Abstract
Italy was one of the first industrialized countries to introduce a program of universal vaccination against hepatitis B in 1991. Twenty years later we verified the impact of universal immunisation on the epidemiology of hepatitis B infection by analyzing the prevalence of specific viral markers (anti-HBs, anti-HBc and HBsAg). The ELISA tests were performed on residual blood samples collected by 0.05% of the resident population aged 1-50 years in Tuscany (Italy). About 63% of subjects aged < 30 years were anti-HBs positive compared to about 25% in older subjects, without differences between genders. About 22% of subjects over 40 years were anti-HBc-positive compared to 5% in the younger age groups. The number of HBsAg-positive subjects was almost 10 fold higher in the unvaccinated age groups than in the cohorts involved in the universal vaccination program. The results of our study show the persisting high anti-HBs reactivity in vaccinated cohorts, while HBV markers related to natural exposure or persistent infection remain remarkably higher in older age groups. This sero-epidemiological study supports with prevalence data the downward incidence trend of acute hepatitis B highlighted by epidemiological surveillance systems, and corroborates the forecast for elimination of hepatitis B in Italy in a few decades.
Introduction
The World Health Organization (WHO) estimates that currently at least 2 billion people have been infected with hepatitis B virus (HBV) worldwide, and more than 350 million have chronic (long-term) liver infections. An estimated 500,000–700,000 people die each year from acute and chronic sequelae of hepatitis B infection, making it a major cause of morbidity and mortality in human beings.Citation1
Hepatitis B is traditionally most prevalent in Asia and Sub-Saharan Africa, in the Amazon Basin, and is less prevalent in the United States, Northern Europe, Australia and parts of South America; the Middle East, some countries of Eastern Europe and the Mediterranean Basin were considered areas of intermediate endemicity. Therefore, hepatitis B results one of the major public health problems in the world. Vaccination is considered the most effective measure to reduce the incidence of HBV infection and to decrease the prevalence of HBV markers (anti-HBc and HBsAg), with a subsequent later positive impact on chronic liver disease.
In recent years, the European Centre for Disease Prevention and Control reported a decrease of new cases of hepatitis B in Western and Eastern Europe.Citation2 Particularly, the reduction of hepatitis B incidence can be explained by the adoption of effective vaccination programs against HBV infection, to a greater emphasis on the use of condoms to prevent sexually transmitted infections, to implementation of health promotion campaigns and to the compliance to universal precautions and use of disposable health devices. As a matter of facts, in 1995, 6.7 cases of hepatitis B per 100,000 inhabitants were reported in Europe; in 2007, that number dropped to 1.5 cases per 100,000 inhabitants.Citation2
Vaccination strategies for control of HBV infection were initially focused on the immunization of high-risk groups (like men who have sex with men, health care workers, intravenous drug users, people with multiple sex partners, people living in households with chronic carriers etc.) and newborns to HBsAg carrier mothers who were screened during pregnancy. In 1992, the WHO recommended that all countries should introduce HBV universal vaccination in their national immunization programs, in addition to immunization of people at increased behavioral or professional risk of exposure to HBV.Citation3,Citation4
Substantial progress has been made in implementing this recommendation worldwide in the last decades, starting from 1% of global vaccination coverage of infants with three doses of hepatitis B vaccine in 1990: by 2010, hepatitis B routine vaccination had been introduced into national infant immunization schedules in 179 countries (including parts of India and the Sudan). Today global hepatitis B vaccination coverage is estimated at 75% and is as high as 91% in the Western Pacific and 89% in the Americas, while in the South-East Asia Region reached 52% in 2010.Citation5 In the WHO European region, 46 out of the 53 countries have implemented universal programss of HBV vaccination.Citation6
In 1991, Italy was one of the first countries to introduce a vaccination policy against hepatitis B, adopting universal immunization of infants and 12-year-old adolescents.Citation7-Citation10 The double cohort strategy of universal immunisation aimed at reducing, and, in the long term, at eliminating the transmission of HBV by rapidly creating 24 generations of immune subjects within the first 12 years of vaccination implementation. Law n.165, which introduced compulsory vaccination against HBV infection, was issued in Italy on 27 May 1991, but it was implemented in the whole country as of the beginning of 1992. According to an “ad hoc” survey, HBV vaccination coverage with three doses, at 24 months of life, increased in some Italian Regions from 63% in the 1991 birth-cohort, to 91-99% in the 1996 birth-cohort.Citation11,Citation12 According to the Italian Ministry of Health database, in the last decade the national HBV vaccination coverage at 24 months of age increased from 94% (2000) to 96% (2011).Citation13
In Italy, the surveillance of acute viral hepatitis relies both on the official notification system of the Ministry of Health, and to the national surveillance system SEIEVA (Epidemiological Integrated System of Acute Viral Hepatitis) run by the National Institute of Heath. Acute hepatitis B showed a robust reduction of incidence, from 11 cases per 100,000 inhabitants reported in 1987 to 0.9 cases per 100,000 inhabitants in 2010. The decrease in incidence was particularly sharp in subjects in the age group 15-24 years. Presently, individuals who develop hepatitis B are mainly males in the age group 35-54 years; 17% of acute HBV infections affect mainly immigrants from Eastern Europe. Moreover, 20 years after the implementation of hepatitis B vaccination in Italy, a generation of children and young adults (involving 30 age-cohorts) is emerging with practically no markers of HBV infection.Citation14-Citation17
Particularly, the decrease of acute hepatitis B cases was evident in some previously hyper-endemic areas of Southern Italy: for example in Afragola, located in Campania Region, the rate of HBsAg-positive subjects among the population was 13.4% before the implementation of HBV vaccination and dropped to 0.9% twenty years after the introduction of vaccination. The prevalence of anti-HBc antibody in the same population and in the same time period decreased from 66.9% to 7.6%.Citation18
In Tuscany (an Italian Region located in the center of the country) the number of reported hepatitis B cases in the resident population decreased from 3.06 cases per 100,000 inhabitants in 1996 to 2.43 cases in 2009, with the most striking reduction in subjects 15-24 years old (from 6.46 to 1.89 cases per 100,000 inhabitants). In the Florence area, the number of cases dropped from 2.96 to 1.73 cases per 100,000 subjects in the same period; also in this province, the decrement was mainly relevant in subjects 15-24 years old.Citation19
The aim of our study was to detect the current rate of susceptible, immune and carrier subjects 20 years after the implementation of HBV universal vaccination in a representative sample of the population living in Tuscany (with particular attention to the difference between vaccinated and non-vaccinated cohorts), by means of qualitative immune-enzymatic tests (ELISA). To do this, we analyzed the prevalence of specific viral markers (anti-HBs, anti-HBc and HBsAg).
Results
The age and gender distribution of 1,071 serum samples (530 females and 541 males) collected from non-hospitalized patients and analyzed in the study are shown in . Particularly, 500 sera were collected from subjects belonging to vaccinated cohort (1–30 years of age) and 570 from subjects of cohorts not involved in the program (31–50 years of age).
Table 1. Demographic data of study population
Prevalence of anti-HBs antibody
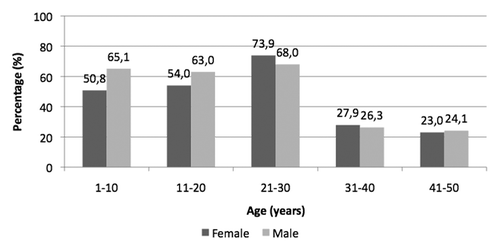
About 43% of tested samples (461/1071) were anti-HBs positive: particularly, 317/501 samples (63.3%) in the vaccinated cohorts (1–30 years of age) and 144/570 samples (25.3%) in the unvaccinated cohorts (31–50 years of age) tested positive for anti-HBs (p < 0.001). ().
Figure 1. Prevalence of anti-HBs reactivity (≥ 10 mIU/ml) in anonymous sera collected from subjects aged 1–50 years living in Florence (year 2009).
Positive results for anti-HBs were comparable by gender in each age group, without statistically significant differences (p > 0.05) ().
Prevalence of anti-HBc antibody
Two serum samples were repeatedly equivocal to anti-HBc, and were excluded in the following analysis. About 9.3% of samples (102/1069) tested positive to anti-HBc: particularly, 27/500 samples (5.4%) in the vaccinated cohorts (1–30 years of age) and 75/569 samples (13.2%) in the non-vaccinated cohorts (31–50 years of age) were anti-HBc positive (p < 0.001).
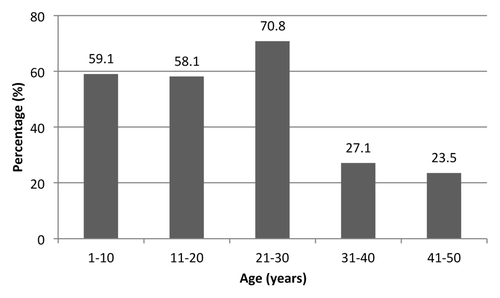
The search for anti-HBc by age groups showed that the first 40 birth-cohorts, divided into four age groups, have a comparable percentage of positive subjects for anti-HBc (ranging from 4.1% to 6.8%). The 41–50 years age group, instead, shows a higher rate of seropositive subjects (21.6%) ().
Figure 3. Prevalence of anti-HBc reactivity in anonymous sera collected from subjects aged 1–50 years living in Florence (year 2009).
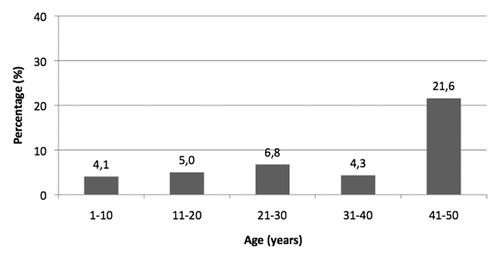
A slight difference in the percentage of positive samples between genders occurred in subjects aged 21–30 years and 41–50 years: prevalence of seropositivity for anti-HBc is higher among males compared to females; however, that difference was not significant (p > 0.05) ().
Positive-negative samples for anti-HBs and anti-HBc antibodies
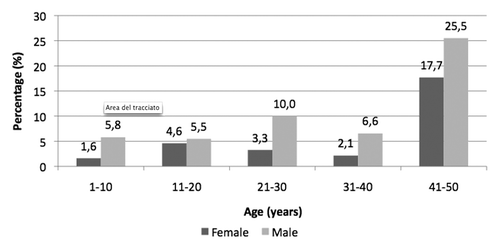
The distribution of positive-negative results for both anti-HBs and anti-HBc were evaluated in vaccinated and unvaccinated cohorts. As underpinned in , the highest rates of anti-HBs+/anti-HBc- subjects were in the following age groups: 1–10 (56.1%), 11–20 (53.8%) and 21–30 years (66.7%). In subjects ≥ 31 years of age, the frequency of anti-HBs+/anti-HBc- samples decreased proportionally to the increase of age (24.5% in 31–40 years age groups and 11.3% in 41–50 years age groups). Conversely, negative samples for both anti-HBs and anti-HBc were mainly in people aged 31–40 years and 41–50 years (71.1% and 67.1%, respectively), while such pattern ranged between 27% and 41% in lower age groups (49.9% between 1 and 10 years, 41.3% in the 11–20 year age group and 26.6% in 21–30 year-olds).
Figure 5. Prevalence of anti-HBs and anti-HBc reactivity in anonymous sera collected from subjects aged 1–50 years living in Florence (year 2009).
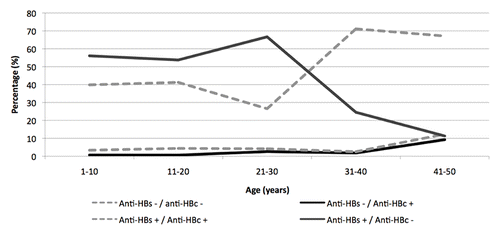
Anti-HBs and anti-HBc positive sera were 3.4% in 1–10 year-olds, 4.4% in the 11–20 year age group, 4.2% between 21-30, 2.5% in those aged 31–40 years; a higher percentage of naturally-immune subjects was found between 41 and 50 years (12.3%).
The rate of subjects who tested negative for the detection of anti-HBs and positive for anti-HBc is higher among 41-50 year-old people (9.2%), while in the younger age groups, the frequency of that pattern ranges between 0.7% and 2.6%.
Prevalence of HBsAg
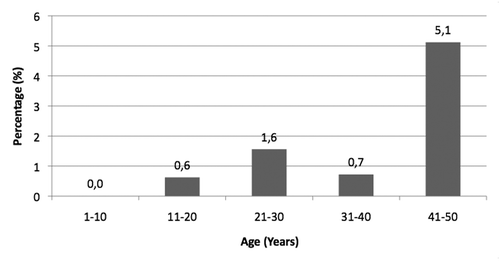
All anti-HBs-/ anti-HBc+ samples (39 samples out of 1071, 3.6% of the study population) were tested for the detection of HBsAg: particularly, 4/501 samples (0.8%) in the vaccinated cohorts (1–30 years of age) and 17/570 samples (3.0%) in the unvaccinated cohorts (31–50 years of age) tested HBsAg positive. About 54% (21/39 samples) of samples specifically analyzed for HBsAg were positive.
HBsAg was found sporadically in the study population (21/1071 samples, 2%) very rarely in younger people: 1 subject in the 11–20 year age group, 3 in those aged 21-30 years, 2 between 31 and 40 years, and 15 in the 41–50 year age group. Particularly, the number of HBsAg-positive subjects was almost 10 fold higher in the unvaccinated compared to vaccinated cohorts ().
Discussion and Conclusion
Italy was one of the first industrialized countries to introduce a routine, simultaneous double-cohort vaccination program against HBV in 1991, before WHO recommended universal immunization in all countries.Citation10 Twenty years after the implementation of this notable immunization activity, an evaluation of the clinical impact due to the adoption of HBV vaccination program in Italy needed to be carried out in order to assess the effects of those impressive efforts.
According to available national epidemiological data, in Italy the introduction of HBV universal vaccination has resulted in a significant decrease of the incidence of acute hepatitis B cases in the last two decades. The reduction involved especially subjects in the age group 15–24 years, who previously were considered the most exposed to the risk of HBV infection. However, the incidence of hepatitis B remains relatively high in unvaccinated cohorts. Also in Tuscany, the introduction of universal hepatitis B vaccination has resulted in a relevant decrease in incidence of hepatitis B cases, especially in the age group 15–24 years.
Undoubtedly, improvements in hygiene conditions, safer blood transfusions, safer sex and use of disposable syringes determined a relevant reduction of HBV infection in the past years in Italy.Citation18,Citation20 However, the incidence of acute hepatitis B cases, which was already declining before 1991, was largely decreased after the introduction of HB universal vaccination program.
The Italian surveillance data on hepatitis B incidence are confirmed by the results of this sero-epidemiological study on prevalence of HBV markers in the Tuscan population. Particularly, the outcomes confirm a high prevalence of immune individuals towards HBV infection in subjects aged 1–30 years, especially due to the implementation of HBV universal vaccination of newborns and adolescents (double cohort program, 1991–2003).
Almost all subjects in the first 30 cohorts of life were theoretically vaccinated at birth or at 12 years of life (given the permanently extremely high vaccination coverage in Italy in both age groups): the sero-epidemiological study shows the higher rate of protective antibody against HBV in those individuals. However, it should be stressed that subjects who tested anti-HBs and anti-HBc negative in the vaccinated cohorts are likely to be all the same immune to HBV thanks to immunological memory. As a matter of fact, subjects who are sero-negative to both anti-HBs and anti-HBc assay are likely to be still immune to HBV infections, since anti-HBs titre could have decreased over time to undetectability. However, a small fraction of anti-HBs negative subjects found in the 1-30 years age group may be represented by immigrants or by people who have not responded to vaccination.
The majority of individuals aged 31–50 years, not involved in the hepatitis B universal vaccination program, have theoretically no vaccine-related anti-HBs and are susceptible to the virus and at risk for HBV infection unless naturally immune. A reported higher frequency of cases of hepatitis B and a higher prevalence of HBsAg in this group (also due to a cohort effect) confirm this hypothesis. In fact, the majority of people in the age group 31–50 years resulted negative for the detection of anti-HBs and anti-HBc. Moreover, in subjects older than 30 years, compared to individuals in vaccinated cohorts, the prevalence of sero-positive individuals for HBsAg test is higher; it is likely that those subjects got infected in the past.
The results of this study can be compared with a similar sero-epidemiological study carried out previously, 10 years after the implementation of HBV vaccination, in the same area: it analyzed the prevalence of anti-HBs reactivity (≥ 10 mIU/ml) in anonymous sera collected in the year 2000 from subjects aged 1–23 years living in Florence.Citation10 Seroprotection of subjects in the 1–4 year age group ranged between 97% and 100% and decreased to 79%-93% in 5–8 years old individuals due to the increasing time interval between vaccination and blood drawing. The percentage of positive subjects to anti-HBs in the 9–11 year age group dramatically dropped (17–50%) due to the fact that such samples belonged to cohorts not yet subject to mandatory immunization. Successively, the rate of seroprotection increased in 12–14 year-olds due to the progressive completion of the vaccination course in adolescent cohorts. The proportion of seropositive subjects was high between 15 and 20 years (79–100%), and markedly decreased in individuals aged 21–23 years (22–50%), who belonged to cohorts not subject to compulsory vaccination. In brief, that study showed the high level of HBV immunity in vaccinated cohorts 10 years after the implementation of HBV vaccination.Citation10
Today, 20 after the adoption of immunization program, all 30 first birth cohorts are largely immune to HBV infection, well correlating with surveillance data of reduction in the incidence of acute hepatitis B cases, especially in the age group 15–24 years. In the unvaccinated cohorts, instead, subjects are mainly susceptible to HBV infection: in fact, in this age groups hepatitis B incidence remains relatively high.
Data from the current study showed a lower percentage of anti-HBs positive subjects compared to the data of the previous study: this difference could be explained with the previous use of HBV monovalent vaccine (until 2003), instead of the hexavalent vaccine used today (since 2001) in the national immunization program. As a matter of fact, several studies showed that the combined DTPa-HBV-IPV-Hib was less immunogenic than the monovalent HB vaccine. However, the combined vaccine induces a lasting immune memory against hepatitis B; therefore, long term protection is likely to be similar to that observed following priming with monovalent HBV vaccines.Citation21-Citation25 At present time, routine booster doses of hepatitis B vaccine do not seem necessary to sustain immunity in children vaccinated with hexavalent vaccines.
In conclusion, hepatitis B is one of the most common liver diseases in the world. In Italy, a clear reduction in the incidence of hepatitis B is reported after the adoption of universal immunization. In particular, a high prevalence of immune subjects to HBV in the age group 1–30 years of life was highlighted. The results of this study show the deep impact that a 20-year vaccination program with initial catch-up of adolescents obtained not only on incidence of acute disease, but also on prevalence of HBV markers in the general population, thus making elimination of hepatitis B from Italy a foreseeable and realistic goal.
Materials and Methods
Collection of serum samples
Serum samples from the general population living in Tuscany were collected from two regional hospitals in Florence (Careggi University Hospital and Anna Meyer Pediatric Hospital). The collected samples are representative not only of the city of Florence, but of the Tuscany Region in general, because individuals from the entire Regional territory apply every day to those structures for diagnosis and care.
All samples were collected in an anonymous way, recording only age, sex and day of collection. Serum samples drawn from subjects of pediatric age were collected from the emergency department of the Pediatric Hospital Meyer, and sera belonging to individuals of pediatric age affected by immunosuppressive conditions were excluded. Serum samples from other older age groups were collected at the outpatient laboratory of the regional Hospital Careggi in Florence and, in this case, only sera withdrawn from outpatients whose blood was taken for routine investigations were included.
A total of 1,071 anonymous residual sera (530 females and 541 males), corresponding to 0.05% of the resident Tuscan population at 1 January 2009 (3.7 million total inhabitants) was collected from March to November 2009, in a proportional number to the age structure of the population.
Serum samples were stratified into the following decade age groups: 1–10, 11–20, 21–30, 31–40 and 41–50 years, maintaining in each year-stratum the same sample size (0.05%) of the respective age group of the general Tuscan population. Serum samples were stored at –20°C until tests were performed.
All 1,071 sera were tested for anti-HBs and total anti-HBc. All samples resulting anti-HBs-negative and anti-HBc-positive were subsequently tested for HBsAg.
The research was carried out in compliance with the Helsinki Declaration.
Enzyme-Linked ImmunoSorbent Assay
The commercial Enzyme-Linked ImmunoSorbent Assay (ELISA) ETI-AB-AUK-3, ETI-AB-COREK PLUS, ETI-MAK-4 (DiaSorin, Italy) were used for detection of anti-HBs, anti-HBc and HBsAg, respectively.
The criteria provided by the producer were applied for the qualitative evaluation of antibodies and antigen detection, according to specific instruction manual.
All equivocal sera were tested twice in order to establish the positive or negative value.
Analysis of assay results
The descriptive analysis of assay results was performed using the Microsoft Excel 2010 software, while the statistical analysis (calculation of chi-square test) was carried out with the Epi Info 3.5 software.
| Abbreviations: | ||
| anti-HBs | = | antibody anti-HBs |
| anti-HBc | = | antibody anti-HBc |
| HBsAg | = | antigen HBs |
Acknowledgements
We thank GlaxoSmithKline SpA, Italy for partially supporting our research with a grant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Health Organization (WHO). Hepatitis B. Immunization, Vaccines and Biologicals. WHO website. Available at: http://www.who.int/immunization/topics/hepatitis_b/en. Last accessed: October 2012.
- European Centre for Disease Prevention and Control (ECDC). Hepatitis B and C – World hepatitis day 2010. ECDC website. Available at: http://www.ecdc.europa.eu. Last accessed: October 2012.
- World Health Organization (WHO). Hepatitis B. Immunization surveillance, assessment and monitoring. WHO website. Available at: http://www.who.int/immunization_monitoring/diseases/hepatitis/en/index.html. Last accessed: October 2012.
- Van Damme P, Kane M, Meheus A, Viral Hepatitis Prevention Board. Integration of hepatitis B vaccination into national immunisation programmes. BMJ 1997; 314:1033 - 6; http://dx.doi.org/10.1136/bmj.314.7086.1033; PMID: 9112852
- World Health Organization/UNICEF. Global Immunization Data. Available to: http://www.who.int/immunization_monitoring/Global_Immunization_Data.pdf. Last accessed: October 2012.
- World Health Organization, Regional Office for Europe. Available at: http://www.euro.who.int/mediacentre/PR/2008/20080421_1. Last accessed: 3 April 2010.
- Law 27 May 1991, n. 165. Obbligatorietà della vaccinazione contro l'epatite virale B. Gazz. Uff. 1° giugno 1991, n. 127.
- Zanetti AR, Tanzi E, Romanò L, Grappasonni I. Vaccination against hepatitis B: the Italian strategy. Vaccine 1993; 11:521 - 4; http://dx.doi.org/10.1016/0264-410X(93)90222-J; PMID: 8488702
- Bonanni P. Implementation in Italy of a universal vaccination programme against hepatitis B. Vaccine 1995; 13:Suppl 1 S68 - 71; PMID: 7571837
- Bonanni P, Pesavento G, Bechini A, Tiscione E, Mannelli F, Benucci C, et al. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine 2003; 21:685 - 91; http://dx.doi.org/10.1016/S0264-410X(02)00580-7; PMID: 12531340
- The Italian Vaccine Coverage Survey Working Group. Childhood vaccination coverage in Italy: results of a seven-region survey. Bull World Health Organ 1994; 72:885 - 95; PMID: 7867134
- Salmaso S, Rota MC, Ciofi Degli Atti ML, Tozzi AE, Kreidl P, ICONA Study Group. Infant immunization coverage in Italy: estimates by simultaneous EPI cluster surveys of regions. Bull World Health Organ 1999; 77:843 - 51; PMID: 10593033
- Ministry of Health. Italy. Infection disease and Vaccination. Vaccination coverage. Ministry of Health website. Available at: http://www.salute.gov.it/malattieInfettive/paginaInternaMenuMalattieInfettive.jsp?id=811&menu=strumentieservizi. Last accessed: October 2012.
- Epicentro, Istituto Superiore di Sanità. Aspetti epidemiologici in Italia. 2011. Available at: http//www.epicentro.iss.it/problem/epatite/EpidemiologiaItalia.asp. Last accessed: October 2012.
- SEIEVA. (Sistema epidemiologico integrato dell’epatite virale acuta). Istituto Superiore di Sanità. SEIEVA website: www.iss.it/seieva/docu/cont. Last accessed: October 2012.
- Romano’ L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liver Dis 2011; 43:Suppl 1 S2 - 7; http://dx.doi.org/10.1016/S1590-8658(10)60685-8; PMID: 21195368
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008; 26:6266 - 73; http://dx.doi.org/10.1016/j.vaccine.2008.09.056; PMID: 18848855
- Da Villa G, Romanò L, Sepe A, Iorio R, Paribello N, Zappa A, et al. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine 2007; 25:3133 - 6; http://dx.doi.org/10.1016/j.vaccine.2007.01.044; PMID: 17280750
- Ministry of Health. Italy. Infection disease and Vaccination. Epidemiological bulletin. Ministry of Health website. Available at: http://www.salute.gov.it/malattieInfettive/paginaInternaMenuMalattieInfettive.jsp?id=812&menu=strumentieservizi. Last accessed: October 2012.
- Mele A, Tosti ME, Mariano A, Pizzuti R, Ferro A, Borrini B, et al, National Surveillance System for Acute Viral Hepatitis (SEIEVA) Collaborating Group. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clin Infect Dis 2008; 46:868 - 75; http://dx.doi.org/10.1086/528687; PMID: 18269332
- Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet JM, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis 2010; 10:9; http://dx.doi.org/10.1186/1471-2334-10-9; PMID: 20078876
- Zinke M, Kappes R, Kindler K, Paulus-Koschik A, Goering U, Disselhoff J, et al. Immune memory to hepatitis B virus in 4-9-year old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin 2009; 5:592 - 8; PMID: 19535920
- Zanetti AR, Romanò L, Giambi C, Pavan A, Carnelli V, Baitelli G, et al, study group. Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis 2010; 10:755 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70195-X; PMID: 20884297
- Romanò L, Zanetti AR. Booster hepatitis B vaccination not necessary for long-term protection in children immunised with hexavalent vaccines. J Prev Med Hyg 2011; 52:45 - 6; PMID: 21842704
- Giambi C, Bella A, Barale A, Montù D, Marchisio M, Oddone M, et al. A cohort study to evaluate persistence of hepatitis B immunogenicity after administration of hexavalent vaccines. BMC Infect Dis 2008; 8:100; http://dx.doi.org/10.1186/1471-2334-8-100; PMID: 18662386