I, Olsen CH, Gehl J, Gissel H. Calcium electrotransfer for termination of transgene expression in muscle. Hum Gene Ther 2011; 22:753 - 60; http://dx.doi.org/10.1089/hum.2010.209; PMID: 21470044
- Teissié J, Eynard N, Gabriel B, Rols MP. Electropermeabilization of cell membranes. Adv Drug Deliv Rev 1999; 35:3 - 19; http://dx.doi.org/10.1016/S0169-409X(98)00060-X; PMID: 10837686
- Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 1999; 6:508 - 14; http://dx.doi.org/10.1038/sj.gt.3300847; PMID: 10476210
- Durieux AC, Bonnefoy R, Busso T, Freyssenet D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J Gene Med 2004; 6:809 - 16; http://dx.doi.org/10.1002/jgm.534; PMID: 15241788
- Gothelf A, Gehl J. Gene electrotransfer to skin. In: Kee S, Gehl J, Lee EW, editors. Clinical aspects of electroporation. 1st ed. Springer, 2011: p. 189-202.
- Katz AB, Taichman LB. Epidermis as a secretory tissue: an in vitro tissue model to study keratinocyte secretion. J Invest Dermatol 1994; 102:55 - 60; http://dx.doi.org/10.1111/1523-1747.ep12371732; PMID: 8288911
- Vandermeulen G, Richiardi H, Escriou V, Ni J, Fournier P, Schirrmacher V, et al. Skin-specific promoters for genetic immunisation by DNA electroporation. Vaccine 2009; 27:4272 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.05.022; PMID: 19450641
- Gupta S, Domashenko A, Cotsarelis G. The hair follicle as a target for gene therapy. Eur J Dermatol 2001; 11:353 - 6; PMID: 11399544
- Pavselj N, Préat V. DNA electrotransfer into the skin using a combination of one high- and one low-voltage pulse. J Control Release 2005; 106:407 - 15; http://dx.doi.org/10.1016/j.jconrel.2005.05.003; PMID: 15982778
- Tam JM, Upadhyay R, Pittet MJ, Weissleder R, Mahmood U. Improved in vivo whole-animal detection limits of green fluorescent protein-expressing tumor lines by spectral fluorescence imaging. Mol Imaging 2007; 6:269 - 76; PMID: 17711782
- Odgren PR, MacKay CA, Mason-Savas A, Yang M, Mailhot G, Birnbaum MJ. False-positive beta-galactosidase staining in osteoclasts by endogenous enzyme: studies in neonatal and month-old wild-type mice. Connect Tissue Res 2006; 47:229 - 34; http://dx.doi.org/10.1080/03008200600860086; PMID: 16987755
- Sanchez-Ramos J, Song S, Dailey M, Cardozo-Pelaez F, Hazzi C, Stedeford T, et al. The X-gal caution in neural transplantation studies. Cell Transplant 2000; 9:657 - 67; PMID: 11144962
- Gothelf A, Eriksen J, Hojman P, Gehl J. Duration and level of transgene expression after gene electrotransfer to skin in mice. Gene Ther 2010; 17:839 - 45; http://dx.doi.org/10.1038/gt.2010.35; PMID: 20376097
- Hojman P, Eriksen J, Gehl J. In Vivo Imaging of Far-red Fluorescent Proteins after DNA Electrotransfer to Muscle Tissue. Biol Proced Online 2009; 11:253 - 62; http://dx.doi.org/10.1007/s12575-009-9005-0; PMID: 19495913
- Trochon-Joseph V, Martel-Renoir D, Mir LM, Thomaïdis A, Opolon P, Connault E, et al. Evidence of antiangiogenic and antimetastatic activities of the recombinant disintegrin domain of metargidin. Cancer Res 2004; 64:2062 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-03-3272; PMID: 15026344
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008; 26:5896 - 903; PMID: 19029422
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13:607 - 15; http://dx.doi.org/10.1016/S1470-2045(12)70137-7; PMID: 22575588
- Golzio M, Teissie J, Rols MP. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc Natl Acad Sci USA 2002; 99:1292 - 7; http://dx.doi.org/10.1073/pnas.022646499; PMID: 11818537