Abstract
In 2010, and due to a quality problem identified in the vaccine manufacture, the rotavirus (RV) vaccination was withheld in Spain during 5 months. Our study aimed to evaluate the impact that this sudden cease had on rotavirus acute gastroenteritis (RAGE) hospitalizations. An increase in RAGE hospitalization was observed in parallel to the drop in vaccine coverage. Here, we report the first reverse evidence of rotavirus vaccine impact.
Introduction
The effectiveness of the two licensed rotavirus (RV) vaccines has been widely reported in the literature. Several studies have evaluated the benefit of RV under routine conditions of use, obtaining variable results depending on the population and the methodology applied. Effectiveness of up to 100% in decreasing rotavirus acute gastroenteritis (RAGE) hospitalizations has been documented.Citation1-Citation3 In addition, vaccination-impact studies have shown decreased healthcare utilization due to RAGE, reductions in the magnitude and duration of the RV season assessed by laboratory testing for RV, and the possible induction of herd immunity.Citation1-Citation4
The Spanish National Health System does not reimburse rotavirus vaccination. However, significant coverage rates have been reached. According to dose distribution/census estimation, the national average in 2009 was 37%.Citation5 In 2010, the Spanish Drugs and Health Products Agency (AEMPS) did not authorize the release of new batches of the two rotavirus vaccines onto the Spanish market for 5 months (from June to November 2010), due to the detection of circovirus in both vaccines.Citation5
Our study aimed to evaluate the impact of the temporary and absolute cease of rotavirus vaccination on RAGE hospitalizations in children < 5 years old in Galicia (North-West Spain).
Results
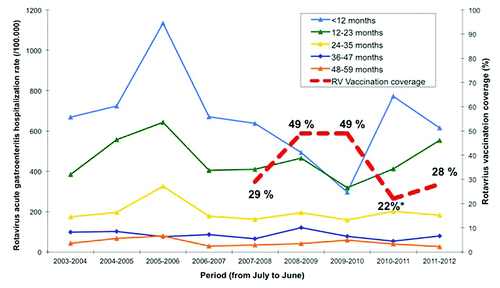
In the pre-vaccination period from July 2003 to June 2007, the median AGE-rotavirus hospitalization rate was of 297.7 per 100,000 children < 5 years of age in Autonomous Community of Galicia (North-West Spain). Hospitalization rates in the post-vaccination period from July 2009 to June 2010 decreased a 44.5% (165.3 per 100,000). Hospitalization rates in the period right after the vaccination sudden cease -i.e., July 2010–June 2011—went back to the pre-vaccination rates (291.0 per 100,000), increasing a 76% compared with the previous season (2009–2010). In the period from July 2011 to June 2012, with rotavirus vaccination resumed, RAGE hospitalization rate (285.6 per 100,000) remained similar to the previous season, and thus, to the pre-vaccination rates.
In children < 1 year of age, RAGE hospitalization rates from July 2009 to June 2010 decreased a 57% (297.1 per 100,000) compared with the pre-vaccination median (696.2 per 100,000). This drop corresponded nicely with an increase in the vaccine coverage (). In the period of July 2010-June 2011—when vaccination was temporarily withheld—the hospitalization rates increased an 11% (771.1 per 100,000) compared with pre-vaccination rates, and a 260% compared with the previous season (2009–2010). In the period from July 2011 to June 2012—once rotavirus vaccination was resumed- RAGE hospitalization rate decreased a 12% (615.5 per 100,000) compared with the median AGE-rotavirus rate for the pre-vaccination period, and a 30% compared with the previous season. There was not a significant variation in the 12–23 months age group RAGE hospitalization rate until the second season after vaccination started: 318.1 per 100,000 (season 2009–2010) compared with 480.1 per 100,000 in the pre-vaccination period (), i.e., a 33.7% decrease. After the vaccination cease, RAGE hospitalization rate increased to 412.4 per 100,000, and continued to increase the next season despite vaccination resume (553.3 per 100,000). No significant changes were observed for the rest of the age intervals analyzed.
Figure 1. Vaccine coverage and rotavirus acute gastroenteritis hospitalization rate per 100,000 children at different age intervals in Galicia (north-west Spain) from June 2003 to July 2012. (*) 22% is the mean RV vaccine coverage for that period. However, for 5 mo within that period, no new batches of vaccine were released into the market, and the coverage estimated for those months was 0–5%.
Discussion
Apart from other factors that are known to influence yearly variations in rotavirus incidence,Citation2 our study suggests that the recent increase in rotavirus hospitalization rate in children < 5 years of age may be related to the sudden drop in vaccination coverage that followed the temporary cease of vaccination in Spain. The rebound increase in the rates of hospitalization for RAGE after the vaccination cease was particularly pronounced and immediate in children < 12 months of age -for whom the vaccine is indicated. This rate decreased again soon after vaccination was resumed. However, for the 12–23 months age group, the increase is maintained despite vaccination was restored, as this group missed the vaccination during the vaccine withhold period and catch-up vaccination is not possible. This pattern is similar to that observed on year after the rotavirus vaccine was initially administered (see ). Thus we might expect again a decrease in the 12–23 mo age group RAGE hospitalization rate during next season.
In Spain, despite the universal rotavirus vaccination is recommended by the Vaccination Advisory Committee of the Pediatric Spanish Association, it is not reimbursed by the Spanish National Health System.Citation6 However, some areas have shown significant coverage rates, and RV introduction has nearly halved the rotavirus-related hospitalization rate.Citation7 From March 29th and June 10th of 2010, the Spanish Medicine and Health Products Agency (AEMPS) did not authorize the release of new batches of Rotarix® and RotaTeq® vaccines, respectively, onto the Spanish market due to problems with the good manufacturing practice (GMP). On November 4th, 2010, AEMPS allowed again the release of batches of the RotaTeq®, while Rotarix® suspension remains up to date. Prior to the vaccines withhold, the average vaccination coverage against rotavirus in Spain, estimated according to dose distribution and census data, was 40%. This coverage fell to 19% in 2010 since June to November, vaccine coverage was nearly 0%. In Galicia, rotavirus vaccine coverage has almost recovered the levels achieved before the vaccine was temporarily withdrawn, although it took three years to reach that level: 49% (2009), 22% (2010), 28% (2011) and 45% (2012). We had previously estimated that in Spain during the 5 month period in which no rotavirus vaccine was available, 84.450 children were not vaccinated against rotavirus, 497 hospitalizations due to RAGE were not avoided, and 2,172,941 euros were spent in both direct and indirect related costs.Citation5
Other factors such as the natural secular variability in rotavirus disease, the year-to-year variability in gastroenteritis caused by rotavirus, or even changes in the coding or clinical practices, may have also influenced our findings. The strengths and weakness of the national administrative database for hospital admissions used as data source in our study have been previously discussed.Citation7 In addition, although estimation of vaccination coverage using sales is a usual practice, the percentage obtained may be overestimated, as the distributed doses may not necessarily reflect the actual number of doses that have been administered. In any case, these limitations have been present through the study period. Continued surveillance and further population-based studies are needed to corroborate and follow-up our findings.
In conclusion, the impact of the temporary withdrawal of rotavirus vaccines in Spain may constitute the first reverse evidence of rotavirus vaccine impact. Rotavirus vaccines work, as far as they are used.
Material and Methods
A retrospective, observational and hospital-based surveillance study of rotavirus gastroenteritis in children was conducted in the Autonomous Community of Galicia, Spain. Total population for the region as a whole (January 2006 census) is 2,767,524 people (approximately 100,000 under 5 years of age). The official surveillance system for hospital data (CMBD-HA, Minimum Set of Acute Hospitalizations Database) was used as the information source. This database includes personal, administrative, and medical data of all patients admitted to the hospital network in Galicia (SERGAS), with diagnoses codified according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The CMBD-HA has maintained a coded valid discharge rate and, since 2003, virtually 100% of the hospitalizations are coded. A search strategy was applied based on disease category codes corresponding to diagnosis at discharge of intestinal infectious gastroenteritis (ICD-9-CM codes 001 to 009) and non-infectious gastroenteritis (ICD-9-CM code 558.9), in any diagnostic position. Among these hospitalizations with ICD-9-MC codes 001–009, the code 008.6, which specifically relates to rotavirus infection, was selected. Diagnosis, age, and year of admission were collected for each case identified. The study protocol was reviewed and approved by the institutional review board of Galicia (Comité Ético de Investigación Clínica de Galicia). The annual hospitalization rates for RV-AGE during the study period were calculated either for the whole study population (children < 5 y of age) or by age groups (< 12, 12–23, 24–35, 36–47, 48–59 mo). The population used as denominator for the calculation of rates was obtained from the Galician Institute of Statistics. Rotavirus vaccination coverage was estimated according to the number of distributed doses of each vaccine per month (Source: IMS Health) divided by 2 or 3 doses depending on the vaccine used, and by the registered number of children born for each age interval and that same period (Source: Spanish National Statistics Institute).
| Abbreviations: | ||
| RAGE | = | rotavirus acute gastroenteritis |
| CMBD-HA | = | Minimum Set of Acute Hospitalizations database |
| ICD-9-CM | = | International Classification of Disease, 9th Revision, Clinical Modification |
Conflicts of Interest and Financial Disclosure
F.M.-T. has received research grants and/or honoraria as consultant/advisor and/or speaker from GlaxoSmithKline, Sanofi Pasteur MSD, Pfizer Inc., Wyeth, Novartis, and Medimmune Inc. No conflicts of interest to be declared for the remaining authors.
References
- Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TT, Maldonado YA, Spoulou V, et al. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin 2011; 7:734 - 48; http://dx.doi.org/10.4161/hv.7.7.15511; PMID: 21734466
- Tate JE, Parashar UD. Monitoring impact and effectiveness of rotavirus vaccination. Expert Rev Vaccines 2011; 10:1123 - 5; http://dx.doi.org/10.1586/erv.11.94; PMID: 21854307
- Gray J. Rotavirus vaccines: safety, efficacy and public health impact. J Intern Med 2011; 270:206 - 14; http://dx.doi.org/10.1111/j.1365-2796.2011.02409.x; PMID: 21668823
- Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980 - 6; http://dx.doi.org/10.1093/infdis/jir492; PMID: 21878425
- Bouzón Alejandro M, Diez Domingo J, Martinón-Torres F. Circovirus and impact of temporary withdrawal of rotavirus vaccines in Spain. Hum Vaccin 2011; 7:798 - 9; http://dx.doi.org/10.4161/hv.7.7.15683; PMID: 21715979
- Bernaola Iturbe E, Giménez Sánchez F, Baca Cots M, de Juan Martín F, Díez Domingo J, Garcés Sánchez M, et al, Asociación Española de Pediatría. [Immunization schedule of the Spanish Association of Pediatrics: recommendations 2008]. An Pediatr (Barc) 2008; 68:63 - 9; http://dx.doi.org/10.1157/13114474; PMID: 18194631
- Martinón-Torres F, Martinón-Torres N, Bouzón Alejandro M, Redondo Collazo L, Pértega-Díaz S, Seoane-Pillado MT, et al. Acute gastroenteritis hospitalizations among children aged < 5 years before and after introduction of rotavirus vaccines: a hospital-based surveillance study in Galicia, Spain. Hum Vaccin Immunother 2012; 8:946 - 52; PMID: 22484280