Abstract
Hypertension is a serious worldwide public health problem. The aim of this study is to design anti-hypertension angiotensin II (Ang II) vaccine using molecular biology and immunological method. This novel anti-hypertension vaccine, which is a chimeric protein named pHAV–4Ang IIs, presents four successive repeated Ang IIs as the functional epitope on the surface of the hepatitis A virus-like particle(HAVLP). In this study, pHAV–4Ang IIs was expressed using Bac-to-Bac Baculovirus Expression System. With the RT-PCR analysis, SDS-PAGE, western blot, IFA, electron microscope methods for identification of expression products, these results confirmed that stable expression of pHAV-4Ang IIs can be effectively achieved in infected sf9 cells. Spontaneous hypertensive rats (SHRs) were immunized with pHAV–4Ang IIs to test immunogenicity and pharmacodynamic action. The results showed that this anti-hypertension vaccine can induce high titer Ang II -specific IgG antibody for almost 10 weeks. When antibody titer reached the peak at 8th week, the mean systolic blood pressure (SBP) degraded approximately 23 mmHg compared with the PBS control group, and the mean diastolic blood pressure (DBP) degraded approximately 12 mmHg compared with the PBS control group. These results suggest that this anti-hypertension vaccine has good immunogenicity and good effect on reduction of blood pressure in SHRs, which provide reliable base for large-scale preparation of this hypertension vaccine in the future, and a new direction of exploration for the development of anti-hypertension therapeutic vaccine.
Introduction
Hypertension is a serious worldwide public health problem. High rates of disability and mortality are associated with its complications, which include stroke, myocardial infarction, cardiac failure and chronic kidney diseaseCitation1. Currently, there are approximately one billion hypertensive patients worldwide, and the number of hypertensive patients is projected to reach 1.5 billion worldwide by the year 2025Citation2. Currently, hypertensive patients are treated with α blockers, calcium channel blockers (CCBs), ACE inhibitors (ACEIs) and angiotensin II receptor antagonistsCitation3. However, hypertension cannot be effectively treated with these medicines due to their serious side-effects and to the poor compliance of patientsCitation4,Citation5. Hence, an efficient and safe vaccine for hypertension would represent a great public health advance.
The renin-angiotensin-aldosterone system (RAAS) is the most important system involved in the onset of hypertensionCitation6. Ang II plays an important role in this system; it causes strong vasoconstriction and increases blood pressure by binding with the Ang II type I receptor on the cell surfaceCitation7–Citation10. Ang II is composed of 8 amino acid residues that the host cannot recognize and induce an immune response for this weak immunogenicity. In this study, we integrated the expression of Ang II and HAVLP to elevate the immunogenicity of Ang II and to activate the immune response of the host. Furthermore, the neutralizing antibody of Ang II blocked the RAAS system, and blood pressure subsequently decreased.
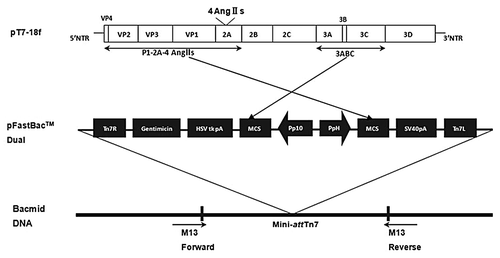
HAVLP is a good vector used to present exogenous antigens because of its stable structure and superior immunogenicityCitation11,Citation12. Moreover, the partial deletion of nucleotides in the 2A region has no significant effect on the expression and translation of proteins as well as the structure and function of VLP. Hence, some genes can be deleted in this region, and exogenous genes can be inserted into the region. Additionally, the 2A region is located on the surface of HAVLP, which is advantageous for the antigen presentation of the exogenous geneCitation13. In our study, four-artificial-repeated Ang IIs sequence was inserted into the 2A region of pT7–18f. And pT7–18f was obtained by modifying the HAV genome, and two restriction enzymes, Afl II and Stu I, were introduced into the 2A region, respectively. P1–2A–4Ang IIs and 3ABC were coexpressed using the Bac-to-Bac Baculovirus Expression System (Invitrogen, Life Technologies, Carlsbad, CA, USA) in sf9 cells. Furthermore, P1–2A–4Ang IIs was incised into three coat proteins by 3ABC, including VP0, VP3 and VP1–2A–4 Ang IIs. These three coat proteins were used to bind HAVLP with Ang II, which were subsequently named pHAV–4Ang IIs. Finally, 240 copies of Ang II were transferred onto the surface of HAVLP, and the antigenicity and immunogenicity of Ang II were significantly increased.
Results
Construction of bacmid–P1–2A–4AngIIs–3ABC
P1–2A–4Ang IIs and 3ABC fragments were specifically amplified, respectively, by PCR using plasmid pT7–18f as template. As shown in , the lengths of P1–2A–4Ang IIs and 3ABC fragments were approximately 3 kb and 1 kb, respectively, as expected. These two genes were cloned into the multiple clone sites of pFastBacTM Dual vector located downstream of the PH and the P10 promoters, respectively. The recombinant plasmids were identified by double digestion. As shown in , the two fragments were successfully inserted into a pFastBacTM Dual vector and the recombinant plasmids pFastBac™ Dual–P1–2A–4Ang IIs–3ABC was obtained. The positive clones were sequenced by TaKaRa (Dalian, China). The results suggest that the sequences of P1–2A–4Ang IIs and 3ABC were correct and that these sequences presented correct open reading frames (ORF).
Figure 1. (A) PCR products. Lane 1: DL5000 DNA marker; Lane 2: P1–2A-4Ang IIs; and Lane 3: 3ABC. (B) Double digestion of pFastBac™ Dual-P1–2A-4Ang IIs-3ABC.Lane 1: DL5000 DNA marker; Lane 2: P1–2A-4Ang IIs; and Lane 3: 3ABC. (C) PCR analysis. Lane 1: DL1000 DNA marker; Lane 2: primary M13; Lane 3: DL10000 DNA marker; and Lane 4: recombinant M13.
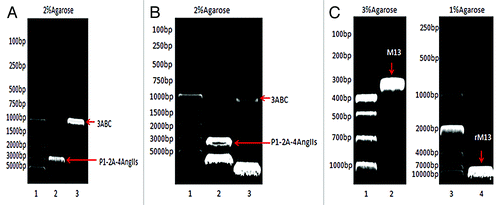
pFastBac™ Dual–P1–2A–4Ang IIs–3ABC were transformed into DH10 Bac™ E. coli,and then the recombinant bacmids were extracted after blue–white spot screening. M13 gene was specifically amplified by PCR. The positive clone presented a band at ~7 kb, and the negative clone presented a band at ~300 bp, as shown in . These data indicate that recombination successfully occurred between pFastBac™ Dual–P1–2A–4Ang IIs–3ABC and bacmid in DH10 Bac™ E. coli. P1–2A–4Ang IIs and 3ABC were integrated into the bacmid, and recombinant bacmid-P1–2A–4AngIIs–3ABC was obtained.
Generation and identification of the recombinant baculovirus
Cytopathic effect (CPE)
Seven days after sf9 cells were transfected with bacmid–P1–2A–4Ang IIs–3ABC, sf9 cells produced an obvious CPE and presented split and necrosis as well. There were no CPE to be found in normal sf9 cell. Four days after sf9 cells were infected with P1 viruses, the same CPE was observed clearly. These findings suggest that sf9 cells were successfully transfected with bacmid–P1–2A–4Ang IIs–3ABC, and the recombinant baculovirus was further assembled.
Observation of virus particles using a transmission electron microscope
Using 2% phosphotungstic acid as a negative stain, the viruses were observed using an electron microscope. The diameter and length of these viruses were approximately 50 nm and 370 nm, respectively. Sf9 cells that were infected with the recombinant baculovirus for 72 h were collected, sectioned and stained using colloidal gold. Large amounts of baculoviruses were observed in nuclei of the infected sf9 cells and no baculovirus was observed in normal sf9 cells.
The titer of the recombinant baculovirus
The plaque assay suggests that the titer of the P3 viruses was approximately 4 × 108.
Expression and identification of pHAV–4Ang IIs
RT-PCR analysis
As shown in , P1–2A–4Ang IIs and 3ABC gene fragments were specifically amplified using infected sf9 cells by RT-PCR analysis, suggesting that P1–2A–4Ang IIs and 3ABC gene fragments were successfully transcribed in infected sf9 cells.
SDS-PAGE and western blot
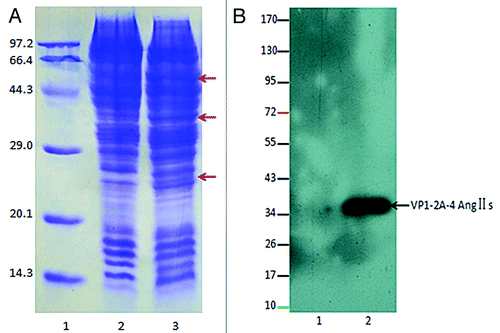
The sf9 cell lysates were analyzed by 12% SDS-PAGE and stained by Coomassie brilliant blue. The novel protein bands were observed at molecular mass of approximately 60, 37 and 26 kDa (). Western blot exposed a specific protein bands at a molecular mass of~37 kDa (), and the protein band may represent the VP1–2A–4Ang IIs subunit of pHAV–4Ang IIs. These data imply that pHAV–4Ang IIs were expressed in the infected sf9 cells and induced good immunoreactivity.
Figure 3. (A) 12% SDS-PAGE analysis. Lane 1: protein molecular weight marker (kDa); Lane 2: normal sf9 cells; Lane 3: infected sf9 cells. The arrows (A) indicate the new foreign proteins. (B) Western blot identification for Ang II using an enhanced chemiluminescence (ECL) assay. The numbers to the left indicate positions of protein molecular weight marker (kDa). Lane 1: normal sf9 cells; Lane2: infected sf9 cells. Western blot exposed a specific protein bands at a molecular mass of~37 kDa, and the protein band may represent the VP1–2A-4Ang IIs subunit of pHAV-4Ang IIs.
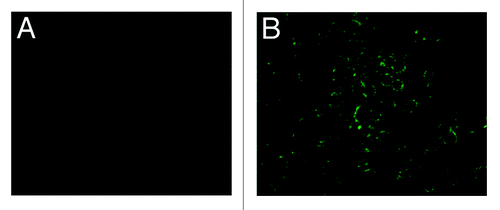
IFA
Specifically, green fluorescence were observed in the infected sf9 cells (), and no fluorescence was observed in the normal sf9 cells (), suggesting that pHAV–4Ang IIs was successfully expressed in the infected sf9 cells, which provides further evidence of good immunoreactivity.
Observation of VLP using a transmission electron microscope
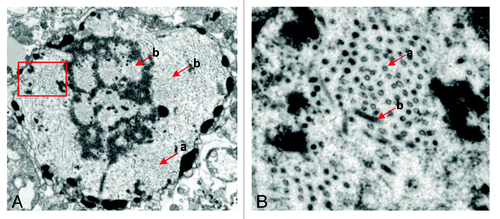
The infected sf9 cells were observed using a transmission electron microscope. As shown in , large amounts of HAV empty-shell-like VLPs were located in the cytoplasm, and the diameter of these VLPs was approximately 22 nm. The particles were not observed in normal sf9 cells. These results suggested that P1–2A–4Ang IIs and 3ABC were coexpressed using the Bac-to-Bac Baculovirus Expression System, and 3C hydrolyzed P1–2A–4Ang IIs into three coat proteins, including VP0, VP3 and VP1–2A–4 Ang IIs. These three coat proteins were assembled into VLP.
Figure 5. The particles of pHAV-4Ang IIs observed using an electron microscope. The red (a) arrows indicate the particles of pHAV-4Ang IIs. The red (b) arrows indicate the recombinant baculovirus. (A) The particles in whole infected sf9 cells observed (×2,000 zoom in). (B) Large amounts of pHAV-4Ang IIs particles observed in the cytoplasm, which amplified from the rectangle delineation in (A)(×5,000 zoom in).
Efficacy of immunization with pHAV–4Ang IIs
Measurement of blood pressure
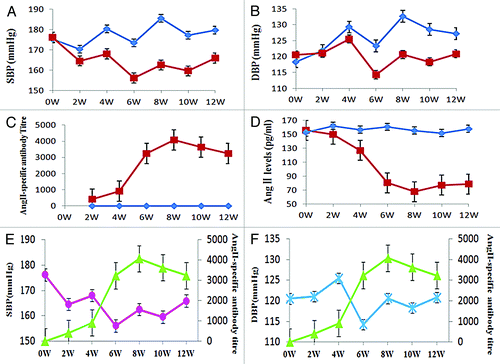
The effect of immunization on blood pressure was tested in the SHRs using the tail-cuff method at 0th, 2th, 4th, 6th, 8th, 10th and 12th week. The mean SBP in the pHAV–4Ang IIs-vaccinated SHRs was significantly lower compared with the PBS control group at 4th -12th week (). At 8th week, a difference of approximately 23 mmHg (p < 0.05) in SBP was observed in comparison with the PBS control group, and approximately 12 mmHg (p < 0.05) in DBP ().
Figure 6. Efficacy of immunization with pHAV-4Ang IIs. Groups of SHR were immunized on days 0 and 21 with PBS (◆, n = 10), pHAV-4AngIIs (■, n = 10) as described. Blood pressure (BP) was measured by tail-cuff arterial blood pressure measurement method; the AngII-specific antibody titers and the Ang II levels in sera were determined by enzyme-linked immunosorbent assay (ELISA). Data of BP and the Ang II levels are presented as the average of each group; Data of antibody titers are presented as the geometric mean titer (GMT) of each group. Error bars indicate the standard error of the mean. (A) Effect of vaccination on SBP. Significant differences (p < 0.05) between the pHAV-4AngIIs(■) and the PBS(◆) group were found at weeks 4–12 by statistics analysis . (B) Effect of vaccination on DBP. Significant differences (p < 0.05) between the pHAV-4AngIIs(■) and the PBS(◆) group were found at weeks 6–12 by statistics analysis.(C) Effect of vaccination on antibody titers. The AngII-specific IgG antibody titers were tested in the sera. The Significant differences (p < 0.05) between the pHAV-4AngIIs(■) and the PBS(◆) group were indicated at weeks 2–12 by statistics analysis. (D) Effect of vaccination on the Ang II levels in sera. The Significant differences (p < 0.05) between the pHAV-4AngIIs (■) and the PBS(◆) group were indicated at weeks 4–12 by statistics analysis.(E) The correlation between SBP(●) and the AngII-specific IgG antibody titer(▲) in the pHAV-4AngIIs group. There is an inverse correlation between antibody titer and SBP(r = -0.734, p = 0.006). (F) The correlation between DBP( × ) and the AngII-specific IgG antibody titer(▲) in the pHAV-4AngIIs group. There is also an inverse correlation between antibody titer and DBP(r = -0.477, p = 0.027).
Detection of anti-AngII in sera
The sera of SHRs were collected at 0th, 2th, 4th, 6th, 8th, 10th and 12th week. The specific antibody levels induced by vaccine were evaluated with ELISA. The serum AngII-specific IgG antibody obviously increased in SHRs immunized with pHAV–4Ang IIs, and achieved the peak at 8th week. Negative ELISA result of AngII-specific antibody was detected in PBS control group ().
Detection of AngII in sera
The total serum Ang II levels in SHRs were measured at 0th, 2th, 4th, 6th, 8th, 10th and 12th week. Reductions in Ang II levels were found in the pHAV–4AngIIs-vaccinated SHRs at the 2th week, achieved statistical significance (p < 0.05) at 4th -12th week as compared with the PBS control group. At 8th week, a lower of approximately 87 pg / ml in Ang II level was tested ().
Discussion
It has been reported that the RAAS system is excessively activated in vivo and that the production of Ang II significantly increases during the development of hypertension. Furthermore, Ang II causes strong contraction of blood vessels by binding with AT1. Finally, blood pressure increases. Blood pressure can be decreased by inhibiting the RAAS system by reducing the expression or inhibiting the biological function of Ang II in hypertensive patients. Based on this assumption, Cytos Biotechnology AG Co., Ltd. (Schlieren, Switzerland) developed a hypertensive vaccine named CYT006–AngQβ. They obtained an eight-peptide sequence of Ang II, and a linker gene CGG was introduced into its N-terminal. Then Ang II sequence was anchored onto VLP of Qβ phage using this linker gene. A total of 180 copies of Ang II were located on the surface of Qβ phage-VLP, which was composed of 180 coat-protein monomers. The immunogenicity of vaccine was verified by animal experiments, phase I and II clinical trials; this vaccine successfully induced the generation of anti-Ang II neutralizing antibodies. Finally, the blood pressure was effectively decreased by blocking AngIICitation14–Citation16. Xie et al. also desigened an anti-hypertension DNA vaccine using Ang II as functional epitopeCitation17. Six-repeat Ang IIs (6AngIIs) was the core of this vaccine, and the hepatitis B virus core protein (HBc) was provided as an immune carrier. Furthermore, six Ang IIs were inserted between the 80th and 81st amino acid residues of HBc. As an adjuvant, interleukin IV was mixed with an equal volume of the vaccine. The vaccine created good immunity in SHRs. The above studies showed that AngII, as functional epitope, is feasible and effective in the treatment of hypertensive disease. In our study, Ang II was also selected as a functional epitope, but the carrier and protein expression system were different with previous studies.
The immunogenicity of Ang II is so weak that the host can induce immunological tolerance. To elevate the immunogenicity of Ang II and to activate the immune response of the host, we selected HAVLP as a carrier in this study. The HAV genome contains approximately 7.5 kb and an ORF, and it encodes a polyprotein of approximately 250 kDa. The structural gene P1 encodes four coat proteins (VP4, VP2, VP3 and VP1) and non-structural genes P2 and P3 encode seven non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C and 3D). As a proteolytic enzyme, 3C can hydrolyze the macromolecular protein into multiple polypeptides. VP0 (VP4 and VP2), VP3, VPX (VP1 and VP2A) constituted a protomer, and five protomers constituted a pentomer. Ultimately, the viral coat was composed of 12 pentomers Citation18. It has been reported that VP1 of the most particles with immature HAV combined with 2ACitation19; even a few 2A proteins are contained in mature HAV particlesCitation20. Beneduce et al. found that 2A was exposed on the surface of immature viral particles through gene analysis Citation11. Harmon et al. reported that deletion of amino acid residues at the middle fragment of HAV 2A had no effect on generating polyprotein, assembling viral particles and even the infection of HAVCitation12. Beneduce et al. replaced the 44th to 53rd amino acid residues of HAV 2A with the immunodominant determinant of exogenous HIV, gp41. Gp41 was successfully localized onto the surface of HAV particles. Furthermore, Yuri Y. Kusov et al. reported that the chimeric HAVLP carrying the HIV gp41 have a good antigenicity, immunogenicity and safety, can induce a mucosal immune response protecting both against HAV and HIV infections in marmosets and guinea pigsCitation11. Christian Savard et al. found that the HAVLP conjugated to NP of influenza viruses can induce a stronger TH1 humoral response to the HAV and NPCitation21. These findings indicated that HAVLP was a good carrier to express exogenous protein. Our study replaced the 8th to 39th amino acid residues of HAV 2A with 4AngIIs; the ORF of 2A and sequence of C-terminal were not changed. P1–2A–4Ang IIs and 3ABC were cloned by recombinant PCR using pT7–18f–4Ang IIs. These two gene fragments were inserted into the multiple clone sites of pFastBacTM Dual vectors located downstream of the PH and P10 promoters, respectively. P1–2A–4Ang IIs and 3ABC were co-expressed using the Bac-to-Bac Baculovirus Expression System. Furthermore, 3C hydrolyzed P1–2A–4Ang IIs into three coat proteins, including VP0, VP3 and VP1–2A–4 Ang IIs. These three proteins assembled a protomer containing 4Ang IIs. Five protomers constituted a pentomer, and 12 pentomers constituted a VLP containing 240 Ang IIs. The chimeric VLP pHAV–4Ang IIs effectively localized Ang II onto its surface. The antigen epitope of Ang II was easily recognized by the antigen receptor of lymphocytes because of the increase of Ang II antigen epitopes and the exposure of Ang II on the surface of VLP. Finally, the strong immune response was induced. The results of indirect immunofluorescence and western blot analyses suggest that pHAV–4Ang IIs present good biological activity and immune response in vitro.
The pFastBac™ Dual vector in a Bac-to-Bac Baculovirus Expression System can accommodate large fragments of exogenous gene, and multiple genes can be inserted into the plasmidCitation22. This facilitates the expression of these two genes. P1–2A–4Ang IIs and 3ABC were successfully inserted into two multiple clone sites of pFastBac™ Dual vector. The recombinant pFastBac™ Dual P1–2A–4AngIIs–3ABC plasmids were identified by double digestion. P1–2A–4Ang IIs and 3ABC genes were amplified using sf9 cells infected with recombinant baculoviruses. These two genes could not be amplified using normal sf9 cells. These findings suggest that the recombinant viral genome carried the two genes and that these two genes can be transcribed in sf9 cells. Simultaneously, the modification of proteins in sf9 cells was similar to that in mammalian cellsCitation23. The signal peptide can be recognized, removed, phosphorylated and glycosylated. The products presented high biological activation. Their configuration, antigenicity and immunogenicity were similar to the corresponding natural proteinsCitation24. Data from indirect immunofluorescence and western blot analyses suggest that pHAV–4Ang IIs present good biological activity and immune response. Additionally, 22-nm-diameter protein particles were clearly observed using a microscope in cytoplasm, suggesting that pHAV–4Ang IIs can simulate the natural configuration of HAVLP by Bac-to-Bac Baculovirus Expression System. Baculoviruses cannot infect vertebrates due to their high species specificity. The safety of baculoviruses facilitates wide application of vaccine development.
The AngII-specific antibody in the pHAV-4AngIIs-vaccinated SHRs were detected at 2th week after the prime immunization, and had been increasing after the boost and peaked at 8th week, then started to reduce at 10th week. This result suggests that pHAV-4AngIIs can break immunological tolerance and induce AngII-specific IgG antibody successfully in vivo, and also suggest that the antigenicity and immunogenicity of Ang II were significantly increased due to use the HAVLP carrier. With increase of serum AngII-specific IgG antibody titer, the concentration of serum AngII in the pHAV-4AngIIs-vaccinated SHRs reduced gradually accompanied When AngII-specific IgG antibody titer reached the peak at 8th week, a decrease trend of ~87 pg / ml in Ang II level was performed in the pHAV-4AngIIs-vaccinated SHRs as compared with the PBS control group. Simultaneously, the blood pressure in the pHAV-4AngIIs-vaccinated SHRs reduced gradually with the increase of antibody level. When antibody titer in the pHAV-4AngIIs-vaccinated SHRs reached the peak, the mean SBP degraded 23 mmHg compared with the PBS control group, and the DBP degraded 12mmHg compared with the PBS control group. These date showed that there is a direct correlation between AngII level and blood pressure, and an inverse correlation between antibody titer and AngII level or blood pressure. Further, these founding suggested that the antibody induced by pHAV-4AngIIs can effectively neutralize the serum AngII in vivo, shield the biological function of the serum AngII, block RAAS to cause blood pressure down.
Although pHAV-4AngIIs were obtained and induced AngII-specific IgG antibody successfully in vivo, and the BP was significantly lower compared with the PBS control group, there are some shortcomings in this study. First, the SHRs in the study were terminated at 12th week, there was no sera from further timepoints being available for long-term antibody titer tests. Long-term follow-up of antibody titer should be performed in future studies to study the decay of antibody from the immunological viewpoint. Second, the blood pressure in SHRs after two immunizations was still higher than normal rats (SBP: 120`150mmHg) although the antibody induced by pHAV-4AngIIs vaccine significantly can reduce the level of the serum AngII. So the dose and procedure of immunization should be further studied for treatment effectiveness. This study has proved the concept that the anti-hypertension AngII vaccine using HAVLP as carrier has good immunogenicity and good effect on reduction of BP in SHRs. However, a concern may exist, that any pre-existing immune responses that were induced as the result of HAV infection or vaccination may reduce the vaccine potency in the presence of HAV-specific antibodies, or alter the kinetics of responses by helper T - cells specific for the virus. Although we didn’t find that this concern had been an issue in our another unpublished study, in which strong antibody responses to IL-13 were successfully induced by HBcAg VLP-IL-13 immunization in mice with the presence of pre-existing anti-HBcAg immune responses, to fully address this point, an experiment will be designed and performed in our future study. The SHRs will be immunized with HAV vaccine first followed by pHAV–4Ang IIs immunizations. If an impact on VLP-Ang II immunization is found, passive transfer of anti-HAV antibodies or T cells will be given to animals before performing pHAV–4Ang IIs immunizations to further demonstrate which arm of and how the pre-existing anti-HAV immune responses affect the vaccine potency. We believe that a study designed like that will be helpful, at the level of an animal model, to clarify the clinical potency of a HAV VLP –based vaccine, but also we are aware of that more convincing conclusions would come from clinical trials.
In conclusion, the recombinant bacmid-P1–2A–4Ang IIs–3ABC was successfully constructed, and pHAV-4AngIIs were obtained using Bac-to-Bac Baculovirus Expression System in this study. This anti-hypertension vaccine can induce high titer AngII-specific antibody for almost 10 weeks accompanied with the degradation of serum angiotensin II level. The BP significantly decreased in the pHAV-4AngIIs-vaccinated SHRs compared with the PBS control group. These findings provide that the pHAV-4Ang IIs would be a new direction of exploration for the development of anti-hypertension therapeutic vaccine.
Materials and Methods
Bacteria, plasmids, cells, SHRs
The pT7–18f was kindly provided by Dr. Graziella Morace. DH5α, DH10 E. coli, pFastBacTM Dual vector and sf9 cells were kept in our laboratory. SHRs were purchased from Vitalriver Experimental Animal Technology Co., Ltd. (Beijing, China).
Reagents
Restriction enzymes, DNA polymerase, T4 DNA ligase, DNA marker and protein molecular weight marker were purchased from TaKaRa Biotechnology Co., Ltd. (Dalian, China). Goat anti-Ang II were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Fluorescein isothiocyanate (FITC)-tagged monkey anti-goat IgG was purchased from Invitrogen and HRP-tagged rabbit anti-goat IgG, HRP-tagged goat anti-rat IgG was purchased from KPL. A human angiotensin II (ANGII) ELISA kit was obtained from Qisong Biology, Co., Ltd. (Beijing, China).
Construction and identification of expressed plasmids
A four-repeat sequence of human Ang II (4Ang IIs) was genetically engineered. Afl II and Stu I were introduced at the 5′ and 3′ ends of the sequence. Furthermore, the sequence was inserted into pMD18-T, and the recombinant plasmid pMD18-T–4Ang IIs was obtained. Next, both pMD18-T–4Ang IIs and pT7–18f were double digested by Afl II and Stu I. 4Ang IIs and pT7–18f fragments were obtained, and the 4Ang IIs fragment was ligated with pT7–18f fragment. Finally, recombinant plasmid pT7–18f–4Ang IIs was obtained, and the 4Ang IIs fragment was inserted into the 2A region of HAV genome. P1–2A–4Ang IIs (2940 bp) and 3ABC (945 bp) fragments were cloned using pT7–18f–4Ang IIs as template through recombinant polymerase chain reaction (PCR). These two fragments were respectively inserted into the multiple clone sites of polycistron pFastBacTM Dual vector located downstream of the PH promoter and the P10 promoter. The recombinant plasmid pFastBac™ Dual P1–2A–4Ang IIs–3ABC was obtained and identified by double digestion. Furthermore, the plasmids were transformed into DH10 Bac™ E. coli for transposition into the bacmid. And these transposition bacteria were screened using blue-white spot assay. The recombinant bacmid- P1–2A–4AngIIs–3ABC were extracted from the bacteria with typical white spot. The plasmids were identified by polymer chain reaction (PCR) using the primers of M13: 5′-GTTTTCCCAGTCACGAC-3′ (sense) and 5′- CAGGAAACAGCTATGAC-3′ (anti-sense). Blue spot was used as a negative control. This construction of plasmids study design was illustrated in .
Culture of sf9 cells
As described previouslyCitation25, sf9 cells were cultured in Grace’s Insect Cell Culture Medium (pH≈6.2, Invitrogen, USA) containing 10% fetal bovine serum (FBS, Sijiqing, China) at 27°C without CO2. The cells were passaged using fresh complete medium.
Transfection of sf9 cells and harvest of recombinant baculovirus
When the cell confluent reached to 70%, sf9 cells were transfected with bacmid–P1–2A–4Ang IIs–3ABC using Cellfectin II reagent (Invitrogen, USA). The transfection was performed following the manufacturer’s instructions for the Bac-to-Bac® Baculovirus Expression System (Invitrogen, USA). A CPEof these transfected cells was observed using a common microscope. Medium was collected when the survival rate of cells decreased to 30–40%. Cells and fragments were removed by centrifuging at 3000 g for 10 min (Thermo, USA). Then the P1 recombinant baculoviruses were suspended in the supernatant. Next, sf9 cells were infected with P1 viruses, and P2 viruses with higher titers were obtained. By succession, the viruses with the required titer level were obtained.
Plaque assay
The 10-fold serial diluted viruses infected sf9 cells that were cultured in two six-well plates. One hour after the absorption, the first layer of plaquing medium was added. These cells and viruses were cultured at 27°C for 4 d, and a second layer of the neutral red (Biyuntian, China) overlay was added. The plaques were observed and counted 7–10 d later. To improve the visualization of plaques, the cells were fixed with pre-chilled 10% formaldehyde at 4°C for 30~40min,and the both of layers were discarded. Then the plaques were stained with 0.5% crystal violet (Xilong, China) for 5 min. Virus titer was calculated using the following equation: Virus titer (pfu/ml) = number of plaques × dilution factor × 1/ml of inoculum/well.
Observation of virus particles using a transmission electron microscope
The virus to condense solution was mixed with 2% phosphotungstic acid staining solution, and the mixed solution was dropped onto a membrane using a capillary. The recombinant baculoviral particles were observed using a transmission electron microscope (HITACHI H-600, Japan) after removing redundant solution. Sf9 cells that were infected with recombinant baculoviruses for 72 h were harvested and centrifuged at 1000 g for 10 min (Thermo, USA). The supernatant was discarded, and the cell precipitation was fixed using 2% glutaraldehyde pre-chilled to 4°C. The fixed precipitation was sectioned and stained with colloidal gold. The existence of baculoviruses in sf9 cells was observed using a transmission electron microscope (HITACHI H-600, Japan). Uninfected sf9 cells were used as a negative control.
RT-PCR analysis
Seventy-two hours after the sf9 cells were infected by the P3 recombinant baculovirus, the total RNA was isolated using the RNApure RNA Extraction Kit(Bio Teke, China) and used as template for reversetranscriptase PCR .The cDNA was synthesized using a reverse transcription PCR kit (Fermentas, USA)according to the manufacturer’s instructions. The profile of reverse transcription was as follows: 25°C for 5 min, 42°C for 60 min and 70°C for 5 min. Next, The obtained cDNA was used as PCR template using the same previous P1–2A–4Ang IIs and 3ABC primers and cycling conditions. Uninfected sf9 cells were used as a negative control.
Protein detection by SDS-PAGE and western blot
Sf9 cells that were infected by the P3 recombinant baculovirus for 72 h were harvested and centrifuged at 1000 g for 10 min (Thermo, USA). The supernatant was discarded, and the precipitation was resuspended in TNE buffer (40 mM Tris, 100 mM NaCl, 2 mM MgCl2, 1 mM EDTA, pH 7.4). The mixture was frozen to -80°C and thawed four times. The mixture was centrifuged at 10000 g for 30 min at 4°C (Thermo, USA). The supernatant containing pHAV–4Ang IIs was collected and analyzed using 12% sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE). Uninfected sf9 cells were used as a negative control.
The recombinant proteins were analyzed using 12% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk for 2 h and incubated with primary antibodies of goat anti-Ang II and secondary antibodies of HRP-tagged rabbit anti-goat IgG. The hybridized protein bands were detected using an enhanced chemiluminescence (ECL) assay. Uninfected sf9 cells were used as a negative control.
Indirect immunofluorescence assay
Forty-eight hours after the sf9 cells were infected by the P3 recombinant baculovirus, the medium was removed. The cells were fixed with pre-chilled 10% formaldehyde at 4°C for 30 min. Then the cells were blocked with 5% skimmed milk for 2 h. The expression of pHAV–4Ang IIs was detected using primary antibodies of goat anti-Ang II and secondary antibodies of FITC-tagged monkey anti-goat IgG. Uninfected sf9 cells were used as a negative control. The specific fluorescence in the infected cells was observed using a fluorescence microscope.
Observation of VLP using a transmission electron microscope
VLPs of pHAV–4Ang IIs in sf9 cells were observed using a transmission electron microscope (HITACHI H-600, Japan), and the uninfected sf9 cells were used as a negative control.
Preparation of pHAV–4Ang IIs particles
Sf9 cells that were infected by the P3 recombinant baculovirus at a MOI of approximately 1 for 72 h were harvested and centrifuged at 1000 g for 10 min (Thermo, USA). The supernatant was discarded, and the cells were resuspended in TNE buffer. The infected sf9 cells was frozen to -80°C and thawed four times. The cell debris was pelleted by centrifugation at 10000 g for 30 min (Thermo, USA) at 4°C and the supernatant containing pHAV–4Ang IIs was collected. To enhance protein extraction, the cell debris was incubated for 30 min at 4°C with TNE buffer supplemented with 1% NP-40 and a proteinase inhibitor cocktail. After centrifugation at 10000 g for 30 min (Thermo, USA) at 4°C, the supernatants were pooled and loaded onto a no-linear sucrose / glycerol gradientCitation26,Citation27. After centrifugation at 4°C for 6h at 180000 g (himac CP56G, HITACHI, Japan), the supernatant was discarded, and the precipitation were resuspended in PBS buffer. The mixture containing high- purity pHAV–4Ang IIs particles was obtained for immunization of SHRs.
Immunization of SHR
Twenty 12-week-old male SHRs weighing approximately 250 g, obtained from Vitalriver Experimental Animal Technology Co., Ltd. (Beijing, China), were housed and cared in compliance with relevant guidelines and requirements. SHRs were divided into two groups in a randomized fashion and immunized in the quadriceps muscle (i.m.) with 250 μg purified pHAV–4AngIIs or 250ul PBS as control, respectively. Primary immunization was administrated once accommodation. After 3 weeks, the SHRs were boosted one dose. Complete Freund's adjuvant was used in the primary immunization and incomplete Freund's adjuvant for the boost.
Measurement of blood pressure
Blood pressure in SHRs was measured by tail-cuff arterial blood pressure measurement method, using BP-98A blood pressure meter (Softron, China) according to the manufacturer’s instructions.
Detection of Ang II in sera
A human angiotensin II (ANGII) ELISA kit, obtained from Qisong Biology, Co., Ltd. (Beijing, China), was used to test Ang II level in SHR’s sera, according to the manufacturer’s instructions.
Detection of anti-AngII in sera
Anti-AngII antibody level in sera was measured by ELISA. ELISA plate wells were coated with the AngII (conjugated to bovine serum albumin,BSA) diluted final concentration 10μg/ml in carbonate-bicarbonate buffer (PH 9.6) overnight at 4°C. The coated wells were washed three times with PBS containing 0.03% Tween-20 (PBS-T).The plates were then blocked with PBS-T containing 1% w/v BSA for 2h at 37°C. Diluted sera (range from 10- to 20480-fold in PBS) from the vaccinated SHRs were then incubated for 1h at 37°C, followed by the addition of goat anti-rat IgG-HRP diluted 2000-fold in PBS for 1h at 37°C. TMB (TIANGEN, China) was used as the peroxidase chromogenic substrate to react for 3 min at room temperature. The reaction was blocked by the addition of 2 mol / L H2SO4. Optical densities (OD) at 450 nm were measured, using an automated plate reader (Model 550, Bio-Rad). Sera collected at 0th week were used as negative control. A sample was considered positive if the OD was greater than or equal to 2.1 times that of negative sera. Antibody levels were defined as the reciprocal of the highest dilution.
Acknowledgments
The authors greatly thank Dr Graziella Morace of National Center for Immunobiologicals Research and Evaluation (CRIVIB)-Instituto Superiore di Sanità (Rome, Italy) offering pT7–18f. And also thank Dr ZHONG Jiang and LI Binbin of Department of Microbiology and Microbial Engineering, School of Life Sciences, Fudan University, Shanghai, China for expert technical assistance. The authors thank all members of Department of Molecular Biology, Institute of Medical Biology, Chinese Academy of Medical Science, Peking Union Medical College (Kunming, China), for their kind assistance. This work was supported by the Research Fund for Doctor Innovation of Beijing Union Medical College.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Liu LS. Chinese guidelines for the management of hypertension. 3th ed. Beijing: Chinese center for disease control and prevention press. 2011.
- World Health Organization. Non communicable diseases. Hypertension 2011;
- Shu YH, Xiao WX. Advances in Pharmacotherapy for Hypertension. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2012; 20:385 - 6
- North of England Hypertension Guideline Development Group (UK).. Essential Hypertension: Managing Adult Patients In Primary Care [Internet]. National Institute for Health and Clinical Excellence: Guidance. 2004; PMID: 20945577
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206 - 52; http://dx.doi.org/10.1161/01.HYP.0000107251.49515.c2; PMID: 14656957
- Volpe M, Savoia C, De Paolis P, Ostrowska B, Tarasi D, Rubattu S. The renin-angiotensin system as a risk factor and therapeutic target for cardiovascular and renal disease. J Am Soc Nephrol 2002; 13:Suppl 3 S173 - 8; http://dx.doi.org/10.1097/01.ASN.0000032549.36050.78; PMID: 12466309
- González A, López B, Díez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am 2004; 88:83 - 97; http://dx.doi.org/10.1016/S0025-7125(03)00125-1; PMID: 14871052
- Krum H, Gilbert RE. Novel therapies blocking the renin-angiotensin-aldosterone system in the management of hypertension and related disorders. J Hypertens 2007; 25:25 - 35; http://dx.doi.org/10.1097/HJH.0b013e3280113950; PMID: 17143168
- Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet 2007; 369:1208 - 19; http://dx.doi.org/10.1016/S0140-6736(07)60242-6; PMID: 17416265
- Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 2006; 7:3 - 14; http://dx.doi.org/10.3317/jraas.2006.003; PMID: 17083068
- Kusov YY, Zamjatina NA, Poleschuk VF, Michailov MI, Morace G, Eberle J, et al. Immunogenicity of a chimeric hepatitis A virus (HAV) carrying the HIV gp41 epitope 2F5. Antiviral Res 2007; 73:101 - 11; http://dx.doi.org/10.1016/j.antiviral.2006.08.003; PMID: 17014915
- Beneduce F, Kusov Y, Klinger M, Gauss-Müller V, Morace G. Chimeric hepatitis A virus particles presenting a foreign epitope (HIV gp41) at their surface. Antiviral Res 2002; 55:369 - 77; http://dx.doi.org/10.1016/S0166-3542(02)00073-6; PMID: 12103436
- Harmon SA, Emerson SU, Huang YK, Summers DF, Ehrenfeld E. Hepatitis A viruses with deletions in the 2A gene are infectious in cultured cells and marmosets. J Virol 1995; 69:5576 - 81; PMID: 7637003
- Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008; 371:821 - 7; http://dx.doi.org/10.1016/S0140-6736(08)60381-5; PMID: 18328929
- Ambühl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens 2007; 25:63 - 72; http://dx.doi.org/10.1097/HJH.0b013e32800ff5d6; PMID: 17143175
- Miller SA, Accardi JR, St Onge EL. Angiotensin II vaccine: a novel approach in the treatment of hypertension. Expert Opin Biol Ther 2008; 8:1669 - 73; http://dx.doi.org/10.1517/14712598.8.11.1669; PMID: 18847303
- Xie YF, Wu RF, Wu J, Li TM, Cao RY, Liu JJ. The construction and pharmacodynamic action of a novel anti-renal hypertension DNA vaccine targeted angiotensin. Pharm Biotechnol 2008; 15:79 - 85
- Jin Q. Medical& Molecular virology. Beijing: Science Press. 2001.
- Probst C, Jecht M, Gauss-Müller V. Intrinsic signals for the assembly of hepatitis A virus particles. Role of structural proteins VP4 and 2A. J Biol Chem 1999; 274:4527 - 31; http://dx.doi.org/10.1074/jbc.274.8.4527; PMID: 9988685
- Anderson DA, Ross BC. Morphogenesis of hepatitis A virus: isolation and characterization of subviral particles. J Virol 1990; 64:5284 - 9; PMID: 2170672
- Savard C, Laliberté-Gagné MÈ, Babin C, Bolduc M, Guérin A, Drouin K, et al. Improvement of the PapMV nanoparticle adjuvant property through an increased of its avidity for the antigen [influenza NP]. [influenza NP] Vaccine 2012; 30:2535 - 42; http://dx.doi.org/10.1016/j.vaccine.2012.01.085; PMID: 22326774
- Xu L, Yang D, Chen T, Su K, Ning Q. Expression of hKCTD9 protein in insect- baculovirus expression system in vitro. J Clin Hepatol 2010; 13:249 - 52
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 2005; 23:567 - 75; http://dx.doi.org/10.1038/nbt1095; PMID: 15877075
- Liu YP, Wang FH, Su ZJ, Li GH, Pang Y. Progress of studies and application on baculovirus expression vector system. J Chinese Bulletin of Entomology. 2006; 51:1 - 7; http://dx.doi.org/10.1007/s11434-006-8201-4
- Jin XH, Meng ZF, Chen CG. The Comparative Study on calf serum and fetal bovine serum on Sf9 cells growth and recombinant protein expression. Chinese Journal of Pathophysiology 2006; 22:415 - 6
- Anderson DA, Ross BC. Morphogenesis of hepatitis A virus: isolation and characterization of subviral particles. J Virol 1990; 64:5284 - 9; PMID: 2170672
- Bishop NE, Hugo DL, Borovec SV, Anderson DA. Rapid and efficient purification of hepatitis A virus from cell culture. J Virol Methods 1994; 47:203 - 16; http://dx.doi.org/10.1016/0166-0934(94)90078-7; PMID: 8051227