Abstract
Background: Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) was previously shown to be immunogenic and well tolerated in Malian children. Data on booster vaccination with a fourth consecutive dose of PHiD-CV are available for Europe, Asia and Latin America but are lacking for Africa. The present study evaluated further the safety, reactogenicity and immunogenicity of a fourth consecutive (booster) dose of PHiD-CV.
Results: Low incidences of AEs with grade 3 intensity (2.1% of subjects) were observed. There were no reports of large swelling reactions and serious adverse events. One month post-booster vaccination, for each vaccine pneumococcal serotype, at least 97.8% of subjects had antibody concentrations ≥ 0.2 μg/ml, and at least 97.1% of subjects had opsonophagocytic activity ≥ 8. From pre- to post-booster, a 12.3-fold increase in anti-protein D geometric mean concentration was observed.
Methods: This phase III, open-label study was conducted in Ouelessebougou, Mali, between November 2009 and June 2010. The study population consisted of Malian children previously primed (3 doses) with PHiD-CV in study NCT00678301 receiving a fourth consecutive (booster) dose of PHiD-CV in the second year of life. The incidences of adverse events (AEs) with grade 3 intensity (primary objective) or of any intensity (secondary objective), and the immunogenicity (secondary objective) of the PHiD-CV booster dose were assessed.
Conclusion: A booster dose of PHiD-CV was well tolerated when administered to Malian children in the second year of life and was highly immunogenic for all 10 vaccine pneumococcal serotypes and NTHi protein D. (ClinicalTrials.gov identifier: NCT00985465)
Introduction
Globally, approximately 1.6 million children under 5 years of age die from pneumonia annually, and nearly half of pneumonia-related deaths occur in sub-Saharan Africa.Citation1 To date, donors such as the Global Alliance for Vaccines and Immunization (GAVI) support the introduction of pneumococcal conjugate vaccines in low-income countries in Africa.Citation2
Among circulating pneumococcal serotypes, serotype 1 is one of the most common causes of invasive pneumococcal disease (IPD) and an important cause of pneumonia and empyema in many developing countries, potentially contributing to high mortality rates.Citation3,Citation4
For pneumococcal vaccines, the World Health Organization (WHO) recommends a 3-dose primary vaccination schedule at 6, 10, 14 weeks of age without booster vaccination.Citation5 However, a previous 3-dose primary vaccination study in Gambian infants receiving a 9-valent pneumococcal conjugate vaccine at 6, 10, 14 weeks of age showed that IPD caused by pneumococcal serotype 1 could not be prevented despite high antibody geometric mean concentrations (GMCs) and a high proportion of children with antibody concentrations above 0.35μg/ml.Citation6 The timing of serotype 1 pneumococcal disease further suggests the need for evaluation of a booster dose.Citation7 Since pneumococcal conjugate vaccines (PCVs) have been rolled out in Africa and since the WHO is currently reviewing its vaccination schedule recommendations, an evaluation of booster responses to PCVs in African children in the second year of life could provide some valuable insight.
The 10-valent pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine [PHiD-CV; Synflorix™, GlaxoSmithKline (GSK) Vaccines] contains pneumococcal serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F.
Given the antibiotic resistance of pneumococcal serotype 6A,Citation8 the observed reduction of IPD due to serotype 6A following vaccination with the 7-valent pneumococcal CRM197 conjugate vaccine (7vCRM; Prevenar/Prevnar™, Pfizer Inc.) and the cross-reactivity with serotype 6B in PHiD-CV,Citation9 immune cross-reactive responses against serotype 6A are considered clinical relevant. Similarly, cross-reactive responses against serotype 19A following PHiD-CV vaccination might also be clinically relevant.
A 3-dose primary vaccination course with the PHiD-CV vaccine was previously shown to be well tolerated and immunogenic in Malian and Nigerian infants.Citation10 Various booster vaccination studies with PHiD-CV conducted in Europe, Asia and Latin America have shown that a fourth consecutive (booster) dose was well tolerated and immunogenic in young children.Citation11-Citation19 The objectives of this booster study in Malian children were to assess the safety in terms of adverse events (AEs) with grade 3 intensity (primary objective), AEs of any intensity and the immunogenicity (secondary objectives) of a fourth consecutive (booster) dose of PHiD-CV.
Results
Study population
A total of 158 subjects who completed the three-dose primary vaccination course with PHiD-CV in study NCT00678301 were invited to participate in the present booster study. Of these, 11 subjects who were planned to be enrolled in this booster study did not participate due to the following reasons: parent/guardian could not be contacted (n = 1), parents/guardians not willing to participate (n = 5), or moved out of the study area (n = 5). Out of the 147 remaining children, 141 received a booster dose of PHiD-CV and 140 completed the study. Six subjects did not receive the booster dose of study vaccine due to history of seizures or progressive neurological disorder (n = 5) or heart murmur diagnosed after the primary vaccination course (n = 1). One subject had consent withdrawal not due to an AE. All subjects were part of the total vaccinated cohort (TVC) and the according-to-protocol (ATP) immunogenicity.
The mean age (± Standard Deviation [SD]) at the time of booster vaccination was 17.0 ± 1.11 months and the mean weight (± SD) was 9.30 ± 1.09 kg. All children were African and 53.2% were female.
Reactogenicity and Safety
During the 31-d post-booster vaccination period, at least one AE (solicited and/or unsolicited, local and/or general) was reported for 92.2% of subjects. Events considered causally related to vaccination and those followed by a medically attended visit were reported for 68.1% and 92.2% of subjects, respectively. Concerning the primary endpoint, at least one AE with grade 3 intensity (solicited and/or unsolicited, local and/or general) was reported for 2.1% of subjects.
Swelling was the most frequently reported solicited local symptom (47.5% of subjects). Redness was the only reported grade 3 symptom (1.4% of subjects) (). No large swelling reactions were reported.
Table 1. Incidence of solicited local and general symptoms reported during the 4 d post-vaccination period following PHiD-CV booster dose (total vaccinated cohort)
Incidences of solicited general symptoms were low (≤ 5.7% of subjects), apart from fever which was reported for 24.1% of subjects. There were no reports of solicited general symptoms with grade 3 intensity ().
At least one unsolicited AE was reported for 67.4% of subjects. Allergic bronchitis (27% of subjects) and rhinitis (18.4% of subjects) were the most frequently reported AEs. One unsolicited AE (malaria) with grade 3 intensity was reported for one subject; this was not considered causally related to vaccination. All reported unsolicited AEs were followed by a medically attended visit.
No subject was withdrawn due to an AE. No serious adverse events (SAEs) were reported during the study.
Use of antipyretic medication during the 4 days following booster vaccination was reported for 29.8% of subjects. There were 6 reports (4.3% of subjects) of prophylactic use of antipyretics.
Immunogenicity
Persistence 11–18 months post-primary vaccination
As expected, a decline in antibody GMCs was observed in the time period after primary vaccinationCitation10 and before booster vaccination for all the vaccine pneumococcal serotypes with the exception of serotype 6B. A decline was also observed for the geometric mean titers (GMTs) of opsonophagocytic activity (OPA) with the exception of serotype 7F ().
Figure 2. Kinetics of serotype-specific OPA geometric mean titres (GMTs) for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, post-primary, pre-booster and post-booster vaccination with PHiD-CV (ATP immunogenicity cohort). ATP, according-to-protocol; OPA , opsonophagocytic activity; GMT, geometric mean titre.
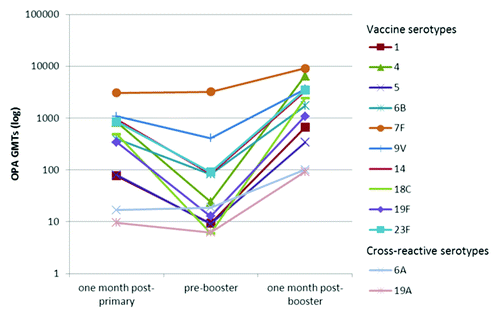
Prior to booster vaccination, for each of the vaccine pneumococcal serotypes, at least 54.3% of subjects had persisting antibody concentrations ≥ 0.20 μg/ml and at least 32.4% had OPA titers ≥ 8 except for serotype 18C (14.7%) ().
Table 2. Percentage of children with antibody concentrations ≥ 0.2 μg/ml (22F-inhibition ELISA) and OPA titers ≥ 8 for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, seropositivity rates and GMCs for anti-protein D antibodies pre- and 1 months post-booster dose (ATP immunogenicity cohort)
Immune response to booster vaccination
Robust increases in antibody GMCs and OPA GMTs were observed one month post-booster vaccination. Depending on the serotype, antibody GMCs and OPA GMTs increased 6.7- to 32.6-fold and 2.8- to 402.2-fold, respectively, compared with pre-booster values. Post-booster antibody GMCs and OPA GMTs exceeded post-primary levels ( and ).
Figure 1. Kinetics of serotype-specific antibody geometric mean concentrations (GMCs, GSK’s 22F-inhibition ELISA) for vaccine pneumococcal serotypes and cross-reactive serotypes 6A and 19A, post-primary, pre-booster and post-booster vaccination with PHiD-CV (ATP immunogenicity cohort). GMC, geometric mean concentration; ATP, according-to-protocol; GSK’s 22F-ELISA, GlaxoSmithKline’s 22F-inhibition enzymelinked immunosorbent assay. Note: Immunogenicity results related to one month post- primary were derived from the primary vaccination study NCT00678301 by re-calculation based on the ATP immunogenicity cohort of the booster study.
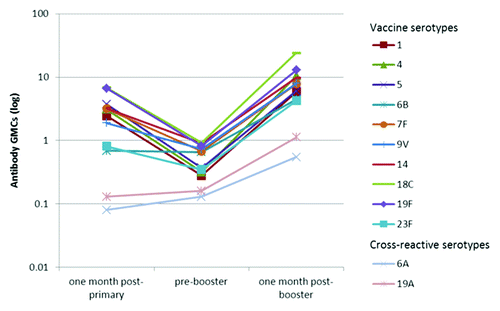
For each of the vaccine pneumococcal serotypes, at least 97.8% of subjects had antibody concentrations ≥ 0.2 μg/ml and at least 97.1% of subjects had OPA titers ≥ 8, one month post-booster vaccination ().
Both IgG antibodies measured with an enzyme-linked immunosorbent assay (ELISA) and OPA against the cross-reactive serotypes 6A and 19A were induced ( and and ).
After the PHiD-CV booster dose, all subjects had measurable antibodies against protein D. The anti-protein D antibody GMC was 3710.1 (95% CI: 3109.0–4427.4) EL.U/ml, with a 12.3-fold increase with respect to the pre-booster time point ().
Discussion
A fourth consecutive (booster) dose of PHiD-CV in the second year of life was shown to be well-tolerated and highly immunogenic in Malian children. The observed safety and reactogenicity profile of a fourth consecutive (booster) dose of PHiD-CV is in line with previous PHiD-CV booster vaccination studies conducted in Europe, Asia and Latin America.Citation11,Citation15-Citation19 The most commonly unsolicited AEs reported were in line with what could be expected in the pediatric age group studied. No SAEs were reported.
The decline in antibody GMCs and OPA GMTs prior to booster vaccination may indicate that subjects could benefit from a booster dose. The robust increases in antibody GMCs and OPA GMTs between pre- and post-booster time points are indicative of adequate priming of the immune system during primary PHiD-CV vaccination. One month after PHiD-CV booster vaccination in the second year of life, for each of the 10 vaccine pneumococcal serotypes, high percentages of children had antibody concentrations ≥ 0.2 μg/ml and an OPA titer ≥ 8. Although some biological effect of protein D against NTHi was previously observed with a candidate 11-valent pneumococcal conjugate vaccine,Citation20 anti-protein D immune responses are difficult to interpret since no serological correlate of protection exists.
The effect of PHiD-CV on cross-reactive serotypes 6A and 19A is not unexpected, given the structural similarity of the serotype specific capsular polysaccharides of cross-reactive serotypes 6A and 19A with polysaccharides from serotypes 6B and 19F contained in PHiD-CV.Citation21,Citation22 Previous experience has shown significant reductions of serotype 6A disease following introduction of 7vCRM not containing serotype 6A, but only 6B. These cross-reactive immune responses might therefore be of clinical significance.
Overall, post-booster immune responses in the present study were higher than those reported in previous booster studies with PHiD-CV, with the exception of OPA booster responses in Asian children.Citation12,Citation14-Citation19 Although regional variations in immune responses to vaccines have been seen previously, the underlying mechanisms are still not fully understood.Citation12,Citation14,Citation16,Citation23-Citation26
A potential limitation of the present booster study includes the fact that assessment of the reactogenicity and safety were not blinded and could be subject to observer bias. Another possible limitation of the study could be the lack of control for the prevalence of antibodies due to environmental transmission and/or exposure, and for the reactogenicity and safety of PHiD-CV since the group of unprimed PHiD-CV subjects of the primary vaccination study NCT00678301 was assigned to receive catch-up vaccination. Nevertheless, it appears unlikely that the lack of a control group would have impacted the conclusions.
In conclusion, a fourth consecutive (booster) dose of PHiD-CV administered to Malian children in the second year of life, was well tolerated and highly immunogenic for all 10 vaccine pneumococcal serotypes and NTHi protein D.
Materials and Methods
Study design and participants
This phase III, open-label, single-center study was conducted in Mali between November 2009 and June 2010. The study population consisted healthy children who were previously primed with PHiD-CV (3 doses) co-administered with DTPw-HBV/Hib and OPV in study NCT00678301 conducted between, June 2008 and December 2009 and who received a booster dose of PHiD-CV at 15–21 months of age.
The study was undertaken according to Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of the Faculty of Medicine, Pharmacy and Dentistry of the University of Bamako. Authorization to conduct the study was obtained from the Ministry of Health. Before enrolment in the study, written informed consent was obtained from the parent(s)/legally acceptable representative(s) [LAR(s)], countersigned by an independent literate witness when the parent(s) /LAR(s) were illiterate.
Vaccines not included in the routine immunization schedule could be given at least one month before or after study vaccine administration. Vaccines recommended through national immunization campaigns were allowed at any time. Vaccines and date of administration were recorded by the study investigator. There was a follow-up visit 1 months post-booster vaccination at 16–22 months of age.
Study vaccine
PHiD-CV (Synflorix™) manufactured by GSK Biologicals S.A., contained 1 μg of each capsular polysaccharide for pneumococcal serotypes 1, 5, 6B, 7F, 9V, 14 and 23F, and 3 μg for serotype 4 conjugated individually to protein D, 3 μg of serotype 18C capsular polysaccharide conjugated to tetanus toxoid, and 3 μg of serotype 19F capsular polysaccharide conjugated to diphtheria toxoid. The vaccine was administered intramuscularly into the deltoid or anterolateral region of the thigh.
Safety and reactogenicity assessment
Local (pain, redness, swelling at the injection site) and general (fever, drowsiness, irritability, loss of appetite) symptoms were actively solicited for 4 days (day 0–3) following vaccination and were evaluated in all subjects using diary cards that were completed by study staff.
Unsolicited AEs were recorded within a 31-d (day 0–30) follow-up period after vaccination and SAEs, defined as any medical event resulting in death, any life-threatening event or any event causing disability, or requiring hospitalization or prolongation of hospitalization, were recorded during the entire study period up to 1 months following booster vaccination. This was done through active questioning by the investigator at each study visit.
For each solicited and unsolicited symptom, medically attended visits defined as hospitalisation, an emergency room visit or a visit to or from medical personnel (medical doctor) for any reason, were recorded.
The intensity of solicited symptoms was graded on a scale from 0 to 3. Pain at the injection site was considered to have a grade 3 intensity if the child cried when the limb was moved/was spontaneously painful, redness and swelling at the injection site if the diameter was > 30 mm and fever if axillary temperature was > 39.5°C. Grade 3 irritability/drowsiness was defined as crying that could not be comforted/prevented normal activity, and loss of appetite was considered grade 3 if the child did not eat at all. Grade 3 intensity for all other AEs was defined as preventing normal everyday activity and/or causing parents/guardians to seek medical advice.
All solicited local symptoms were defined in the protocol to be considered causally related to vaccination. For all other AEs, assessment of causal relationship to vaccination was based on the investigator’s clinical judgment. Use of therapeutic and prophylactic antipyretic medication was recorded within 4 days following booster vaccination. A use of antipyretic was considered to be prophylactic when it was given in the absence of fever and any other symptom, to prevent them from occurring.
Immunogenicity assessment
Blood samples were collected before and one month after vaccination. Serum samples were stored at -20°C until analyzed at GSK Biologicals’ Laboratory (Rixensart, Belgium) and SGS Laboratory (Ghent, Belgium).
GSK Biologicals’ 22F-inhibition ELISA was used to measure anti-pneumococcal serotype-specific total IgG concentrations using a threshold antibody concentration of 0.2 μg/ml, as described previously.Citation27-Citation29
OPA was measured by a pneumococcal killing assay with a cut-off opsonic titer of 8 as described previously.Citation30 Antibodies against NTHi protein D were measured with an ELISA assay developed by GSK Biologicals with an assay cut-off of 100 EL.U/ml. Serological results of blood samples collected one month post-primary vaccination in study NCT00678301 were re-calculated to show kinetics of immune responses over time. Data quality was assured by internal quality control procedures. Additionally, the same laboratory testsCitation27-Citation30 for which quality procedures are in place to ensure stability of the test results were used in the primary and booster vaccination studies.
Statistical analysis
The analysis of safety and reactogenicity was performed on the TVC. The immunogenicity analysis was done on the ATP immunogenicity cohort comprising all children with assay results available and who complied with the procedures and interval for blood sampling as defined in the protocol.
Immunogenicity results one month post-primary were derived from the primary vaccination study NCT00678301 by re-calculation based on the ATP immunogenicity cohort of the booster study.
The sample size was contingent on the number of Malian subjects who received a full vaccination course (i.e., 3-dose primary vaccination) in study NCT00678301 with the PHiD-CV vaccine.
The primary safety endpoint was the occurrence of grade 3 AEs (solicited and/or unsolicited, local and/or general) within 31 days (Day 0–30) after vaccination.
Secondary safety endpoints were occurrences of solicited symptoms (local and general) within 4 days (day 0–3) after vaccination, unsolicited AEs within 31 days (Day 0–30) after vaccination, and SAEs during the entire study period.
Secondary immunogenicity endpoints included concentrations of antibodies and opsonophagocytic titers against vaccine pneumococcal serotypes, cross-reactive pneumococcal serotypes 6A and 19A, and anti-protein D antibody concentrations.
Antibody GMCs, OPA GMTs, and percentages of children reaching the predefined immunological thresholds, were determined with 95% confidence intervals (95% CIs) for each vaccine pneumococcal serotype and cross-reactive serotypes 6A and 19A. Anti-protein D antibody GMCs and seropositivity rates were calculated with 95% CIs.
Incidences of symptoms and AEs were calculated with exact 95% CI.
The statistical analyses were performed using the Statistical Analysis System (SAS) Drug and Development (SDD), web portal version 3.5 with SAS version 9.2.
| Abbreviations: | ||
| AE(s) | = | adverse event (s) |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| DTPw-HBV-IPV/Hib | = | diphtheria-tetanus-whole-cell pertussis hepatitis B virus and Haemophilus influenzae type b vaccine |
| ELISA | = | enzyme-linked immunosorbent assay |
| GAVI | = | Global Alliance for Vaccines and Immunization |
| GMC | = | geometric mean concentration |
| GMT | = | geometric mean titre |
| GSK | = | GlaxoSmithKline |
| IPD | = | invasive pneumococcal disease |
| LAR | = | legally acceptable representative |
| OPA | = | opsonophagocytic activity |
| OPV | = | oral polio vaccine |
| PCV | = | pneumococcal conjugate vaccine |
| PHiD-CV | = | pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine |
| SAE | = | serious adverse event |
| SAS | = | statistical analysis system |
| SD | = | standard deviation |
| 7vCRM | = | 7-valent pneumococcal CRM197 conjugate vaccine |
| TVC | = | total vaccinated cohort |
| WHO | = | World Health Organization |
Acknowledgments
The authors would like to thank the parents and their children who participated in this study and the community leaders of Ouelessebougou. The authors also acknowledge the assistance of all the investigators, study nurses, clinicians, laboratory personnel and other staff members in conducting this study, in particular Nathalie Annez, Veronique Mazarin Diop and Tineke Ryckaert (Harrison Clinical Research Benelux n.v for GlaxoSmithKline Vaccines) for their contribution to the clinical operational aspects of the study at the Sponsor site. Dr. Ann Dhoest (freelance writer for GlaxoSmithKline Vaccines) is acknowledged for drafting the manuscript and Dr. Aneta Skwarek-Maruszewska (XPE Pharma and Science for GlaxoSmithKline Vaccines) for manuscript coordination.
Submitted
07/30/12
Revised
10/19/12
Accepted
10/27/12
Trademark Statement
Synflorix is a trademark of the GlaxoSmithKline group of companies. Prevenar/Prevnar is a trademark of Pfizer, Inc.
Previous Publications
Parts of the results of this study were presented at the 7th World Congress of the World Society for Paediatric Infectious Diseases (WSPID), Melbourne, Australia, November 16–19, 2011.
Authors’ Contribution
AD helped plan the reported study, collected data and provided interpretation of the results. A.D., G.S., A.M., Y.S., A.B., Y.D., A.Diallo, A.Dolo, contributed to the data collection. A.D. and O.D. oversaw the conduct of the study. NF helped design the study, FS and NF did the statistical analyses and interpret the results; L.S., D.B. designed and planned the study, L.S., A.S. and D.B. helped interpret the results. All authors critically reviewed the different drafts of the manuscript and approved the final version.
Financial Disclosure
This study was sponsored by GlaxoSmithKline Biologicals SA. GSK Biologicals was involved in all stages of the study conduct and analysis and took in charge all costs related to the development of this manuscript.
Potential Conflicts of Interest
A.D., A.M., Y.S., A.B., A.Diallo, A.Dolo, G.S., Y.D. and O.D. declare that their institution has received grants from the GlaxoSmithKline group of companies; A.D. has also received travel fees from the GlaxoSmithKline group of companies; AS works as consultant for GlaxoSmithKline Biologicals; L.S., N.F., F.S. and D.B. are employed by the GlaxoSmithKline group of companies, and L.S. and D.B. own stocks.
References
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al, Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375:1969 - 87; http://dx.doi.org/10.1016/S0140-6736(10)60549-1; PMID: 20466419
- Global Alliance for Vaccines and Immunization (GAVI): Advance Market Commitments for Vaccines Update: World's Poorest Children Among First to Receive New Life-Saving Pneumococcal Vaccines. 2010 [http://www.vaccineamc.org/updatemar23_10.html]
- Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005; 5:83 - 93; PMID: 15680778
- Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Hance LF, Reithinger R, et al. Systemic evaluation of serotypes causing invasive pneumococcal disease among children under five: the global serotype project. PLoS Med 2010; 7:10; http://dx.doi.org/10.1371/journal.pmed.1000348
- World Health Organization (WHO). Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec 2007; 82:93 - 104; PMID: 17380597
- Saaka M, Okoko BJ, Kohberger RC, Jaffar S, Enwere G, Biney EE, et al. Immunogenicity and serotype-specific efficacy of a 9-valent pneumococcal conjugate vaccine (PCV-9) determined during an efficacy trial in The Gambia. Vaccine 2008; 26:3719 - 26; http://dx.doi.org/10.1016/j.vaccine.2008.04.066; PMID: 18514974
- Klugman KP, Madhi SA, Adegbola RA, Cutts F, Greenwood B, Hausdorff WP. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine 2011; 29:3372 - 3; http://dx.doi.org/10.1016/j.vaccine.2011.02.089; PMID: 21396901
- Liñares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect 2010; 16:402 - 10; http://dx.doi.org/10.1111/j.1469-0691.2010.03182.x; PMID: 20132251
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368:1495 - 502; http://dx.doi.org/10.1016/S0140-6736(06)69637-2; PMID: 17071283
- Dicko A, Odusanya OO, Diallo AI, Santara G, Barry A, Dolo A, et al. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health 2011; 11:882 - 93; http://dx.doi.org/10.1186/1471-2458-11-882; PMID: 22112189
- Chevallier B, Vesikari T, Brzostek J, Knuf M, Bermal N, Aristegui J, et al. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis J 2009; 28:Suppl S109 - 18; http://dx.doi.org/10.1097/INF.0b013e318199f62d; PMID: 19325447
- Wysocki J, Tejedor JC, Grunert D, Konior R, Garcia-Sicilia J, Knuf M, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009; 28:Suppl S77 - 88; http://dx.doi.org/10.1097/INF.0b013e318199f609; PMID: 19325450
- Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, et al. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009; 28:Suppl S97 - 108; http://dx.doi.org/10.1097/INF.0b013e318199f61b; PMID: 19325452
- Silfverdal SA, Hogh B, Bergsaker MR, Skerlikova H, Lommel P, Borys D, et al. Immunogenicity of a 2-dose priming and booster vaccination with the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine. Pediatr Infect Dis J 2009; 28:e276 - 82; http://dx.doi.org/10.1097/INF.0b013e3181b48ca3; PMID: 20118683
- Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009; 374:1339 - 50; http://dx.doi.org/10.1016/S0140-6736(09)61208-3; PMID: 19837254
- Vesikari T, Karvonen A, Lindblad N, Korhonen T, Lommel P, Willems P, et al. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with measles-mumps-rubella-varicella vaccine in children aged 12 to 16 months. Pediatr Infect Dis J 2010; 29:e47 - 56; http://dx.doi.org/10.1097/INF.0b013e3181dffabf; PMID: 20508478
- Bermal N, Szenborn L, Edison A, Hernandez M, Pejcz J, Majda-Stanislawska E, et al. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr Infect Dis J 2011; 30:69 - 72; http://dx.doi.org/10.1097/INF.0b013e3181f2da06; PMID: 20980933
- Lagos RE, Muñoz AE, Levine MM, Lepetic A, François N, Yarzabal JP, et al. Safety and immunogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Chilean children. Hum Vaccin 2011; 7:511 - 22; http://dx.doi.org/10.4161/hv.7.5.14634; PMID: 21441782
- Kim CH, Kim JS, Cha SH, Kim KN, Kim JD, Lee KY, et al. Response to primary and booster vaccination with 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine in Korean infants. Pediatr Infect Dis J 2011; 30:e235 - 43; http://dx.doi.org/10.1097/INF.0b013e31822a8541; PMID: 21817957
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740 - 8; http://dx.doi.org/10.1016/S0140-6736(06)68304-9; PMID: 16517274
- Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?. BMC Pediatr 2010; 10:4; http://dx.doi.org/10.1186/1471-2431-10-4; PMID: 20122261
- De Wals P, Lefebvre B, Defay F, Deceuninck G, Boulianne N. Invasive pneumococcal diseases in birth cohorts vaccinated with PCV-7 and/or PHiD-CV in the province of Quebec, Canada. Vaccine 2012; 30:6416 - 20; http://dx.doi.org/10.1016/j.vaccine.2012.08.017; PMID: 22921290
- Hoppenbrouwers K, Lagos R, Swennen B, Ethevenaux C, Knops J, Levine MM, et al. Safety and immunogenicity of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual-chamber syringe to infants in Belgium and Chile. Vaccine 1998; 16:921 - 7; http://dx.doi.org/10.1016/S0264-410X(97)00303-4; PMID: 9682338
- Lagos R, Hoffenbach A, Scemama M, Dupuy M, Schodel F, Hessel L, et al. Lot-to-lot consistency of a combined hexavalent diptheria-tetanus-acellular-pertussis, hepatitis B, Inactivated polio and haemophilus B conjugate vaccine, administered to healthy chilean infants at two, four and six months of age. Hum Vaccin 2005; 1:112 - 7; http://dx.doi.org/10.4161/hv.1.3.1848; PMID: 17012870
- Bermal N, Szenborn L, Chrobot A, Alberto E, Lommel P, Gatchalian S, et al. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr Infect Dis J 2009; 28:Suppl S89 - 96; http://dx.doi.org/10.1097/INF.0b013e318199f901; PMID: 19325451
- Lee HJ, Huang LM, Togashi T, Juergens C, Amdekar YK, Kieninger DM, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine: population differences observed in Asian, European, and American paediatric studies. Abstract presented at the 8th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD), Brazil, March 11-15, 2012.
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266 - 72; PMID: 11238206
- Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006; 13:356 - 60; http://dx.doi.org/10.1128/CVI.13.3.356-360.2006; PMID: 16522777
- Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134 - 42; http://dx.doi.org/10.1128/CVI.00289-09; PMID: 19889940
- Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4:415 - 22; PMID: 9220157