Abstract
This commentary summarizes the laboratory investigations and clinical trials published recently involving per-oral application of IgY supplemented food for specific orogastrointestinal disease prevention and control purposes. The prolonged use and misuse of conventional antibacterial drugs has spawned antibiotic resistant microbes prompting scientists to search for other germ-killing options. In particular, the use of IgY as a novel mode of immunotherapy using oral chicken immunoglobulin (IgY) to confer passive immunity has gained much interest as an inexpensive non-antibiotic alternative for the prophylaxis and treatment of a wide variety of infectious diseases. The stability of IgY in the orogastrointestinal tract and its safety profile has been well-documented. IgY has been used in the treatment or prevention of dental caries, periodontitis and gingivitis, gastritis and gastric ulcer, oral thrush and infant rotavirus diarrhea. The recent clinical trials on IgY with encouraging results has catapulted into the market novel nutraceutical or health supplements for therapeutic or prophylactic intervention based on the consumption of mono-specific or mixed IgY formulations. With recent trends in consumer preference for natural materials to alleviate health concerns, the increasing healthcare costs and the recent advances in drug delivery systems, IgY is likely to shift from its mainly functional food status toward pharmaceuticalization in the foreseeable future.
Introduction
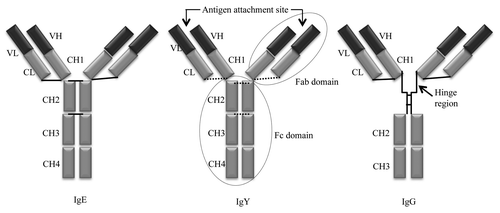
The concept of passive immunity by transferring the specific antibodies from hen to chick via egg for chick protection was first demonstrated by Klemperer in 1893.Citation1 It was in 1969Citation2 that Leslie and Clem coined the term “IgY” to refer to antibodies of poultry including those found in egg yolk. IgY is the major serum antibody of amphibians, reptiles and birds and shares a common ancestor with both mammalian IgG and IgE.Citation3 Among avian IgY, chicken IgY has been the most frequently studied, described and characterized. IgY glycoprotein was first identified by Williams (1962)Citation4 as gamma-globulin in a gamma-livetin fraction of yolk which are produced in egg yolks (10–25 mg/ml) as well as in blood (5–6 mg/ml). The antibody fragment (Fab) domain containing a structure of the IgY with no hinge region, () gives IgY less flexibility to antigen binding with a broad array of antigenic epitopes (e.g., proteins, carbohydrates and nucleic acids). Compared with mammalian IgG, chicken IgY has 3 to 5 times more affinity and reacts more rapidly to the same antigensCitation5-Citation7 when tested in competition assays.
Figure 1. Basic structure of IgY. IgY molecule containing two heavy and two light chains. The heavy chain consists of a variable domain (VH) and four constant domains (CH1, CH2, CH3 and CH4). The two heavy chains are connected by disulfide bonds are shown as solid (known) or dashed (putative) lines. The light chain has one variable domain (VL) and only one constant domain (CL). Fragment antibody (Fab) domain binds to antigenic epitopes, Fragment crystallizable (Fc) domain of IgY has biological effector functions, are circled. The domain structure of IgG shown here with the hinge region represented by a zigzag line linking CH1 and CH2.
Passive immunity is the transfer of active humoral immunity in the form of ready-made antibodies from one individual to another. As such, passive immunotherapy by antigen-specific IgY acquires a special value as a tool for infection control and immunologic research with global commercial application as raw material for nutraceutical and pharmaceutical products and for applications in numerous medical and research fields since the 1980s. Specific IgY antibodies are obtained by immunizing the hen with the antigen of interest. A small amount of antigen in the milligram or microgram range usually elicits enough IgY response and the antibody titers persist over several weeks to several months. The advantages of using chicken IgY have been recognized by many authors.Citation8 Inasmuch as antibiotics are commonly used or misused for the treatment of orogastrointestinal infections, the frequency of antibiotic-resistance organisms has been on the rise at an alarming rate against a backdrop of decreasing numbers of new antibiotics being developed and added to the market. We are therefore compelled to fall back to simple and yet effective natural remedies of which IgY comprises the most potent and easily generated substitute to antibiotics.
IgY immunotherapy has several attractive featuresCitation9 including: (1) lack of reactivity with the human complement system and human Fc-receptors thereby preventing non-specific inflammationCitation10 (2) excludes the use of toxic compounds or additives for their preparation from egg yolks (3) egg cholesterols and triglycerides can be controlled to infinitesimally low levelsCitation11 and (4) IgY exerts beneficial antimicrobial and immuno-stimulatory effects in conjunction with other egg proteins.Citation12 Egg allergies usually involve egg albumin components which may explain why no reactivity issues have been encountered in consumer use of several products now in the market containing purified IgY. Compared with vaccination, passive immunotherapy using IgY has distinct advantages such as: (1) rapid and local onset of action, (2) highly specific activity, (3) applicability to a broader age range of patients from infants to adults including immunodeficient patients and (4) it is nontoxic being a normal part of the human diet. While immunity derived from passive immunization lasts for only a short period of time co-terminous with the presence of antibodies in the recipient, it nonetheless provides immediate and efficient host protection when given in proper concentration onto the target organ.
Discussed in this commentary are salient information pertaining to IgY as a therapeutic and prophylactic regimen including stability and safety issues, as well as the current trend that shows the way forward for future utilization of IgY.
Mechanism of IgY Therapy
Generally the action of orally administered IgY is intended to be achieved within a specific localized site along the alimentary tract, is highly target specific and relies on the largely predictable and usually efficient antigen-antibody interaction. Several mechanisms of action is proposed in host protection: (1) inhibition of microbial adhesion to cell surfaces, (2) suppression of viral colonization by preventing cell-to-cell spread, (3) bacterial agglutination with resulting microbial immobilization and death or ease of being flushed down the gut, (4) inhibition of enzyme activity and (5) neutralization of toxin activity. The cumulated literature on IgY covers in vitro and animal model studies which comprised the foundation upon which current mucosal disease protection among domestic animals were based. The initial success of IgY in animal subjects has provided impetus toward human clinical trials and immunotherapeutic applications.
Effects of Heat, Atmospheric Pressure, pH, Pepsin and Gut Passage on IgY Stability
IgY is the predominant immunoglobulin isotype in chicken egg and as such acts as a major immunoglobulin fraction that confers passive gut immunity. IgY is proteinaceous and is therefore sensitive to heat, pH and pepsin, properties that pose real challenges to its oral application for various digestive disorders. Within the past decade, several studies have been conducted to overcome these problems with various degrees of success. Shin et al. evaluated the heat, pH and pepsin stabilities of anti-Helicobacter pylori IgY (IgY-Hp) ().Citation13
Figure 2. Effect of heat, pH and pepsin on IgY-Hp. IgY-Hp was treated at various temperatures for 10 min (A), at various pHs for 4 h (B) and with pepsin (15 ml/ml) (C) at pH 2, 4 and 6 for 0.5, 1, 2 and 4 h. Remaining activities after the treatments were measured using ELISA and are expressed as a percentage of the initial activity. Adapted with permission from Shin et al.Citation13
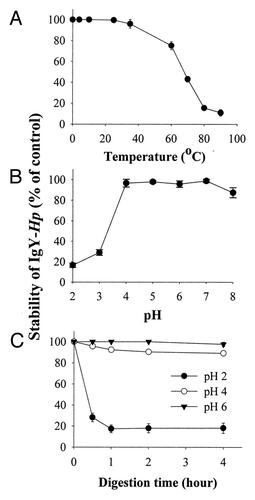
The binding activity of IgY with antigen decreased with increasing temperature and heating time. IgY is stable at temperature ranging between 30°C and 70°C. The activity of IgY decreased by heating for 15 min at 70°C or higher and IgY was denatured significantly when treated at temperatures higher than 75°C. IgY is relatively stable to pressure up to 4,000 kg per cm2. The addition of high levels of sucrose, maltose, glycerol or glycine conferred additional protection against pressure and thermal denaturation of IgY.
The stability of IgY to acid and alkali has been studied under various conditions. It was found that the activity range of IgY for pH was pH 3.5 ~11.The stability of IgY at pH 3 was increased in the presence of sorbitol.Citation14
IgY is quite resistant against trypsin and chymotrypsin inactivation, but degraded by pepsin.Citation15 The stability of IgY against pepsin appears to be highly dependent on pH and the enzyme/substrate ratio. At pH 5 or higher, IgY was fairly resistant to pepsin and retained its antigen-binding and cell-agglutinating activities. However, at pH 4.5 or below, both activities were lost. IgY digested with pepsin at pH 4 retained 91% and 63% of its activity after 1 h and 4 h incubation time, respectively.
Several strategies to protect IgY from hydrolysis by gastric enzymes and acidic condition have been investigated like dissolving in sodium carbonate buffer, encapsulation with liposomes, egg lecithin/cholesterol liposomes and chitosan-alginate. Encapsulated IgY released smoothly in in-vitro studies () and was found to cure enteric colibacillosis in pigs more rapidly than non-coated IgY.Citation16 Encapsulated IgY were more resistant both to pepsin and gastric conditionsCitation17 but the uncoated IgY showed a better effect than the commonly used antibiotic. Another report showed that IgY and freeze-dried IgY coated with gum arabic was protected against hydrolysis by trypsin, chymotrypsin and pepsin.Citation18
Figure 3. In vitro IgY release from IgY loaded microcapsules. Samples were first incubated in stimulated gastric fluid for 2 h, and then transferred to stimulated intestinal fluid for 4 h. The accumulative release percentages was calculated by equation Data are presented as mean SD (n = 3). Adapted with permission from Li et al.Citation16
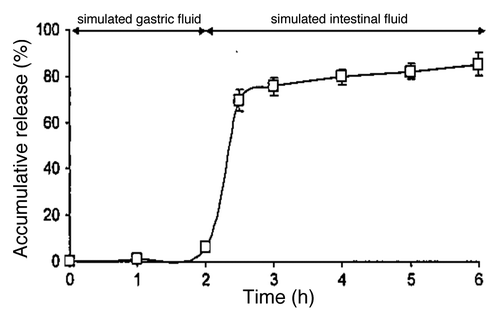
IgY is naturally protected by the yolk granules. The addition of high levels of sucrose, maltose, glycerol or glycine displayed effective additional protection against thermal denaturation of IgY. If encapsulated, they are particularly resistant to pH and digestive enzymes. Encapsulation of IgY with egg lecithin/cholesterol liposomes reduced the activity loss of IgY under gastric conditions. IgY may be stable in 0.9% NaCl, 0.02% NaN3 at 4°C for 20 y without any significant loss of antibody titer. The activity of IgY was also well preserved after freeze-drying. Generally Recognized As Safe (GRAS) status from both the US. Department of Agriculture (USDA) and the Food and Drug Administration (FDA) for IgY has been obtained. Approval of individual products by the FDA for the use of egg antibodies in human patients is relatively easy. Since the activity of IgY was well preserved and easy to apply for human patients, we have started to develop the various food products with this IgY like tablets, pastilles, straws and sachets. This would be easier to handle, both for the patients and for the pharmacy or hospital due to the ease storing IgY at room temperature.
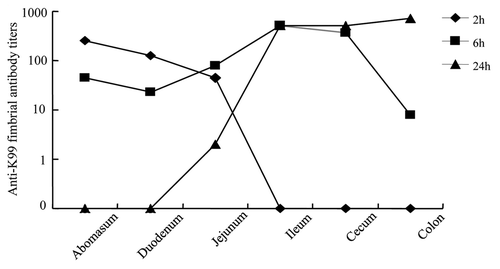
Our group has investigated the in vivo passage and the efficacy of IgY in the gastrointestinal tract of pigletsCitation19 and calves.Citation20 Results indicated that IgY powder was transported as immunologically functional molecules from the stomach down to the small intestine of calves while retaining much of their original biological activity ().
Figure 4. In vivo passage of IgY in the gastrointestinal tract of calves. Anti-K99 fimbriae antibody titers of IgY in the gastrointestinal tract of calves after 2, 6 and 24 h post administration. Adapted with permission from Ikemori et al.Citation20
Safety of Oral IgY
There are several properties that make IgY attractive for oral immunotherapy. While the mouth is the portal of entry for many infectious agents, it is therefore logical to use this as the route for IgY to target specific infectious entities within the alimentary tract. IgY does not pass as intact molecules from the intestines to the blood circulation thus precluding any systemic effect.Citation21 IgY use is associated with much lower risk of inducing specific resistance among pathogenic microorganisms since it is directed to multiple antigenic targets that require multiple genes for their synthesis. Being an ingredient in our regular diet, poultry eggs are considered generally safe. Allergic reactions may occur upon ingestion of egg-derived components particularly those that contain appreciable amount of egg white. However, the water-soluble IgY materials purified from egg yolk (devoid of lipids) are not usually associated with allergic reactions based on our own experience, which conforms to the general perception that egg white materials are the ones responsible for the common egg allergy. The risk of allergy is lower when administering the antibodies orally than by other routes.Citation22 Moreover, oral administration of egg protein (mainly ovalbumin) has been shown to induce systemic tolerance.Citation23
IgY for Prophylactic or Therapeutic use in Human Medicine
Acute microbial orogastrointestinal infections destroy the body’s first line of defense in the alimentary tract, which includes the innate component of the immune response particularly the epithelial barriers, as well as the adaptive mucosal immunity resulting in the development of severe and complicated forms of the infectious disease. Orogastrointestinal infections with various pathogens are mainly treated with antibiotics or antimicrobials. However, a dramatic increase in antibiotic resistance among common bacterial pathogens has impacted negatively impact on the efficacy of antimicrobial chemotherapyCitation24 and is re-shaping the topography of research for novel and alternative infection control modalities. The past decade or so has seen increasing numbers of studies on and use of IgY in the treatment and prevention of infectious diseases in a variety of animal speciesCitation25 as well as in the development of functional food for human application.Citation26 It has been established that oral administration of antimicrobial immunoglobulins derived from bovine milkCitation27 and poultry egg is an effective way to provide protective immunity against a variety of viral or bacterial pathogensCitation28 which might reduce the clinical use of antibiotics and thereby minimize the risk of bacteria developing antibiotic resistance. Compared with bovine milk immunoglobulins, however, IgY has the advantage of being easier and cheaper to produce.
Prophylactic Use of IgY in Dental Caries in Children
Dental caries is one of the most common infectious diseases among children and adolescents affecting up to 90% of the world’s inhabitants.Citation29 The economic burden of the disease is therefore quite high with dental caries costs alone exceeding the total healthcare budget for children in many low-income countries.
Overgrowth of Streptococcus mutans in the oral cavity is recognized as the primary cause of dental caries. Most treatments are now aimed at either elimination of this bacterium or suppression of its virulence. Adverse feedbacks involving potential toxicities and microbial resistance in the use of fluoride and antibiotics for treatment and prevention of dental caries has re-directed efforts toward passive immunotherapy using IgY. Suppression of salivary mutans was achieved with anti-cell-associated glucosyltransferase (anti-CA-GTAse) IgY. In a randomized, double blind, placebo-controlled clinical trial, Nguyen et al.Citation30 reported that lozenges containing anti-CA-GTase IgY can significantly and selectively suppress oral colonization by salivary mutans (). Prior to this, effective local protection against dental caries was achieved with anti-Streptococcus mutans IgY in an animal model.Citation31 In this latter trial, a direct correlation was found between a given IgY dose and a reduction in the incidence of dental caries. Hatta et al.Citation32 evaluated the efficacy of oral IgY anti-S. mutans rinses in human volunteers. This IgY inhibited S. mutans adherence to saliva-coated hydroxyapatite discs by 59%, while the control IgY from non-immunized hens only gave an 8% inhibition. All these results strongly support the efficacy of oral treatments with anti-S. mutans IgY as a valid alternative for preventing dental plaque in humans.
Table 1. Changes in salivary MS scores in volunteers during the trial
Prophylactic Use of IgY in Periodontitis
A review on the effectiveness of mechanical, chemical and antibiotic plaque removal in subjects with periodontal disease has outlined their various degrees of limitations. While oral care products containing chlorhexidine exert anti-plaque effects as indicated by meta-analyses,Citation33 IgE antibodies against chlorhexidine have been detected in the majority of sera from a small group of predominantly Japanese individuals showing anaphylactic-type adverse reactions directed against chlorhexidine. The use of chlorhexidine at a concentration effective for oral care has thus been banned in Japan.
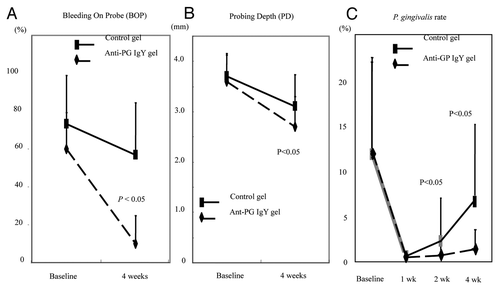
Meanwhile, the emergence of antimicrobial resistance is currently posing a major challenge globally, with an increasing number of strains, including commensal and pathogenic oral bacteria, becoming resistant to commonly used antibiotics. Due to these current limitations, new therapeutic approaches for the control of biofilm are clearly required. The search for adjuncts in biofilm control has led to the exploration of oral passive immunotherapy by IgY as a biological plaque controller. A clinical trial using egg yolk antibody against gingipains (IgY-GP) was performed in five patients with chronic periodontitis. IgY-GP containing ointment was administered directly into the periodontal pocket. Scaling and root planning (SRP) combined with the use of IgY-GP reduced the probing depth, bleeding on probing and levels of P. gingivalis at 4 weeks as compared with SRP only ().Citation34 Sugano N also investigated the effect of IgY-GP on periodontitis by IgY-GP supplemented tablets in 42 patients after scaling and root planing employing a double-blind placebo-controlled approach.Citation35 A significant improvement in mean probing depth was noted in the IgY-GP group at 12 weeks after therapy. Parallel to the clinical changes, the number of P. gingivalis cells in subgingival plaque from the deepest pocket was significantly reduced. These results indicated that daily administration of tablet containing IgY-GP, in conjunction with scaling and root planing, in patients produced significantly better clinical and microbiological results.
Figure 5. Effect of Anti-gingipain IgY on clinical parameters in the periodontitis patients: (A) Bleeding On Probe (BOP) (B) Probing Depth (PD) (C) P. gingivalis rate (P. gingivalis/Total) (%). Adapted with permission from Yokoyama et al.Citation34
Prophylactic Use of IgY in Oral Candidiasis
Candidiasis is one of the most common oral fungal infections in patients with impaired immune system and has a high morbidity with approximately 85% of patients being infected at some point during the course of their illness. Treatment of oral candidiasis is relatively simple and effective for the healthy patient. Typically, topical medications are adequate usually involving the use of a commonly prescribed anti-fungal agent, nystatin oral suspension. To be effective, topical medications must be in contact with the organism to eliminate it. Since patients are usually unable to hold liquids in their mouth cavity, antibiotic-supplemented lozenge tablets are used wherein the tablet dissolves slowly allowing the drug to be present for a longer length of time in the oral cavity.
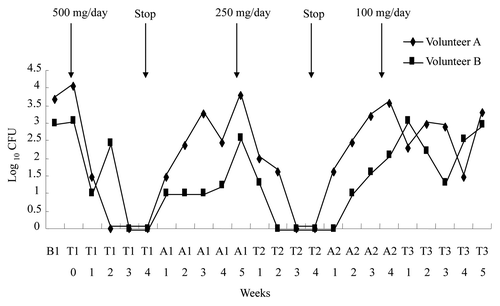
From another perspective, development of cross resistance has primarily been a problem with fluconazole in AIDS treatment. Inasmuch as antibiotic resistance correlates with clinical failure, oral passive immunization with IgY for the control of oral candidiasis acquires a special relevance. Toward this end, a clinical trial was performed by our group in 2 healthy elderly volunteer subjects. A tablet supplemented with egg yolk antibody against Candida albicans (CA-IgY) was prescribed for 4 weeks daily and treatment was stopped for 4 weeks. This treatment cycle was repeated 3 more times with both subjects being examined every week. The patient who received tablets containing anti-CA IgY revealed reduced the number of salivary Candida CFU. With each 4-week pause in treatment, Candida albicans count gradually increased to previous level ()Citation36 indicating that the mode of action was specific for the anti-CA IgY and reduction of Candida CFU is feasible with regular treatment.
Figure 6. Effect of Anti-CA IgY on growth of C. albicans in saliva in volunteer study. B1 = Before treatment; T1 = First time treatment; A1 = Stop treatment after T1; T2 = Second time treatment; A2 = Stop treatment after T2; T3 = Third time treatment. Adapted with permission from Ibrahim et al.Citation36
IgY Use on Treatment of Helicobacter pylori-Infected Patients
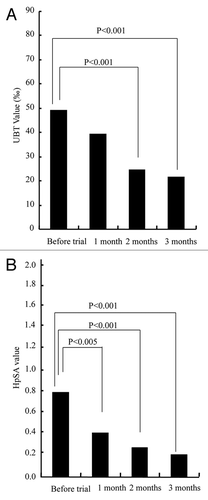
Helicobacter pylori infection may lead to gastric cancer which is the fourth most common cancer and second leading cause of cancer-related deaths worldwide.Citation37H. pylori infects approximately 50% of the world’s inhabitants and the number of newly diagnosed cases was calculated as 750,000 persons per year. H. pylori is the first bacterium to be classified as a class 1 carcinogen by the World Health Organization. Eradication of H. pylori infection both in animal models and in human subjects invariably fails when using an antibiotic as a monotherapeutic regimen even when the organism is susceptible to said antibiotic in vitro. Current first-line treatment regimens generally employ a potent acid-suppressing agent plus two antibiotics (such as amoxicillin, metronidazole, or tetracycline) but this approach is also associated with a variety of problems including induction of antimicrobial resistance and high cost of treatment. Recently, IgY have been used successfully to reduce H. pylori colonization and diminish the severity of mucosal inflammation in the stomach in a mouse model of infection.Citation38 In a clinical trial performed in 16 volunteers and designed to evaluate the protective effect of a yogurt drink fortified with anti-H. pylori urease IgY, values of urea breath and H. pylori stool antigen (HpSA) among the treatment group decreased significantly ().Citation39 Interestingly, the number of volunteers with complaint of gastric pain decreased over the three-month treatment period. Although reduction and not elimination of H. pylori load upon oral treatment with IgY against H. pylori urease was observed, such reduction may have been enough to improve the quality of life of H. pylori infected patients. Another clinical studyCitation40 on anti-urease IgY involving 42 volunteers revealed significant reduction in urea breath values among patients in the treated group. Protection by anti-H. pylori IgY has also been investigated in animalsCitation41 and humans.Citation42 Some studies on anti-H. pylori IgY in animals also demonstrated a prophylactic effect. While IgY does not bring about a total eradication of H. pylori, it may serve as an adjunct to standard treatment of H. pylori infection.
Figure 7. Effect of Anti-HP IgY on Average value of: (A) UBT (carbon urea breath test) and (B) Stool antigen detection in volunteer study. Adapted with permission from Yamane et al.Citation39
IgY in the Treatment of Intestinal Infections
Intestinal infections are considered to be a public health problem of global importance by the World Health Organization and also the major cause of morbidity and mortality, particularly among neonates and immunocompromised patients. Cases of gastroenteritis reported annually worldwide are attributable to a broad spectrum of viral, bacterial and protozoan pathogens. In particular, the children, the elderly and people with compromised immune system are at the receiving end of these infectious diseases. The World Health Organization (WHO) estimates that diarrhea due to gastrointestinal infection is one of the leading causes of deaths worldwide. Among the etiologic agents of diarrhea, rotavirus is the most important being responsible for over two million diarrhea episodes among infants with 600,000 deaths annually, mainly in developing countries.Citation43 Although mortality rate from diarrhea have decreased, morbidity rates remain high. Several independent guidelines based on systematic reviews of the best available evidence related to rotavirus vaccination of infants and to the management of acute gastroenteritis among infants and young children were recently published.Citation44,Citation45 There is agreement in the scientific community that antimicrobials should not be routinely administered to children with gastroenteritis. The uptrend in the frequency of antibiotic-resistant bacteria, the widespread treatment of diarrhea with antimicrobials that sometimes do not respond to antibiotics, and the increasing number of immuno-compromised individuals has prompted much research into alternative approaches to management of diarrhea.
The oral administration of IgY specific for any of the causative agents of diarrhea has proved successful for treatment of a variety of gastrointestinal infections. Considering that patients afflicted with uncomplicated viral infections will not benefit much from antimicrobials, IgY against specific enteric viral infections emerges as a fitting adjunct to standard supportive treatment of such infectious diarrhea. It has been shown that specific IgY against rotaviral antigens are able to inhibit in vitro adhesion of this virus to intestinal epithelial cells. The efficacy of IgY in the treatment of acute rotaviral gastroenteritis in infants and children has been investigated in a randomized double blind clinical trial.Citation46 In this study, researchers evaluated the therapeutic efficacy of IgY specific to human rotavirus, in children with proven rotavirus diarrhea. They observed a modest improvement of diarrhea in association with IgY therapy in the form of reduction of stool volume and earlier clearance of rotavirus from stool in children indicating a potential role of chicken IgY in the management of this infection. Recently, our group evaluated the anti-human rotavirus IgY with higher antibody titer as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients.Citation47 Two natural HRV reassortant clinical strains were used as mixed immunizing antigens to generate anti-HRV IgY (Rotamix IgY). Rotamix IgY exhibited multiserotypic cross neutralization activities along with synergistic effects against major global serotypes G1, G2, G3, G4 in vitro.
Out of 114 children with diarrhea upon admission in a Myanmar hospital, 52 dehydrated and rotavirus-positive children were randomized into Rotamix IgY group and placebo IgY group with n = 26 children per study group. Ninety-two percent of patients in each of these groups were positive for co-infecting enteric non-cholera pathogens and all patients received standard supportive therapy for diarrhea.
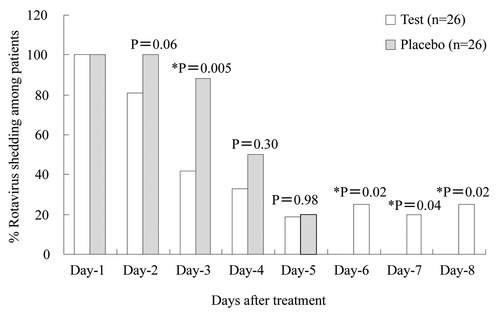
Significant reduction in principal outcomes of diarrhea () i.e., mean ORF intake (p = 0.004), mean duration of intravenous fluid administration (p = 0.03), mean duration of diarrhea from day of admission (p < 0.01), mean stool frequency, mean duration of rotavirus clearance from stool from day of admission (p = 0.05) and frequency of rotavirus shedding () were significantly associated with the IgY intervention group in contrast to the placebo group. These significant observations may translate into real benefits in terms of earlier termination of IV fluid by 3 d, earlier recovery from diarrhea by 2 d, and earlier cessation of rotavirus shedding via stool by 1 d (). Overall, our novel approach using oral Rotamix IgY for rotavirus-infected children mostly with non-cholera enteric pathogen co-infection appears to be a promising, safe and effective adjunct to management of acute diarrhea in pediatric patients.
Table 2. Comparative analysis of Rotamix IgY and placebo effects on study groups according to outcome measures
Figure 8. Effect of Rotamix IgY on daily frequency of rotavirus shedding in stools of children. *Significant differences between Rotamix IgY and placebo IgY groups (*p ≤ 0.05, chi-square test). Adapted with permission from Rahman et al.Citation47
Conclusion
IgY is an effective immunologic tool to fight infection, involving microbes colonizing the alimentary tract of humans. It is relatively safe being a functional foodstuff found in the daily human diet, and is able to exert its activity within the entire length of the alimentary tract in predictable fashion. Oral administration of IgY has been successfully used to prevent or treat specific diseases including dental caries (Streptococcus mutans), E. coli-induced diarrhea, infant rotavirus diarrhea, gastritis (H. pylori), periodontitis (P. gingivalis) and oral candidiasis (C. albicans). With a mechanism of action that depends on direct intermolecular contact, specific IgY has been shown to reduce bacterial or viral load and their accompanying symptoms. While it may not exert total microbial eradication, it may significantly reduce infectious pathogen load to a point where the patient's own immunity can finish the job of host protection. A major force that is drawing more and more attention to IgY's reliable and customizable anti-microbial mechanism is the gloomy prospect in the long-term fight against pathogenic microbes whose resistance to many antimicrobials is thwarting current treatment efforts. Together with other developments in recent antimicrobials and chemotherapeutic research, IgY has the potential to play a contributory role in delaying the advent of the dreaded post-antibiotic era.
Future Directions
Within the biopharmaceutical disciplines, the IgY technology constitutes a relatively novel field that started to draw serious attention only about more than two decades ago with pioneering efforts largely coming from Japan. With the availability of IgY products as functional foodstuffs delivered via lozenge tablets or food carriers such as fermented milk product in the past decade in the Japanese and other East Asian markets, the practical impact of IgY for human application has created ripples in the biomedical sphere, with its broad potential only now starting to unfold. The relatively low cost of producing antibodies from poultry eggs is an attractive side of IgY technology. The need for an inexpensive alternative to anti-infectious regimens has become even more urgent with sharply escalating costs of healthcare, the prospect of an aging population in many industrialized and newly developed countries and scarcity of financial resources among Third World economies. Likewise, the current trend among consumers shifting from synthetics to natural materials to alleviate medical concerns has provided further impetus to the growth of the IgY market. As a functional foodstuff, IgY is well positioned to expand its niche in both pharmaceutical and dietary supplement areas. With the expected application of advances in drug delivery systems for IgY delivery, IgYs are destined for pharmaceuticalization and are expected to devolve toward other important clinical targets including microbial toxins or other high value targets such as metabolic syndrome.Citation48
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Klemperer F. Ueber natürliche Immunität und ihre Verwerthung für die Immunisirungstherapie. Arch Exp Path Pharm 1893; 31:356 - 82; http://dx.doi.org/10.1007/BF01832882
- Leslie GA, Clem LW. Phylogeny of immunoglobulin structure and function. J Exp Med 1969; 130:1337 - 52; http://dx.doi.org/10.1084/jem.130.6.1337; PMID: 5352783
- Taylor AI, Gould HJ, Sutton BJ, Calvert RA. Avian IgY binds to a monocyte receptor with IgG-like kinetics despite an IgE-like structure. J Biol Chem 2008; 283:16384 - 90; http://dx.doi.org/10.1074/jbc.M801321200; PMID: 18400746
- Williams J. Serum proteins and the livetins of hen’s-egg yolk. Biochem J 1962; 83:346 - 55; PMID: 14040270
- Ikemori Y, Peralta RC, Kuroki M, Yokoyama H, Kodama Y. Research note: avidity of chicken yolk antibodies to enterotoxigenic Escherichia coli fimbriae. Poult Sci 1993; 72:2361 - 5; http://dx.doi.org/10.3382/ps.0722361; PMID: 7906035
- Lemamy GJ, Roger P, Mani JC, Robert M, Rochefort H, Brouillet JP. High-affinity antibodies from hen’s-egg yolks against human mannose-6-phosphate/insulin-like growth-factor-II receptor (M6P/IGFII-R): characterization and potential use in clinical cancer studies. Int J Cancer 1999; 80:896 - 902; http://dx.doi.org/10.1002/(SICI)1097-0215(19990315)80:6<896::AID-IJC16>3.0.CO;2-J; PMID: 10074924
- Stuart CA, Pietrzyk RA, Furlanetto RW, Green A. High affinity antibody from hen’s eggs directed against the human insulin receptor and the human IGF-I receptor. Anal Biochem 1988; 173:142 - 50; http://dx.doi.org/10.1016/0003-2697(88)90171-6; PMID: 2973262
- Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz-Asplund J, Terzolo HR. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim 2005; 33:129 - 54; PMID: 16180988
- Nguyen HH, Tumpey TM, Park HJ, Byun YH, Tran LD, Nguyen VD, et al. Prophylactic and Therapeutic Efficacy of Avian Antibodies Against Influenza Virus H5N1 and H1N1 in Mice. PLoS ONE 2012; 7:e42788; PMID: 22880110
- Larsson A, Bålöw RM, Lindahl TL, Forsberg PO. Chicken antibodies: taking advantage of evolution--a review. Poult Sci 1993; 72:1807 - 12; http://dx.doi.org/10.3382/ps.0721807; PMID: 8415358
- Nilsson E, Hanrieder J, Bergquist J, Larsson A. Proteomic characterization of IgY preparations purified with a water dilution method. J Agric Food Chem 2008; 56:11638 - 42; http://dx.doi.org/10.1021/jf802626t; PMID: 19053374
- Kovacs-Nolan J, Phillips M, Mine Y. Advances in the value of eggs and egg components for human health. J Agric Food Chem 2005; 53:8421 - 31; http://dx.doi.org/10.1021/jf050964f; PMID: 16248532
- Shin JH, Yang M, Nam SW, Kim JT, Myung NH, Bang WG, et al. Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection. Clin Diagn Lab Immunol 2002; 9:1061 - 6; PMID: 12204960
- Lee KA, Chang SK, Lee YJ, Lee JH, Koo NS. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J Biochem Mol Biol 2002; 35:488 - 93; http://dx.doi.org/10.5483/BMBRep.2002.35.5.488; PMID: 12359091
- Hatta H, Tsuda K, Akachi S, Kim M, Yamamoto T, Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosci Biotechnol Biochem 1993; 57:1077 - 81; http://dx.doi.org/10.1271/bbb.57.1077; PMID: 7764069
- Li XY, Jin LJ, Uzonna JE, Li SY, Liu JJ, Li HQ, et al. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY): in vivo evaluation in a pig model of enteric colibacillosis. Vet Immunol Immunopathol 2009; 129:132 - 6; http://dx.doi.org/10.1016/j.vetimm.2008.12.016; PMID: 19150135
- Shimizu M, Miwa Y, Hashimoto K, Goto A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci Biotechnol Biochem 1993; 57:1445 - 9; http://dx.doi.org/10.1271/bbb.57.1445; PMID: 7764217
- Chang HM, Ou-Yang RF, Chen YT, Chen CC. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY). J Agric Food Chem 1999; 47:61 - 6; http://dx.doi.org/10.1021/jf980153u; PMID: 10563850
- Yokoyama H, Peralta RC, Sendo S, Ikemori Y, Kodama Y. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am J Vet Res 1993; 54:867 - 72; PMID: 8323054
- Ikemori Y, Ohta M, Umeda K, Peralta RC, Kuroki M, Yokoyama H, et al. Passage of chicken egg yolk antibody treated with hydroxypropyl methylcellulose phthalate in the gastrointestinal tract of calves. J Vet Med Sci 1996; 58:365 - 7; http://dx.doi.org/10.1292/jvms.58.365; PMID: 8741272
- Losonsky GA, Johnson JP, Winkelstein JA, Yolken RH. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis. A pharmacokinetic and functional analysis. J Clin Invest 1985; 76:2362 - 7; http://dx.doi.org/10.1172/JCI112248; PMID: 4077983
- Russo M, Nahori MA, Lefort J, Gomes E, de Castro Keller A, Rodriguez D, et al. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol 2001; 24:518 - 26; PMID: 11350820
- Matsunaga Y, Wakatsuki Y, Tabata Y, Kawasaki H, Usui T, Yoshida M, et al. Oral immunization with size-purified microsphere beads as a vehicle selectively induces systemic tolerance and sensitization. Vaccine 2000; 19:579 - 88; http://dx.doi.org/10.1016/S0264-410X(00)00120-1; PMID: 11027824
- Carlander D, Kollberg H, Wejåker PE, Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res 2000; 21:1 - 6; http://dx.doi.org/10.1385/IR:21:1:1; PMID: 10803878
- Xu Y, Li X, Jin L, Zhen Y, Lu Y, Li S, et al. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol Adv 2011; 29:860 - 8; http://dx.doi.org/10.1016/j.biotechadv.2011.07.003; PMID: 21787857
- Schade R, Zhang XY, Terzolo HR. Use of IgY Antibodies in Human and Veterinary Medicine. Bioactive Egg Compounds 2007;213-22
- Korhonen H, Marnila P, Gill HS. Bovine milk antibodies for health. Br J Nutr 2000; 84:Suppl 1 S135 - 46; http://dx.doi.org/10.1017/S0007114500002361; PMID: 11242458
- Narat M. Production of antibodies in chickens. Food Technol Biotechnol 2003; 41:259 - 67
- World Health Organization. Oral Health Fact Sheet No. 318. http://www.who.int/mediacentre/factsheets/fs318/en/print.html 2007, Accessed on November 9, 2010.
- Nguyen SV, Icatlo FC Jr., Nakano T, Isogai E, Hirose K, Mizugai H, et al. Anti-cell-associated glucosyltransferase immunoglobulin Y suppression of salivary mutans streptococci in healthy young adults. J Am Dent Assoc 2011; 142:943 - 9; PMID: 21804061
- Krüger C, Pearson SK, Kodama Y, Vacca Smith A, Bowen WH, Hammarström L. The effects of egg-derived antibodies to glucosyltransferases on dental caries in rats. Caries Res 2004; 38:9 - 14; http://dx.doi.org/10.1159/000073914; PMID: 14684971
- Hatta H, Tsuda K, Ozeki M, Kim M, Yamamoto T, Otake S, et al. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans.. Caries Res 1997; 31:268 - 74; http://dx.doi.org/10.1159/000262410; PMID: 9197932
- Sugano N. Biological plaque control: novel therapeutic approach to periodontal disease. J Oral Sci 2012; 54:1 - 5; http://dx.doi.org/10.2334/josnusd.54.1; PMID: 22466880
- Yokoyama K, Sugano N, Shimada T, Shofiqur RA, Ibrahim SM, Isoda R, et al. Effects of egg yolk antibody against Porphyromonas gingivalis gingipains in periodontitis patients. J Oral Sci 2007; 49:201 - 6; http://dx.doi.org/10.2334/josnusd.49.201; PMID: 17928726
- Sugano N. Adjunctive effects of anti-Porphyromonas gingivalis egg yolk antibody with scaling and root planning: a randomized, placebo-controlled clinical trial. J Periodontol 2009; 80:1901 - 3
- Ibrahim EM, Rahman AKMS, Isoda R, Umeda K. SA NV, Kodama Y, Maeda N. Anti-Candida Albicans Egg Yolk Immunoglobulin: Cross Activity and Pilot Study. The 55th Annual meeting of Japanese Association for Dental Research (JADR), 2007, November 17~18,Yokohama, Japan.
- Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010; 14:249 - 58; PMID: 20496531
- Ameri Shah Reza M, Mousavi Gargari SL, Rasooli I, Jalali Nadoushan M, Ebrahimizadeh W. Inhibition of H. pylori colonization and prevention of gastritis in murine model. World J Microbiol Biotechnol 2012; 28:2513 - 9; http://dx.doi.org/10.1007/s11274-012-1059-5; PMID: 22806157
- Yamane T, Saito Y, Takizawa S, Goshima H, Kodama Y, Horie N, Kim M.. Development of anti-Helicobacter pylori urease IgY and its application for food product. Food and Development 2003; 38
- Horie K, Horie N, Abdou AM, Yang JO, Yun SS, Chun HN, et al. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J Dairy Sci 2004; 87:4073 - 9; http://dx.doi.org/10.3168/jds.S0022-0302(04)73549-3; PMID: 15545368
- Nomura S, Suzuki H, Masaoka T, Kurabayashi K, Ishii H, Kitajima M, et al. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2005; 10:43 - 52; http://dx.doi.org/10.1111/j.1523-5378.2005.00290.x; PMID: 15691314
- Suzuki H, Nomura S, Masaoka T, Goshima H, Kamata N, Kodama Y, et al. Effect of dietary anti-Helicobacter pylori-urease immunoglobulin Y on Helicobacter pylori infection. Aliment Pharmacol Ther 2004; 20:Suppl 1 185 - 92; http://dx.doi.org/10.1111/j.1365-2036.2004.02027.x; PMID: 15298626
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/10.3201/eid0905.020562; PMID: 12737740
- Guarino A, Dupont C, Gorelov AV, Gottrand F, Lee JK, Lin Z, et al. The management of acute diarrhea in children in developed and developing areas: from evidence base to clinical practice. Expert Opin Pharmacother 2012; 13:17 - 26; http://dx.doi.org/10.1517/14656566.2011.634800; PMID: 22106840
- Szajewska H, Dziechciarz P. Gastrointestinal infections in the pediatric population. Curr Opin Gastroenterol 2010; 26:36 - 44; http://dx.doi.org/10.1097/MOG.0b013e328333d799; PMID: 19887936
- Sarker SA, Casswall TH, Juneja LR, Hoq E, Hossain I, Fuchs GJ, et al. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr 2001; 32:19 - 25; http://dx.doi.org/10.1097/00005176-200101000-00009; PMID: 11176319
- Rahman S, Higo-Moriguchi K, Htun KW, Taniguchi K, Icatlo FC Jr., Tsuji T, et al. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine 2012; 30:4661 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.04.091; PMID: 22575165
- Hirose M, Ando T, Umeda K, Kodama Y, Rahman S, Nagaoka S. Effect of anti-lipase IgY on obesity. The 66th Annual meeting of Japan society of nutrition and food science, 2012, May 18~20, Sendai, Japan. http://eishok66.umin.jp.