Abstract
Herpes simplex virus (HSV) type 2 (HSV-2) is the main cause of genital and neonatal herpes and is highly prevalent worldwide. Previous phase I and II studies showed the immunogenicity and safety of the candidate prophylactic HSV-2 glycoprotein D-based subunit vaccine (gD2-AS04), containing aluminum hydroxide and 3-O-deacylated monophosphoryl lipid A (MPL) as adjuvant (AS04), in healthy adults. The primary objective of the study presented here was to compare the immunogenicity and safety of five different vaccine formulations: 3 different antigen doses [20, 40 or 80 μg of truncated glycoprotein D from HSV-2 strain (gD-2t)], different aluminum salts [AlPO4 or Al(OH)3], different preservatives or different volumes of vaccine (0.5 or 1 ml). One hundred and fifty healthy men and women aged 18–45 years, with negative serological markers for HSV-1 and HSV-2 infection, were vaccinated with one of 5 formulations of the gD2-AS04 candidate vaccine according to a 0-, 1-, 6-month schedule. No statistically significant difference was observed in humoral or cellular immune responses between different antigen doses or the different aluminum salts, preservatives or volumes of vaccine. The gD2-AS04 vaccine was well tolerated by study participants for the duration of the study period. Local symptoms were more frequently reported than general symptoms, with muscle stiffness and/or injection site redness being the most frequently reported. Overall, the incidence of adverse events was comparable in all groups. Based on these results the gD2-AS04 formulation, containing 20 μg of gD-2t, was selected for evaluation of prophylactic efficacy in further clinical trials.
Introduction
Herpes simplex virus (HSV) types 1 (HSV-1) and 2 (HSV-2) cause genital herpes. HSV-2 is highly prevalent: in 2003 there were an estimated 536 million people 15–49 years of age infected with HSV-2 worldwide.Citation1 The prevalence of infection with HSV-2 varies by geographical area, age, sex and socio-economic status.Citation2-Citation5 A nationally representative survey conducted in the US between 1999 and 2004 estimated an overall age-adjusted seroprevalence of HSV-2 ranging from 1.6% among adolescents aged 14 to 19 years to 26.4% among adults aged 40 to 49 years. Seroprevalence in adults was twice as high for women as it was for men, irrespective of age group.Citation5
Although the risk of transmission is greater during initial symptomatic infection, most spread occurs as a consequence of asymptomatic shedding of HSV during recurrent infections.Citation6 Prophylactic administration of antivirals can reduce the risk of HSV transmission,Citation7 but less than 10% of infected people take medication.Citation8 The clinical implications of a vaccine that prevents primary and recurrent herpes simplex disease as well as rare but serious consequences, such as neonatal infection, would be significant.
Until now, no vaccine has been licensed for the prevention of herpes simplex infection or disease. A prophylactic HSV-2 glycoprotein D-based subunit candidate vaccine (gD2-AS04) containing aluminum hydroxide and 3-O-deacylated monophosphoryl lipid A (MPL) as adjuvant (AS04) has been developed by GlaxoSmithKline Vaccines.
Results from clinical trials have shown that the candidate vaccine elicits binding and neutralizing antibodies against HSV as well as HSV-specific cellular immune responses in both men and women.Citation9,Citation10 Based on these promising immunogenicity results, and on an acceptable safety profile, studies to evaluate the efficacy of gD2-AS04 containing 20 μg of truncated glycoprotein D from HSV-2 strain G (gD-2t) were performed. In these studies, conducted in persons whose partners had recurrent HSV genital disease, gD2-AS04 vaccination resulted in a > 70% reduction in HSV genital disease and a 40% reduction in HSV infection in HSV-1 and HSV-2-seronegative women but not in men or in HSV-1-seropositive women.Citation10 To further examine the efficacy of the candidate gD2-AS04 vaccine, a large efficacy trial involving 8,323 HSV-1 and HSV-2 seronegative women aged 18 to 30 years was conducted. Administration of the gD2-AS04 candidate vaccine demonstrated no overall efficacy against genital herpes disease. However, a disparity between efficacy against HSV-1 and HSV-2 was observed: 35% efficacy against HSV-1 infection and 58% efficacy against HSV-1 genital disease (both statistically significant) was shown, but no efficacy against HSV-2 infection (-8%, 95%CI: -59–26).Citation11
The development program of this candidate vaccine was stopped because of the lack of efficacy observed in the HERPEVAC Trial for Women.Citation11 Other candidate vaccines are currently being developed, some of which depend on immune responses to HSV gD. It is therefore important to make all relevant data on previous HSV gD-based vaccines available to the research community, including previously unpublished results from clinical trials conducted as part of a GlaxoSmithKline’s HSV vaccine development program.
The study presented here, which preceded the aforementioned efficacy studies, compared the immunogenicity and safety of 5 different formulations of the herpes simplex candidate vaccine in healthy HSV-seronegative subjects in order to define an optimal formulation for further studies.
Results
Study population
Overall, 80 men and 70 women were enrolled, received the first dose of the study vaccine and were included in the analysis (30 participants in each of the 5 groups) ( and ). Of these, 148 participants completed the study. Two participants withdrew their consent after the first dose: one in group 2 and one in group 4. There was no statistically significant difference in the male/female ratio between groups (p = 0.52, Chi square test). The mean age of the study participants was 22.3 years, ranging from 18 to 42 years (). The two-way ANOVA test showed no statistically significant differences in mean age between groups and gender: group effect (p = 0.93), gender effect (p = 0.46) and interaction group-gender effect (p = 0.98).
Figure 1. Flow diagram illustrating enrolment, randomization and follow-up of study participants. Group 1 = gD-2t 40 μg, Group 2 = gD-2t 80 μg, Group 3 = gD-2t 20 μg, Group 4 = gD-2t 20 μg, Group 5 = gD-2t 20 μg. Vaccine formulations administered to groups 1 to 4 contained phenoxyethanol preservative and those administered to group 5 contained thiomersal preservative. The vaccine formulations administered to groups 1–3 and 5 contained Al(OH)3. The vaccine formulations administered to group 4 contained AlPO4.
Table 1. Anti HSV-2 candidate vaccine formulations administered during the study
Table 2. Demographic characteristics of vaccinated participants
Immunogenicity
Anti-gD antibodies
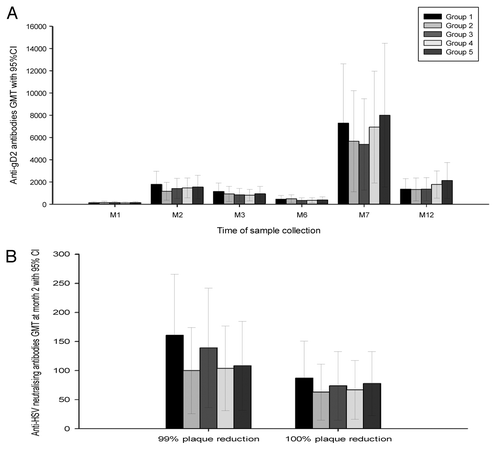
One month after the second dose, at least 97% of the participants in group 1 and all participants in groups 2–5 had anti-gD2 antibodies ≥ 40 enzyme-linked immunosorbent assay (ELISA) units per ml (EL.U/ml). Geometric mean titers (GMTs) ranged from 1,165.3 EL.U/ml in group 2 to 1,791.3 EL.U/ml in group 1. After the third vaccine dose, seropositivity rates were 100% in all treatment groups and GMTs ranged between 5,376.9 EL.U/ml in group 3 and 8,003.4 EL.U/ml in group 5. All vaccine groups were comparable in terms of seropositivity rates and GMTs at all time points for which blood samples were taken (p = 0.89 at one month post dose 2, p = 0.16 at one month post dose 3 and p = 0.11 at month 12). Although lower than between one-month post dose 2 and one-month post dose 3, anti-gD antibody levels remained elevated compared with pre-vaccination levels at month 12 in all subjects ().
Figure 2. Geometric mean gD specific antibodies (panel (A) and HSV neutralizing antibodies (panel (B) levels by study visit in each treatment group. Group 1 = gD-2t 40 μg, Group 2 = gD-2t 80 μg, Group 3 = gD-2t 20 μg, Group 4 = gD-2t 20 μg, Group 5 = gD-2t 20 μg. GMT, geometric mean antibody titer; CI, confidence interval; M, month.
HSV neutralizing antibodies
With an assay endpoint of 99% plaque reduction, HSV neutralizing antibodies at month 2 (one month after the second dose) were detected in at least 65% of participants in each group. All vaccine formulations were comparable in terms of seropositivity rates (p = 0.34) and GMTs (p = 0.74) ().
Cell mediated immune response
A global increase in lymphoproliferation (Integrated Stimulation Index - ISI) and interleukin-2 (IL-2) secretion was observed after the second and the third vaccine doses in all groups ().
Figure 3. Levels of integrated stimulation index (panel (A), interleukin-2 (panel (B), γ interferon (panel (C) by study visit in each treatment group. Group 1 = gD-2t 40 μg, Group 2 = gD-2t 80 μg, Group 3 = gD-2t 20 μg, Group 4 = gD-2t 20 μg, Group 5 = gD-2t 20 μg. ISI, integrated stimulation index; IL-2, interleukin-2; γ-IFN, gamma interferon; M, month.
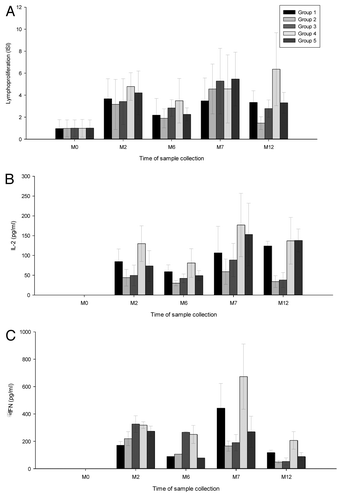
The median ISI ranged from 3.16 in group 2 to 4.78 in group 4 and from 3.48 in group 1 to 5.47 in group 5 at one month after the second and third doses, respectively (). At month 12, six months following dose 3, median ISI was significantly higher in group 4 compared with group 2 (p = 0.001), group 3 (p = 0.0192) and group 5 (p = 0.0118). Also, median ISI was higher in participants in group 1 compared with participants in group 2 (p = 0.0074). No significant difference was observed between median ISI values in groups 5 and 2 at this time point (p = 0.0724). Additionally, no significant differences were observed between the study groups at any other time point.
At all time points the levels of IL-2 in micro-culture supernatants were lower in groups 2 and 3 than in groups 1, 4 and 5 (). The median IL-2 levels ranged from 44 pg/ml in group 2 to 130 pg/ml in group 4 and from 59 pg/ml in group 2 to 177 pg/ml in group 4 at one month after the second and third doses, respectively. At month 2, IL-2 levels were significantly higher in groups 1, 4 and 5 than in group 2 (p values: 0.0263, 0.002 and 0.0243, respectively) and in group 4 than in group 3 (p = 0.0243). These differences remained significant at months 7 and 12 (p values were 0.0097 and 0.0284 for differences between groups 1 and 2 at months 7 and 12, respectively; p = 0.0003 and 0.0004 for differences between groups 5 and 2; p = 0.0002 for differences between groups 4 and 2 at both time points; p = 0.0301 and 0.0111 for differences between groups 4 and 3). Additionally, at month 12, IL-2 levels were higher in group 5 compared with group 3 (p = 0.0110).
Increases in gamma interferon (IFN-γ) secretion were observed after the second vaccine dose in all groups (). After the third dose (month 7) IFN-γ secretion increased relative to month 6 levels in groups 1, 2, 4 and 5, but not in group 3. In group 3, IFN-γ levels were highest after the second dose and then showed a steady decrease through month 12. IFN-γ levels ranged from 170.5 pg/ml in group 1 to 325.50 pg/ml in group 3 and from 165.0 pg/ml in group 2 to 673.0 pg/ml in group 4 at one month after the second and third doses, respectively. At month 7, IFN–γ levels were higher in groups 1 and 4 compared with group 2 (p values: 0.0402 and 0.0080) and in group 4 compared with groups 3 and 5 (p values: 0.0448 and 0.0487). At month 12, IFN–γ levels were higher in group 4 compared with groups 2 and 3 (p values: 0.0039 and 0.0169).
Safety and reactogenicity
The incidence of solicited and unsolicited symptoms is shown in and . The compliance in returning symptom sheets was 100% in groups 1, 3, 4 and 5 and 98.9% in group 2. There was no statistically significant difference between groups in the incidence of unsolicited/solicited local and/or general solicited symptoms reported. The overall incidence of solicited local and general symptoms was similar in all groups (p > 0.05): at least one adverse event (AE) was reported following more than 90% of vaccine doses in each group.
Table 3. Incidence of solicited symptoms (overall/dose) on Days 0–3 post-vaccination
Table 4. Most frequently reported (≥ 5 cases) unsolicited symptoms
The most common solicited local symptoms were muscle stiffness and soreness, reported by at least 78.9% and 57.5% of participants in each group. Sixteen grade 3 solicited local symptoms were reported by 10 participants: one in groups 1 and 3, two in group 2 and three in groups 4 and 5.
Headache and fatigue were the most frequently reported solicited general symptoms. These symptoms were reported after at least 9.1% and 13.3% of doses, respectively (). Seventeen solicited general symptoms were considered to have a causal relationship with the vaccination and 125 of 186 (67.2%) were considered to be possibly related. Six of 186 solicited general symptoms reported were classified as Grade 3 (). One participant in group 5 reported fever > 39.0°C on day 0, which was considered to be unrelated to vaccination.
Overall, 174 unsolicited AEs were reported: 41 AEs reported by 21 participants in group 1, 28 AEs reported by 19 participants in group 2, 30 AEs reported by 15 participants in group 3, 38 AEs reported by 17 participants in group 4 and 37 AEs reported by 19 participants in group 5. Nine serious adverse events (SAEs) were reported (5 in group 1, 1 in group 4 and 3 in group 5). All involved hospitalizations and all were considered to be unrelated to vaccination.
Discussion
Recent advances in understanding the pathophysiology of HSV infections have led to the development of a number of candidate vaccines. Due to safety concerns associated with live attenuated vaccines and poor results in immunogenicity studies of the inactivated vaccines, research has focused on developing vaccines containing viral proteins such as DNA, peptides and virus-like particles. It has been shown that the immune response following HSV infection is primarily directed toward glycoproteins from the viral envelope.Citation12-Citation14 One of these proteins is gD. Many antigenic determinants of the gDs are shared by both HSV types (HSV-1 and HSV-2).Citation15 Antibodies against gD provide protection following passive immunization or vaccination in animalsCitation16 and the majority of virus-neutralizing antibodies are directed toward this glycoprotein.Citation17 In light of such findings, recombinant gD-2t was chosen for inclusion in the prophylactic candidate vaccine developed by GlaxoSmithKline. The immunoenhancer MPL was added to the aluminum salt to enhance the immune response to gD-2t.Citation18
Preclinical studies and early studies performed in humans have indicated that the use of MPL and aluminum as an adjuvant in combination with gD-2t improves stimulation and persistence of humoral and cellular immune responses, while maintaining an acceptable reactogenicity profile.Citation19 Given that both cell-mediatedCitation20 and humoral immune responsesCitation18 are considered to be necessary for the prevention of herpetic infections, GlaxoSmithKline has incorporated MPL into its gD2-AS04 vaccine formulation.
The randomized controlled trial presented here compares five different candidate vaccine formulations in terms of both immunogenicity and safety. All candidate vaccine formulations induced high titers of anti-gD and neutralizing antibodies as well as a robust T-cell response. No dose-related increase in immunogenicity was observed: comparable immune responses were observed after the administration of vaccine formulations containing 20, 40 or 80 µg of gD-2t. Additionally, no statistically significant difference was observed in immune responses (humoral and cellular) induced by different preservatives or the different volumes of vaccine. Cellular immune responses (evaluated through quantification of ISI, IL-2 and IFN–γ secretion) observed at month 12 were significantly higher in participants from group 4 than those observed in group 3 participants. Additionally, significantly higher IL-2 levels were observed at months 2 and 7 and higher IFN–γ secretion was observed at month 7 in participants from group 4 compared with group 3. The vaccines administered in these two study groups had the same quantities of antigen (20 µg of gD-2t), the same immunostimulant (MPL) and the same preservative (phenoxyethanol), but they contained different aluminum salts: AlPO4 in group 4 and Al(OH)3 in group 3. Despite the differences observed in cellular immune responses, both the AlPO4 and Al(OH)3 containing vaccines elicited strong and similar humoral immune responses. It should be noted that both AlPO4 and Al(OH)3 are used in licensed vaccines.
In terms of reactogenicity, the gD2-AS04 HSV vaccine was well tolerated by study participants for the duration of the study period. Local symptoms were more frequently reported than general symptoms, with muscle stiffness and/or soreness at the injection site being the most frequently reported. Overall, the incidence of AEs was comparable in all groups. Based on these results the gD2-AS04 formulation containing 20 μg of gD-2t was selected for evaluation of prophylactic efficacy in further clinical trials.
As this trial was performed more than 10 years ago, the assays employed are no longer considered state-of-the-art and thus represent a limitation of our study. Cellular immune responses to vaccine formulations were evaluated by analyzing human peripheral blood mononuclear cell proliferation and cytokine production in response to antigen stimulation. No additional information about the nature of the cellular immune response to the vaccine formulations could be obtained using these assays.
These immunogenicity results are consistent with results obtained in a number of other clinical studies designed to test the efficacy, immunogenicity and safety of the gD2-AS04 vaccine formulation containing 20 μg of gD-2t.Citation9-Citation11
Results from a randomized controlled trial including 380 vaccine recipients and 176 placebo recipients showed that the candidate vaccine induced anti-gD antibodies in all seronegative recipients and that these antibodies persisted for at least 6 months after vaccination.Citation9 Titers of anti-gD antibodies after vaccination were higher than those induced by natural infection with HSV.Citation9 Furthermore, the HERPEVAC trial, in which participants received the same vaccine formulation, demonstrated that administration of three vaccine doses led to a marked increase in anti-gD antibodies that waned over time.Citation11 Overall, results of that trial showed no vaccine efficacy against HSV disease (20%, 95%CI: -29–50).Citation11
These rather disappointing results in the HERPEVAC trial might raise additional questions regarding the antigen dose that was chosen to be included in the candidate HSV vaccine. Based on the dose-ranging study presented here, the vaccine formulation containing 20 μg of gD-2t antigen was chosen for further development because it elicited humoral and cellular mediated immune responses that were comparable to responses elicited by formulations containing higher doses of antigen (40 or 80 μg of gD-2t antigen). No dose-related increase in immune response was observed. This suggests that the failure of the candidate vaccine to demonstrate efficacy against HSV disease and infection in the HERPEVAC trial was not due to an inadequate gD-2t antigen dose.
The negative results from the HERPEVAC trial, together with the large potential socioeconomic impact of an effective vaccine, have renewed the interest in development of an HSV vaccine. A prophylactic HSV vaccine would be an attractive alternative to existing treatment options and may have considerable impact if it also reduced viral transmission. Different approaches are currently being explored including glycoprotein-based subunit vaccines,Citation21,Citation22 DNA vaccines,Citation23,Citation24 peptide vaccines and live vectored vaccines.Citation25 Data such as those presented here could play an important role in guiding new vaccine approaches that may control or prevent HSV disease.
Materials and Methods
Study design and participants
This was a Phase I/II, double-blind, randomized, single-center, dose-ranging study, with five parallel groups conducted in Belgium. Group 5 was unblinded to the investigators because a 1 ml volume of vaccine was given, compared with 0.5 ml in groups 1–4.
Subjects were randomized on a 1:1:1:1:1 basis to receive one of the five vaccines formulations of the candidate herpes simplex vaccine according to a 0–1-6 month schedule and were followed up to month 12. Monodose vials of the vaccines were coded according to randomization lists prepared by GlaxoSmithKline using an algorithm of pseudo random numbers. Subjects eligible for inclusion in the study were allocated a study number in the order in which they were enrolled. Each subject received only the vaccines labeled with his/her study number.
The study protocol and all study-related documents were approved by the Ethical Committee of the Ghent University Hospital in 1995. The study was conducted in accordance with the Declaration of Helsinki as amended in 1989 and Good Clinical Practice guidelines in operation at the study initiation. Written informed consent was obtained from all the subjects prior to any study procedures.
The study started in March 1995 and was completed in March 1996. The final study report was available from December 4, 1996. A summary of the study protocol is available at www.gsk-clinicalstudyregister.com (Study ID 208141/014).
Study population
One hundred and 50 healthy men and women, aged 18–45 years, were enrolled between March 1995 and March 1996. All women had negative serological markers for HSV-1 and HSV-2 infection as assessed by gD ELISA. Women were required to use effective birth control measures. Exclusion criteria included: pregnancy or lactation, any previous vaccination against herpes simplex, any previous administration of MPL and history of allergic disease likely to be exacerbated by the vaccination, history of convulsions, epilepsy, or any other signs of central nervous system disease, any suspected or confirmed immune disorder or immunosuppressive therapy.
Vaccines
The prophylactic candidate vaccine used in this study was an adjuvanted recombinant subunit vaccine manufactured by GlaxoSmithKline Vaccines. The antigen component of the candidate vaccine was gD-2t from HSV-2 strain G at concentrations of 20, 40 or 80 µg (according to study group). The adjuvant system was AS04, containing 50 µg MPL and 0.5 mg of aluminum salt (see ).Citation18 The vaccine in group 5 contained thiomersal preservative and the other vaccines contained phenoxyethanol preservative. Additionally two different types of aluminum salts were used: AlPO4 in group 4 and Al(OH)3 for the vaccines used in groups 1–3 and 5.
Study objectives
The objective of this study was to compare, in terms of humoral and cellular immune response, the immunogenicity of different formulations of herpes simplex candidate vaccine. Another objective aimed at assessing the safety and reactogenicity of the five vaccine formulations in terms of solicited local and general AEs during study period.
Criteria for evaluation
Immunogenicity
Blood samples were collected for evaluation of anti-gD antibodies, anti-HSV neutralizing antibodies, and CMI at screening, prior to the first vaccination, one month post dose 1 (month 1) and dose 2 (month 2), 2 month post dose 2 (month 3), pre-dose 3 (month 6), one month post dose 3 (month 7) and six months post dose 3 (month 12). Seropositivity rates and GMTs, with 95% confidence intervals for anti-gD antibodies and anti-HSV neutralising antibodies were calculated by group for all time points for which blood samples were taken. Cell mediated immunity was evaluated through lymphoproliferation assays and quantification of IL-2 and IFN–γ secretion.
Reactogenicity
Local and general AEs were recorded by the study participants on a diary card on the day of vaccination and for the three subsequent days. Additionally, unsolicited symptoms were recorded during 30 days after each dose and data regarding any SAEs were collected throughout the study period.
Laboratory assays
Serum was harvested from whole blood samples and kept at -20°C until analyzed at GlaxoSmithKline Vaccines.
Serological status at study enrollment and humoral immune response to vaccine were assessed by two ELISAs for anti-gD antibody titers.Citation9,Citation10 These ELISAs were developed by GlaxoSmithKline Vaccines. Serum samples were incubated in 96-well plates coated with gD-2t antigen. The presence of anti-gD antibodies was revealed by addition of an anti-human conjugate. Human control sera with known titers were included in each assay for validation of results. Anti-gD antibody titers were expressed EL.U/ml, with reference to a standard serum, using four parameter method.Citation26 The cut-off for the assays was set at 40 EL.U/ml. Subjects with titers ≥ the cut-offs were considered as seropositive.
The presence of HSV-neutralizing antibodies was also evaluated using an in-house assay (GlaxoSmithKline Vaccines). Serially diluted serum was incubated with HSV-2 virus particles (4,000 plaque forming units) and complement in 96-well plates. Baby hamster kidney cells were then added. After incubation, the presence of viral plaques was determined by microscopic examination. Two end points were used in this assay: neutralizing titers corresponding to the reciprocal of the highest serum dilution at which no viral plaques were observed (100% neutralization) or to the reciprocal of the serum dilution giving 99% inhibition of cytopathogenic effect (99% neutralization). Human control serum was added to each plate for assay validation. The first dilution of this test was 1/50. Subjects with titers ≥ 50 were considered seropositive.
Peripheral blood mononuclear cells separated from heparinized whole blood samples were suspended in freezing media and kept in liquid nitrogen until cell mediated immune responses were assessed in the laboratory of the Center for Vaccinology (Ghent University and Hospital). The in vitro T cell response to gD-2t was evaluated through lymphoproliferation and data were expressed as integrated stimulation indices as described previously.Citation27 Supernatants were harvested from the lymphoproliferation cultures before addition of 3H-thymidine at 120 h and tested for the presence of IL-2 and IFN–γ by ELISA tests (after 48 and 96 h).
Statistical method
Sample size calculation
Considering 80% power and a type I error of 5%, it was estimated that the inclusion of 20 subjects in each group would allow detection of a 66% decrease in GMTs and a 25% decrease in seropositivity rates, while the inclusion of 30 subjects will allow detection of a 59% decrease in GMTs and a 20% decrease in seropositivity rates. Based on this sample size calculation the inclusion of 20 to 30 subjects in each vaccine group was planned.
Seropositivity rates between groups were compared using Fisher’s exact test and GMTs between groups were compared using one-way ANOVA test. The percentage of doses followed by a report of any symptom during the 4-day-follow-up period (solicited or unsolicited) was reported for each group. The incidence of local and/or general symptoms was compared using Fisher’s exact test. A post hoc analysis was performed to compare cell mediated immune responses between groups. Differences between study groups were assessed using the Kruskall Walllis test. Pairwise comparisons were done using Wilcoxon’s Mann-Whitney test. The level of significance was set to 0.05.
| Abbreviations: | ||
| HSV | = | herpes simplex virus |
| MPL | = | 3-O-deacylated monophosphoryl lipid A |
| gD-2t | = | truncated glycoprotein D from HSV-2 strain G |
| GMTs | = | geometric mean titers |
| AE | = | adverse event |
| ELISA | = | enzyme-linked immunosorbent assay |
| IFN-γ | = | gamma interferon |
| ISI | = | integrated stimulation index |
| IL-2 | = | interleukin-2 |
Acknowledgments
The authors thank the study participants, staff members at the study site and GlaxoSmithKline staff members involved in the study.
Medical writing assistance was provided by Adriana Rusu (XPE Pharma and Science). We thank Linda Gibbs (BD Life Sciences, Belgium) for editorial support and Jérémie Dedessus le Moutier (BD Life Sciences, on behalf of GlaxoSmithKline Vaccines) for manuscript coordination and editorial support.
Funding
GlaxoSmithKline Biologicals SA took responsibility for all costs associated with this study and development and publishing of the present manuscript.
Clinical Trial Registration
208141/014 (HSV-014)
Disclosure of Potential Conflicts of Interest
All participating institutions received compensation for study involvement. Dr G. Leroux-Roels reports payments from Baxter, GlaxoSmithKline Vaccines, Novartis, Immune Targeting Systems and UK for consultancy. Drs G. Dubin, T. Heineman and M. Fourneau are employees of GlaxoSmithKline group of companies. Dr P. Vandepapelière is a former GlaxoSmithKline group of companies employee. Drs G. Dubin, T. Heineman and M. Fourneau report ownership of stock options. Dr G. Dubin reports royalties payments from Wyeth Vaccines.
References
- Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. [A.] Bull World Health Organ 2008; 86:805 - 12; http://dx.doi.org/10.2471/BLT.07.046128; PMID: 18949218
- Bernstein DI. Effects of prior HSV-1 infection on genital HSV-2 infection. Prog Med Virol 1991; 38:109 - 27; PMID: 1647042
- Nahmias AJ, Lee FK, Beckman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl 1990; 69:19 - 36; PMID: 2175939
- Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002; 186:Suppl 1 S3 - 28; http://dx.doi.org/10.1086/343739; PMID: 12353183
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296:964 - 73; http://dx.doi.org/10.1001/jama.296.8.964; PMID: 16926356
- Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 1992; 116:197 - 202; PMID: 1309413
- Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, et al, Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11 - 20; http://dx.doi.org/10.1056/NEJMoa035144; PMID: 14702423
- Marchant J, Roe A. Genital herpes: recognizing and addressing patient's needs. Herpes 1997; 4:36 - 41
- Bernstein DI, Aoki FY, Tyring SK, Stanberry LR, St-Pierre C, Shafran SD, et al, GlaxoSmithKline Herpes Vaccine Study Group. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin Infect Dis 2005; 40:1271 - 81; http://dx.doi.org/10.1086/429240; PMID: 15825029
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al, GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 2002; 347:1652 - 61; http://dx.doi.org/10.1056/NEJMoa011915; PMID: 12444179
- Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al, Herpevac Trial for Women. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 2012; 366:34 - 43; http://dx.doi.org/10.1056/NEJMoa1103151; PMID: 22216840
- Mikloska Z, Cunningham AL. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol 1998; 79:353 - 61; PMID: 9472620
- Schmid DS, Thieme ML, Gary HE Jr., Reeves WC. Characterization of T cell responses to herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) using a TNF-beta ELISpot cytokine assay. Arch Virol 1997; 142:1659 - 71; http://dx.doi.org/10.1007/s007050050187; PMID: 9672626
- Rosenthal KL, Smiley JR, South S, Johnson DC. Cells expressing herpes simplex virus glycoprotein gC but not gB, gD, or gE are recognized by murine virus-specific cytotoxic T lymphocytes. J Virol 1987; 61:2438 - 47; PMID: 3037106
- Eisenberg RJ, Ponce de Leon M, Cohen GH. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol 1980; 35:428 - 35; PMID: 6255183
- Dix RD, Pereira L, Baringer JR. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun 1981; 34:192 - 9; PMID: 6271681
- Eing BR, Kühn JE, Braun RW. Neutralizing activity of antibodies against the major herpes simplex virus type 1 glycoproteins. J Med Virol 1989; 27:59 - 65; http://dx.doi.org/10.1002/jmv.1890270113; PMID: 2466100
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723 - 39; http://dx.doi.org/10.1586/14760584.6.5.723; PMID: 17931153
- Leroux-Roels G, Moreaux E, Desombre I, Crayne O, Francotte M, Pala P. Persistence of humoral and cellular immune response and booster effect following vaccination with herpes simplex (gD2t) candidate vaccine with MPL [abstract]. 34th Interscience Conference on Antimicrobial Agents and Chemotherapy 1994;205.
- Ahmad A, Sharif-Askari E, Fawaz L, Menezes J. Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J Virol 2000; 74:7196 - 203; http://dx.doi.org/10.1128/JVI.74.16.7196-7203.2000; PMID: 10906173
- Khodai T, Chappell D, Christy C, Cockle P, Eyles J, Hammond D, et al. Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid A exhibit differential immunity that is not correlated to protection in animal models. Clin Vaccine Immunol 2011; 18:1702 - 9; http://dx.doi.org/10.1128/CVI.05071-11; PMID: 21852545
- Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 2006; 80:5509 - 15; http://dx.doi.org/10.1128/JVI.02659-05; PMID: 16699031
- Morello CS, Levinson MS, Kraynyak KA, Spector DH. Immunization with herpes simplex virus 2 (HSV-2) genes plus inactivated HSV-2 is highly protective against acute and recurrent HSV-2 disease. J Virol 2011; 85:3461 - 72; http://dx.doi.org/10.1128/JVI.02521-10; PMID: 21270160
- Cattamanchi A, Posavad CM, Wald A, Baine Y, Moses J, Higgins TJ, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin Vaccine Immunol 2008; 15:1638 - 43; http://dx.doi.org/10.1128/CVI.00167-08; PMID: 18784341
- Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, et al. Future of an “Asymptomatic” T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virol 2012; 7:371 - 8; http://dx.doi.org/10.2217/fvl.12.22; PMID: 22701511
- Karpinski KF, Hayward S, Tryphonas H. Statistical considerations in the quantitation of serum immunoglobulin levels using the enzyme-linked immunosorbent assay (ELISA). J Immunol Methods 1987; 103:189 - 94; http://dx.doi.org/10.1016/0022-1759(87)90289-4; PMID: 3668258
- Leroux-Roels G, Van Hecke E, Michielsen W, Voet P, Hauser P, Pêtre J. Correlation between in vivo humoral and in vitro cellular immune responses following immunization with hepatitis B surface antigen (HBsAg) vaccines. Vaccine 1994; 12:812 - 8; http://dx.doi.org/10.1016/0264-410X(94)90290-9; PMID: 7975860