Abstract
Although great efforts have been undertaken for the development of malaria vaccines, no completely effective malaria vaccines are available yet. Despite being clinically silent, the pre-erythrocytic stage is considered an ideal target for the development of malaria vaccines. Sporozoite asparagine-rich protein 1 (SAP1) is a sporozoite-localized protein that regulates the expression of UIS (upregulated in infectious sporozoites) genes, which are essential for the infectivity of sporozoites. In this study, a recombinant DNA vaccine encoding a predicted antigenic determinant region of Plasmodium yoelii SAP1 (PySAP1) was constructed. Immunization of mice with this DNA vaccine construct resulted in significant elevation of cytokines such as IFN-γ, IL-2, IL-4 and IL-10, and total IgG as compared with control groups immunized with either the empty DNA vector or saline. After challenge with sporozoites, the group receiving the DNA vaccine showed delayed development of parasitemia and prolonged survival time compared with the control group. The DNA vaccine provided partial protection against P. yoelii 17XL infection, with an overall protection rate of 20%. In addition, the DNA vaccine did not show integration into the host genome. Further studies of SAP1 are needed to test whether it can be used as subunit vaccine candidate.
Introduction
Malaria, an insect-borne infectious disease, is widely prevalent in tropical, subtropical and the edge regions of the temperate zones. According to the latest data from the World Health Organization (WHO), malaria causes 216 million cases and 655 thousand deaths each year in 106 countries.Citation1 Effective control of malaria requires integrated control of both parasites and vectors, but the development of drug resistance in Plasmodium parasites and insecticide resistance in mosquitoes hampers the control efforts. Thus, the development of an effective and safe malaria vaccine has become one of the main focuses in malaria research.Citation2,Citation3
In the complex life cycle of Plasmodium parasites, the liver stage is considered an ideal target for the development of antimalarial vaccines because effective targeting of the pre-erythrocytic stages could prevent subsequent blood stage infections.Citation4,Citation5 To date, many proteins expressed specifically in the sporozoite and/or liver stage have been identified, and circumsporozoite protein (CSP) is one of the leading antigen candidates. The RTS,S vaccine, consisting of the hepatitis B virus surface antigen fused to the central repeat and the C-terminal portion of the Plasmodium falciparum CSP protein, has been studied for more than 20 y,Citation6 and a phase III clinical trial of the RTS,S/AS01 vaccine is currently underway.Citation7 Other antigen candidates include thrombospondin-related anonymous protein (TRAP) and liver stage antigen-1 (LSA-1).Citation8,Citation9 Thus far, results of clinical trials have demonstrated that the protection levels afforded by these traditional antigens are not sufficiently effective,Citation10 and new candidates for pre-erythrocytic vaccines are needed.
Plasmodium sporozoites invade hepatocytes and develop inside a parasitophorous vacuole (PV), where they multiply to produce thousands of merozoites.Citation11 When the sporozoites in Plasmodium salivary glands obtain infectivity for the mammalian hosts, the expression of the UIS (upregulated in infectious sporozoites) genes, including UIS3 and UIS4, is upregulated.Citation12 Many UIS genes are essential for the early liver stage development.Citation13,Citation14 Immunization with UIS knockout sporozoites leads to complete protection against infectious sporozoite challenge in the rodent model.Citation13-Citation15 Although the mechanism regulating the expression of UIS genes is not completely understood, sporozoite asparagine-rich protein 1 (SAP1) has been shown to be involved in post-transcriptional regulation of the UIS genes as deletion of SAP1 in P. yoelii results in a significant reduction of the UIS transcripts.Citation16,Citation17Pysap1(-) sporozoites can invade host hepatocytes and form PV normally, but development is arrested completely in the early liver stage.Citation16,Citation17 More importantly, Pysap1(-) sporozoites can confer long-lasting protection against infectious sporozoite challenge in mice.Citation17 SAP1 has a large asparagine-rich low-complexity domain which is conserved among Plasmodium species, indicating that they are functionally important regions.Citation16 The low-complexity domain is flanked by non-asparagine-rich N- and C-terminal domains.Citation14 In this study, we sought to investigate the immunogenicity and protective efficacy of SAP1 as a vaccine antigen against P. yoelii using a DNA vaccine strategy. Levels of various cytokines and antibodies induced by the vaccine were investigated, and its protective efficacy and safety were evaluated in a mouse model.
Results
Construction of the recombinant DNA vaccine
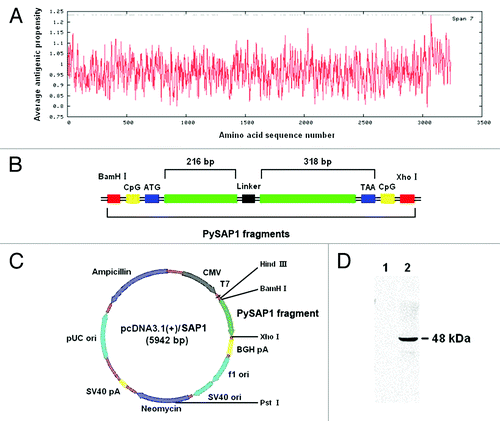
PySAP1 (EU652769) encodes a large protein of 3240 amino acids with a predicted molecular mass of 370 kDa.Citation16 To construct a recombinant SAP1 DNA vaccine, antigenic peptides in the P. yoelii SAP1 protein were analyzed by using an antigen prediction software and a domain with a high average antigenic propensity (score > 1.15) was selected, which corresponds to amino acids 3063–3227 (). In the genomic region, this antigenic domain is interrupted by an intron. To make a two-partite construct, a 216 bp fragment prior to the intron and a 318 bp fragment after the intron were joined by a linker consisting of five-glycine tandem repeat sequences, which were flanked by two CpG motifs to enhance immune responses (). This SAP1 cassette was inserted into the pcDNA3.1(+) vector to generate the recombinant DNA vaccine construct pcDNA3.1(+)/SAP1 (), which was confirmed by restriction digestion and sequencing analysis (data not shown). The predicted size of the truncated SAP1 domain is 22 kDa.
Figure 1. Construction of the recombinant DNA vaccine pcDNA3.1(+)/SAP1. (A) Prediction of the antigenic peptides of PySAP1. (B) Design of the PySAP1 gene fragment. The predicted antigenic polypeptides of amino acids 3063–3227 were constructed in two fragments linked with a linker sequence of five glycines. Outside the initiation codon (ATG) and termination codon (TAA), two CpG motifs were included to enhance immune responses. (C) Construction of the recombinant DNA vaccine pcDNA3.1(+)/SAP1 with the PySAP1 expression directed by the CMV promoter. (D) western blot analysis of the GST-PySAP1 fusion protein. The recombinant GST-PySAP1 expressed in bacteria as the 48 kDa protein band was separated in a 10% SDS-PAGE gel and the blots were probed with control sera from mice immunized with the pcDNA3.1(+)/CpG (lane 1) and sera from mice immunized with pcDNA3.1(+)/SAP1 (lane 2).
Expression of the truncated SAP1 protein in COS-7 cells
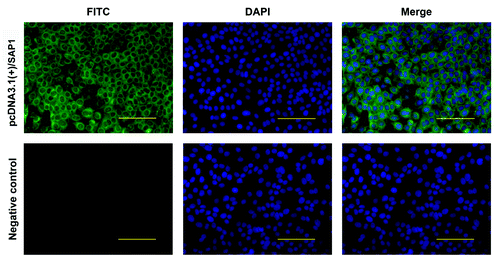
We first evaluated the expression of the recombinant SAP1 protein from the pcDNA3.1(+)/SAP1 plasmid in COS-7 cells. After transfection of the COS-7 cells with either pcDNA3.1(+)/SAP1 or pcDNA3.1(+)/CpG as the negative control, expression of the recombinant SAP1 truncated SAP1 protein was evaluated by indirect immunofluorescence assay (IFA), using a mouse polyclonal antiserum against recombinant SAP1. Whereas no immunofluorescence was detected in COS-7 cells transfected with the control plasmid, IFA detected strong SAP1 expression in pcDNA3.1(+)/SAP1-transfected COS-7 cells, with the fluorescence mostly located in the cytoplasm (). The result demonstrated that the truncated SAP1 protein was expressed efficiently in eukaryotic cells.
Figure 2. Detection of the PySAP1 recombinant protein expression in COS-7 cells by IFA. The PySAP1 protein was detected using a polyclonal mouse antiserum and FITC-conjugated goat anti-mouse IgG antibodies. Immunofluorescence of cells transfected with pcDNA3.1(+)/SAP1 or negative control pcDNA3.1(+)/CpG was compared. Green fluorescence from FITC indicates PySAP1 expression. Nuclei were counterstained with DAPI (blue). Scale bar = 50.0 μm.
T cell responses induced by immunization
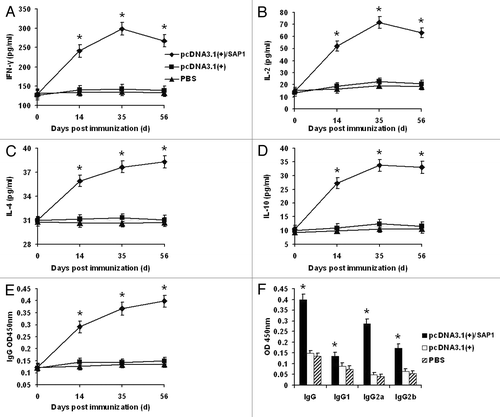
To assess the immunogenicity of the recombinant SAP1 delivered by the DNA vaccine, three groups of mice were injected with either phosphate buffered saline (PBS), pcDNA3.1(+)/CpG, or pcDNA3.1(+)/SAP1. Mice were sacrificed at different time points and splenocytes were collected to measure T cell responses. Levels of IFN-γ, IL-2, IL-4 and IL-10 in the supernatants of cultured splenocytes detected by ELISA were not significantly different between the two control groups (pcDNA3.1(+)/CpG and PBS) and there were no substantial increases after immunization (). However, the levels of these cytokines were increased after the primary immunization in the pcDNA3.1(+)/SAP1 group, and at each time point the cytokine levels were significantly higher than those of the control groups (p < 0.05). Of the cytokines measured, IFN-γ and IL-2 and IL-10 displayed a similar trend, characterized by quick rises after primary immunization and slight declines after the peak levels on day 35 (). In comparison, the level of IL-4 increased persistently throughout the course of the study, but the fold increase was modest among all cytokines analyzed (). These results indicated that the IFN-γ and IL-2 responses induced by the vaccine were very prominent, while the levels of IL-4 and IL-10 were not as high as those of IFN-γ and IL-2.
Figure 3. Levels of cytokines and antibodies in mice immunized with pcDNA3.1(+)/SAP1, pcDNA3.1(+), and PBS. (A–E) Levels of IFN-γ, IL-2, IL-4, IL-10 and total IgG on days 0, 14, 35, and 56 post-immunization. (F) Levels of IgG subtypes two weeks after the final immunization. Data are shown as means ± standard deviation (SD). * indicates significant differences between the pcDNA3.1(+)/SAP1 group with the two control groups (p < 0.05).
Antibody responses induced by immunization
To study the antibody responses in the three immunization groups, sera were collected from immunized mice to determine the total IgG and subtype levels. In the PBS and empty vector control groups, total IgG levels did not experience marked changes during the study (). In contrast, the levels of total IgG antibodies in the pcDNA3.1(+)/SAP1 group were continually elevated and were significantly higher than those in the two control groups (p < 0.05) (). Sera from the immunized mice were collected two weeks after the final immunization to confirm their reactivity with PySAP1. For this purpose, the truncated PySAP1 protein was expressed in bacteria and the 48 kDa GST-PySAP1 fusion protein was purified for western blot analysis. Whereas the immunization control sera did not react with the GST-PySAP1, the immune sera from the pcDNA3.1(+)/SAP1 group detected the 48 kDa recombinant (), which verified that mice immunized with the pcDNA3.1(+)/SAP1 plasmid produced antibodies against recombinant PySAP1. We then compared the levels of three IgG subtypes between the two immunization groups. Levels of IgG subtypes (IgG1, IgG2a, and IgG2b) in the pcDNA3.1(+)/SAP1 group were significantly higher than those in the two control groups (p < 0.05) (). Of particular note is that the level of IgG2a was elevated more remarkably in the pcDNA3.1(+)/SAP1 group ().
Tissue distribution of the pcDNA3.1(+)/SAP1 plasmid in mouse
Distribution of the pcDNA3.1(+)/SAP1 plasmid in various tissues of immunized mice was detected by PCR. The plasmid DNA was found initially in all of the tissues on days 1, 7 and 30 after intramuscular injection. However, on day 60, it was only detected in the muscles at the site of injection. On day 90, it was not detected in any tissues ().
Table 1. Tissue distribution of plasmid DNA
Protective efficacy of the truncated SAP1 protein
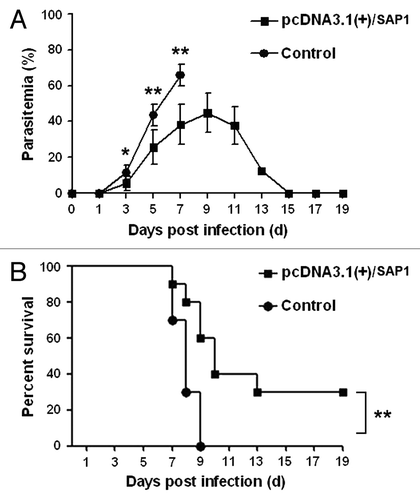
To assess the protective efficacy of the truncated SAP1 protein, mice were immunized three times at three-week intervals intramuscularly in the quadriceps femoris of the posterior limb with 100 μg of the recombinant plasmid pcDNA3.1(+)/SAP1 in a total volume of 100 μl. The control group received 100 μl of PBS. All groups were challenged via bites from Plasmodium-infected mosquitoes two weeks after the final immunization. Examination of tail blood smears showed that parasite-infected erythrocytes in the peripheral blood of all mice (10/10) in the control group began to appear on day 3 post-infection (). Thereafter, the parasitemia rose rapidly and reached the peak level (65.7%) on day 7 (). By contrast, parasitized erythrocytes began to appear in the peripheral blood of 60% (6/10) of the vaccinated mice on day 3 post-infection (). The parasitemia level in vaccinated mice rose more slowly than in the control group, reaching the peak at 44.9% on day 9 (). Mice without parasitemia on day 14 after challenge were considered completely protected.Citation18 On day 14 post-challenge, 80% (8/10) of the vaccinated mice showed parasitemia (). Seven of the eight vaccinated mice with high parasitemias were sacrificed for ethical reasons; one did not suffer severe symptoms of infection and cleared the parasitemia gradually. Therefore, the overall protective rate of the vaccine was 20% (), and the survival rate of the mice was 30% on day 19 post-infection (). The results showed that vaccination with the pcDNA3.1(+)/SAP1 DNA plasmid could delay parasitemia and prolong survival time in mice.
Table 2. Protection of mice from P. yoelii infection (infected/total) by the DNA vaccine pcDNA3.1(+)/SAP1
Discussion
Malaria is a serious threat to human health and economic development. Despite tremendous efforts in malaria vaccine development for over half a century, most of the candidates do not induce significant protection.Citation6,Citation19 One factor that deters the development of effective malaria vaccines is the very complex nature of antigenic structures and d antigen epitopes.Citation20 Vaccines against the liver stage are particularly important because it is the only stage in which parasites can be completely eliminated by sterilizing immune responses.Citation21 However, we know little about liver stage biology because of the low numbers of parasites and their limited experimental accessibility.Citation22 PV has a critical function for successful hepatocyte infection and liver stage development,Citation23 and the UIS3 and UIS4 proteins on the PVM are essential for Plasmodium parasite liver infection.Citation13-Citation15 The main function of SAP1 is to regulate the expression of some genes, including UIS genes, related to the infectivity of sporozoites.Citation16,Citation17 Deletion of the SAP1 gene leads to a dramatic decrease of UIS transcripts and a complete developmental arrest in the early liver stage.Citation16,Citation17 These studies have provided a theoretical and experimental basis for the development of new subunit vaccines against the liver stage.
In our current study, the levels of various cytokines induced by vaccination with SAP1 were significantly higher than those of the control groups. The IFN-γ and IL-2 responses induced by the vaccine were the most prominent. Pre-erythrocytic protection is associated with CD8+ T cells and IFN-γ-mediated responses against infected hepatocytes.Citation24,Citation25 It has been reported that IFN-γ and IL-2 are correlated with protection against parasite infection.Citation26,Citation27 High levels of IFN-γ can inhibit parasite development, prevent reinfection and provide protection against parasitemia and clinical malaria.Citation25,Citation28 In addition, levels of IgG and IgG subtypes were found to be elevated to varying degrees, while the IgG2a response induced by immunization was the most robust. The results suggest that IgG2a might have also participated in the observed vaccine-mediated reduction of parasitemia. It has been reported that IgG2a plays a key role in protective immunity against parasite infection in the P. yoelii models.Citation29,Citation30 T helper type 1 (Th1) cells principally mediate cellular immune responses by secreting IL-2, IFN-γ and TNF-α, and they promote IgG2a synthesis. By contrast, T helper type 2 (Th2) cells principally mediate humoral immune responses by secreting IL-4, IL-6 and IL-10, and inducing B cell proliferation and antibody production. In addition, inhibition of IL-2 and IFN-γ production can promote IgG1 synthesis.Citation31 Protective immunity against intracellular pathogens including P. yoelii generally depends on cellular immune responses, whereas elimination of extracellular pathogens generally depends on humoral immune responses.Citation32 Cellular immune responses have been demonstrated to be important for targeting parasites in the pre-erythrocytic stage,Citation24 as well as for elimination of liver-stage parasites.Citation33,Citation34 CD8+ T cells play an essential role in protection induced by radiation attenuated sporozoites and genetically attenuated parasites, but the specific CD8+ T cell responses induced by single-antigen subunit vaccines that contribute to protection remain unknown.Citation15,Citation35 As a surrogate activation marker, CD8aloCD11ahi has been measured to evaluate protection against infection of liver-stage parasites.Citation35 Unfortunately, it is unclear whether the protection was antibody-mediated or T-cell mediated in this study. A goal of future experiments will be to better define the mechanisms of protection.
The protective efficacy of malaria vaccines is normally assessed by monitoring parasitemia.Citation11,Citation18 If a vaccine against the pre-erythrocytic stage offers full protection, blood stage infection would not occur. In the case of partial protection, reduced blood stage parasite load and prolonged survival time of the host are expected. Our results suggest that immunization with pcDNA3.1(+)/SAP1 offers partial protection. First, parasites were found in 8/10 of the pcDNA3.1(+)/SAP1-vaccinated mice while 20% were fully protected against blood stage infection. In addition, immunized mice had delayed parasitemia and exhibited less severe infection than the controls. Moreover, the vaccinated group had a prolonged survival time and higher survival rate compared with the control group.
DNA vaccines have wide, potential applications in the prevention and treatment of infectious diseases, autoimmune diseases, allergies and cancer.Citation36 However, safety problems unique to DNA vaccines, such as integration into the host chromosome leading to insertion mutations, limit their development.Citation37 In this study, the distribution analysis showed that the plasmid DNA disseminated to all tissues of the mouse in the early stage after immunization. However, the injected DNA was detected only in the muscles at the injection site on day 60 and was not found in any tissues by day 90. Our results are consistent with previous reports demonstrating that intramuscularly administered plasmid DNA is mainly distributed to tissues with a rich blood supply in the early stage after immunization but persists mainly at the injection site in later stages for at least 8 weeks.Citation38 Collectively, these results demonstrated that the SAP1 recombinant plasmid did not integrate into the host genome.
As the number of animals used in this preliminary study was limited, future work should include larger sample sizes to address this shortcoming. In addition to monitoring blood-stage parasitemia for determining the protective efficacy of the vaccine, direct measurement of the parasite burden in the liver by quantitative PCR should also be performed. Also, the numbers of sporozoites injected by mosquito bites are not precise and it is more desirable to initiate liver infections by directly injecting the same numbers of sporozoites into the bloodstream of mice. Furthermore, the mechanism of protection (antibody and/or T-cell mediated) by immunization with PySAP1 should be elucidated. In addition, we want to evaluate whether SAP1 protein is suitable for inclusion as a component of a multiunit vaccine together with blood stage antigens. We will also carry out future experiments to determine whether the mechanism of protection is mediated by humoral vs. cellular immunity.
Materials and Methods
Experimental animals, parasites, and mosquitoes
Six- to eight-week-old female specific pathogen free BALB/c mice were purchased from Vital River Laboratories (Beijing, China). Anopheles stephensi mosquitoes were maintained in our laboratory and P. yoelii 17XL infection was obtained by feeding adult female mosquitoes on P. yoelii 17XL infected mice. This study was conducted in accordance with the guidelines of the laboratory animal ethical standards of China Medical University.
Construction of the recombinant PySAP1 DNA vaccine
PySAP1 (EU652769) encodes a large protein of 3240 amino acids. Antigenic peptides were predicted using an antigen prediction software (http://imed.med.ucm.es/Tools/antigenic.pl), and regions with an average antigenic propensity > 1.15 were considered antigenic determinants and selected for this study. Two small fragments corresponding to amino acids 3063–3227 were amplified from P. yoelii 17XL genomic DNA by PCR with the primer pairs 5′-GCG GGA TCC GAC GTT ATG TGG ACA ATT TGC CCC-3′, 5′-ACC ACC ACC ACC ACC TTG AGT AGA ATG CTT CGA-3′ and 5′-GGT GGT GGT GGT GGT GAA CAC GAA ACC ATA TTC-3′, 5′-GCG CTC GAG AAC GTC TTA ATA AAA TAA GAA TCG-3′. The PCR products were a 216 bp fragment (C-terminus with a linker sequence) and a 318 bp fragment (N-terminus with a linker sequence). The target gene fragment was amplified by overlapping PCR using the first PCR products as templates with the sense primer 5′-GCG GGA TCC GAC GTT ATG TGG ACA ATT TGC CCC-3′ and the antisense primer 5′-GCG CTC GAG AAC GTC TTA ATA AAA TAA GAA TCG-3′. The sense primer sequences included the initiation codon ATG, CpG motif and restriction enzyme site BamHI, while the antisense primer sequences included the termination codon TAA, CpG motif and restriction enzyme site XhoI. PCR was performed under the following conditions: 35 cycles of 94°C for 30 sec, 48°C for 30 sec and 72°C for 1 min. The target gene was cloned into the eukaryotic expression vector pcDNA3.1(+) to construct the plasmids pcDNA3.1(+)/SAP1.
Expression of recombinant PySAP1 protein in bacteria
The PySAP1 fragment from pcDNA3.1(+)/SAP1 was cloned into the prokaryotic expression vector pGEX-6P-1. Recombinant PySAP1 fragment was expressed in Escherichia coli BL21 after induction with 1.0 mM isopropyl β-D-1-thiogalactopyranoside at 28°C for 5 h. The GST-fusion protein was purified by affinity columns and used for immunoblot analysis.
Transfection of the recombinant plasmid in COS-7 cells
Coverslips in six-well tissue culture plates were seeded with COS-7 cells (1 × 106 cells/well). Monolayer cells at 80–90% confluency were transfected with the pcDNA3.1(+)/SAP1 plasmid or pcDNA3.1(+) plasmid (negative control) using Attractene Transfection Reagent (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The expression of the recombinant protein was observed by indirect immunofluorescence as described elsewhere.Citation39 The SAP1 polyclonal mouse anti-serum was used as primary antibodies. FITC-conjugated goat anti-mouse IgG (Sigma, St. Louis, MO, USA) was used as the secondary antibody. COS-7 cells transfected with plasmids on the coverslips were fixed with 4% paraformaldehyde (Sigma) for 30 min and then washed with PBS three times. Cell membranes were then permeabilized with 0.5% Triton X-100 (Sigma) for 15 min. Following three washes with PBS, 1% BSA (Sigma) was added for 1 h, and then the diluted primary antibody was added for incubation at 4°C overnight. The cells were washed with PBS three times, and secondary antibody was added for 1–1.5 h. After further washes with PBS for three times, the nuclei of cells were counterstained with DAPI (Sigma) for 15 min. Protein expression was observed under a fluorescence microscope.
Immunization of mice with the recombinant DNA vaccine
Six- to eight-week-old BALB/c mice were randomly divided into three groups (n = 40/group) and immunized three times at three-week intervals. Each time, 100 μg of the plasmid pcDNA3.1(+)/SAP1 (vaccinated group) or 100 μg of pcDNA3.1(+)/CpG (negative control group) in a total volume of 100 μl were injected intramuscularly in the quadriceps femoris of the posterior limb. The control group only received 100 μl PBS.
Detection of cytokines IFN-γ, IL-2, IL-4 and IL-10 by ELISA
Supernatants of cultured splenocytes were collected to detect cytokines two weeks after each immunization. The immunized mice were sacrificed, and spleens were removed aseptically. Splenocyte suspensions were harvested by standard methods and then seeded in 96-well plates (1 × 106 cells/well) with 200 μl DMEM culture medium (Gibco). Splenocytes were cultured under their normal growth conditions (typically 37°C and 5% CO2) for 48 h, and then supernatant fluids of cultured splenocytes were collected and stored at -70°C until use. Cytokines in the culture supernatant were detected by ELISA (Thermo) according to manufacturer’s instructions, and absorbance was measured at 450 nm.
Western blot
To determine whether mice immunized with the DNA vaccine produced antibodies against PySAP1, we used GSP-PySAP1 protein expressed in bacteria for western blot analysis. Recombinant GST-PySAP1 was separated by 10% SDS-PAGE and transferred to a PVDF membrane. Either pcDNA3.1(+)/CpG control mouse sera or sera from pcDNA3.1(+)/SAP1 immunized mice at 1:40 was used as the primary antibodies, and HRP-labeled goat anti-mouse IgG at 1:2000 was used as the secondary antibody (Sigma). The western blots were developed using an ECL plus chemiluminescence reagent kit (Thermo) according to manufacturer’s instructions.
Quantitation of IgG and IgG subtypes by ELISA
Sera were collected to determine the total IgG levels two weeks after each immunization and to quantify the IgG subtypes two weeks after the final immunization. Blood samples were obtained aseptically from the retro-orbital plexus of the immunized mice. Sera were collected by centrifugation and stored at -70°C until use. IgG and IgG subtypes (IgG1, IgG2a and IgG2b) were detected by ELISA (Thermo) according to manufacturer’s instructions, and absorbance was measured at 450 nm.
Tissue distribution of the recombinant DNA
DNA was extracted from blood, muscle (at the injection site), heart, lung, liver, spleen and kidney tissues on days 1, 7, 30, 60 and 90 after the final immunization. Two pairs of primers covering the beginning and the end of the SAP1 gene fragment were: 5′-CTG TAA TAG TGC CTT GTG CC-3′ and 5′-CGT TGA ATA TGG TTT CGT GT-3′, resulting in a 211 bp product; 5′-GTG GTG GTG AAC ACG AAA C-3′ and 5′-TGG GAG TTC CTT AAT GGA TAT AGA-3′, resulting in a 286 bp product. PCR was performed under the following conditions: 35 cycles of 94°C for 30 sec, 50°C for 30 sec and 72°C for 30 sec. The PCR products were separated in a 1% agarose gel, and DNA was stained by ethidium bromide and visualized by UV light.
Challenge of the immunized mice by P. yoelii infected mosquitoes
Both immunized and control mice (n = 10/group) were challenged two weeks after the final immunization. Each mouse was challenged by bites from five P. yoelii infected A. stephensi mosquitoes.Citation11,Citation18 The mosquitoes used for inoculation of sporozoites were on days 14–17 after an infectious blood meal. The average number of sporozoites per infected mosquito was ~15,000. Three days after the challenge, parasitemia was monitored daily by microscopic examination of Giemsa-stained smears of tail blood. Mice without parasitemia on day 14 after challenge were considered completely protected.
Statistical analysis
Statistical comparison between groups was performed with the Mann-Whitney U test using SPSS software version 16.0. p < 0.05 was considered statistically significant.
| Abbreviations: | ||
| SAP1 | = | sporozoite asparagine-rich protein 1 |
| UIS | = | upregulated in infectious sporozoites |
| P. yoelii 17XL | = | Plasmodium yoelii 17XL (lethal strain) |
| CSP | = | circumsporozoite protein |
| TRAP | = | thrombospondin-related anonymous protein |
| LSA-1 | = | liver stage antigen-1 |
| PV | = | parasitophorous vacuole |
| PVM | = | parasitophorous vacuole membrane |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the staff of the Immunology Department of China Medical University for technical support. We thank Daniel Parker for critical reading of the manuscript. This research was supported by grants from the National Natural Science Foundation of China (30972572 and 30972774).
References
- World Health Organization (WHO). World malaria report 2011; http:// www. who.int/malaria/world_malaria_report_2011/en/index.html.
- Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, et al. The threat of artemisinin-resistant malaria. N Engl J Med 2011; 365:1073 - 5; http://dx.doi.org/10.1056/NEJMp1108322; PMID: 21992120
- Chanda E, Hemingway J, Kleinschmidt I, Rehman AM, Ramdeen V, Phiri FN, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS One 2011; 6:e24336; http://dx.doi.org/10.1371/journal.pone.0024336; PMID: 21915314
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science 2010; 328:862 - 6; http://dx.doi.org/10.1126/science.1184785; PMID: 20466924
- Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol 2009; 63:195 - 221; http://dx.doi.org/10.1146/annurev.micro.091208.073403; PMID: 19575563
- Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest 2010; 120:4168 - 78; http://dx.doi.org/10.1172/JCI44423; PMID: 21123952
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al, RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863 - 75; http://dx.doi.org/10.1056/NEJMoa1102287; PMID: 22007715
- Bejon P, Ogada E, Mwangi T, Milligan P, Lang T, Fegan G, et al. Extended follow-up following a phase 2b randomized trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS One 2007; 2:e707; http://dx.doi.org/10.1371/journal.pone.0000707; PMID: 17710125
- Moormann AM, John CC, Sumba PO, Tisch D, Embury P, Kazura JW. Stability of interferon-gamma and interleukin-10 responses to Plasmodium falciparum liver stage antigen-1 and thrombospondin-related adhesive protein in residents of a malaria holoendemic area. Am J Trop Med Hyg 2006; 74:585 - 90; PMID: 16606988
- Mikolajczak SA, Aly AS, Kappe SH. Preerythrocytic malaria vaccine development. Curr Opin Infect Dis 2007; 20:461 - 6; http://dx.doi.org/10.1097/QCO.0b013e3282ef6172; PMID: 17762778
- Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog 2008; 4:e1000086; http://dx.doi.org/10.1371/journal.ppat.1000086; PMID: 18551171
- Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, Jacobs-Lorena V, et al. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol Cell Biol 2008; 28:6196 - 207; http://dx.doi.org/10.1128/MCB.00553-08; PMID: 18710954
- Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 2005; 433:164 - 7; http://dx.doi.org/10.1038/nature03188; PMID: 15580261
- Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A 2005; 102:3022 - 7; http://dx.doi.org/10.1073/pnas.0408442102; PMID: 15699336
- Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis 2007; 196:608 - 16; http://dx.doi.org/10.1086/519742; PMID: 17624848
- Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, Labaied M, et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol 2008; 69:152 - 63; http://dx.doi.org/10.1111/j.1365-2958.2008.06271.x; PMID: 18466298
- Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH. SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol Microbiol 2011; 79:929 - 39; http://dx.doi.org/10.1111/j.1365-2958.2010.07497.x; PMID: 21299648
- Nganou-Makamdop K, van Roosmalen ML, Audouy SA, van Gemert GJ, Leenhouts K, Hermsen CC, et al. Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar J 2012; 11:50; http://dx.doi.org/10.1186/1475-2875-11-50; PMID: 22348325
- Friesen J, Matuschewski K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine 2011; 29:7002 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.07.034; PMID: 21787828
- Omosun YO, Adoro S, Anumudu CI, Odaibo AB, Uthiapibull C, Holder AA, et al. Antibody specificities of children living in a malaria endemic area to inhibitory and blocking epitopes on MSP-1 19 of Plasmodium falciparum.. Acta Trop 2009; 109:208 - 12; http://dx.doi.org/10.1016/j.actatropica.2008.11.011; PMID: 19081386
- Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002; 185:1155 - 64; http://dx.doi.org/10.1086/339409; PMID: 11930326
- Kaiser K, Camargo N, Kappe SH. Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J Exp Med 2003; 197:1045 - 50; http://dx.doi.org/10.1084/jem.20022100; PMID: 12707302
- Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, et al. Migration of Plasmodium sporozoites through cells before infection. Science 2001; 291:141 - 4; http://dx.doi.org/10.1126/science.291.5501.141; PMID: 11141568
- Doolan DL, Martinez-Alier N. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med 2006; 6:169 - 85; http://dx.doi.org/10.2174/156652406776055249; PMID: 16515509
- McCall MB, Sauerwein RW. Interferon-γ--central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol 2010; 88:1131 - 43; http://dx.doi.org/10.1189/jlb.0310137; PMID: 20610802
- Petritus PM, Burns JM Jr.. Suppression of lethal Plasmodium yoelii malaria following protective immunization requires antibody-, IL-4-, and IFN-gamma-dependent responses induced by vaccination and/or challenge infection. J Immunol 2008; 180:444 - 53; PMID: 18097046
- Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468 - 77; http://dx.doi.org/10.1056/NEJMoa0805832; PMID: 19641203
- D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, et al. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 2008; 47:1380 - 7; http://dx.doi.org/10.1086/592971; PMID: 18947328
- Matsumoto S, Yukitake H, Kanbara H, Yamada H, Kitamura A, Yamada T. Mycobacterium bovis bacillus calmette-guérin induces protective immunity against infection by Plasmodium yoelii at blood-stage depending on shifting immunity toward Th1 type and inducing protective IgG2a after the parasite infection. Vaccine 2000; 19:779 - 87; http://dx.doi.org/10.1016/S0264-410X(00)00257-7; PMID: 11115699
- Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del Portillo HA. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One 2011; 6:e26588; http://dx.doi.org/10.1371/journal.pone.0026588; PMID: 22046311
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996; 17:138 - 46; http://dx.doi.org/10.1016/0167-5699(96)80606-2; PMID: 8820272
- Dwivedi V, Vasco A, Vedi S, Dangi A, Arif K, Bhattacharya SM, et al. Adjuvanticity and protective immunity of Plasmodium yoelii nigeriensis blood-stage soluble antigens encapsulated in fusogenic liposome. Vaccine 2009; 27:473 - 82; http://dx.doi.org/10.1016/j.vaccine.2008.10.054; PMID: 18996429
- Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J Immunol 2009; 183:5870 - 8; http://dx.doi.org/10.4049/jimmunol.0900302; PMID: 19812194
- Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science 2011; 334:475 - 80; http://dx.doi.org/10.1126/science.1211548; PMID: 21903775
- Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog 2010; 6:e1000998; http://dx.doi.org/10.1371/journal.ppat.1000998; PMID: 20657824
- Ulmer JB, Wahren B, Liu MA, UImer JB. DNA vaccines: recent technological and clinical advances. Discov Med 2006; 6:109 - 12; PMID: 17234144
- Martin T, Parker SE, Hedstrom R, Le T, Hoffman SL, Norman J, et al. Plasmid DNA malaria vaccine: the potential for genomic integration after intramuscular injection. Hum Gene Ther 1999; 10:759 - 68; http://dx.doi.org/10.1089/10430349950018517; PMID: 10210143
- Parker SE, Borellini F, Wenk ML, Hobart P, Hoffman SL, Hedstrom R, et al. Plasmid DNA malaria vaccine: tissue distribution and safety studies in mice and rabbits. Hum Gene Ther 1999; 10:741 - 58; http://dx.doi.org/10.1089/10430349950018508; PMID: 10210142
- Zhao C, Sun Y, Zhao Y, Wang S, Yu T, Du F, et al. Immunogenicity of a multi-epitope DNA vaccine against hantavirus. Hum Vaccin Immunother 2012; 8:208 - 15; PMID: 22426376