Abstract
The aim of this study was to gather data on the safety of the HPV-16/18 AS04-adjuvated vaccine among women aged 25, evaluating the frequency and severity of adverse events reported after vaccination and to compare the results obtained with previously published data regarding a sample of Italian preadolescents. Every woman residing in the province of Florence and in the age group targeted by the cervical cancer screening was invited to participate. Participants registered daily, for 14 d post-vaccination, solicited local and systemic reactions, as well as unsolicited adverse events in a developed ad hoc safety diary card. Data were collected in a database in Access and analyzed using STATA 11 SE statistical software. A total of 271 participants were recruited in the study group. All three diary cards were completed and delivered by 186 subjects (85.7% of participants). In all, a total of 616 diary cards were collected: 216 after the 1st dose, 209 after the 2nd dose and 191 after the 3rd dose. No severe symptoms were registered. The most frequently reported adverse reaction proved to be pain at the site of injection (83.4% of doses), followed by local swelling (20.8%) and pyrexia (14.6%). The safety and tolerability of the HPV-16/18 AS04-adjuvated vaccine in this sample of adult women aged 25 did not differ much from that previously observed in a sample of preadolescents Italian girls. Fever and local pain were however more frequently registered in our sample of adult women.
Introduction
Clinical and subclinical infections caused by the Human Papillomavirus (HPV), are the most common sexually transmitted infections, since most sexually-active individuals are likely to be exposed to HPV infection during their lifetimes.Citation1 Most HPV infections in the genital tract are short-lived and asymptomatic and regress on their own, however, about 5% of infections are not cleared within 2 y.Citation2
The demonstration that the persistency of infections by some HPV genotypes (high-oncogenic HPV types) is the necessary cause for the development of cervical cancer, the second most common malignant disease in women and the leading cause of cancer death in developing countries, was one of the most important scientific discoveries of the last century.Citation3 High oncogenic-risk HPV genotypes are also associated with other types of malignancies, in varying proportions: 60% of vaginal, 40–60% of vulvar, 45–95% of anal, 30% of head and neck, and also part of urethra and penis carcinomas are caused by high oncogenic-risk HPV types.Citation4-Citation10
Recently, two highly effective prophylactic vaccines became available: Cervarix® (GlaxoSmithKline), bivalent vaccine able to prevent infections caused by HPV 16 and 18, the types responsible for about 70% of cervical cancers, and Gardasil® (Sanofi Pasteur MSD), quadrivalent vaccine that additionally protects against infection with HPV 6 and HPV 11, that cause around 90% of genital warts.Citation11
In Italy, HPV vaccination was introduced into national immunization schedules in the second half of 2007; its targets are females aged 12 y, since in preadolescence sexual exposure is close to null, the immune system guarantees a better response, it is possible to catch-up missing doses and offer again immunization in case of lack of compliance. Moreover, it is convenient to add such vaccination to the others provided at the same age.Citation12 In Tuscany, following the decisions of the Regional Health Council (DGR n.1020 issued the 27th of December 2007 and DGR n.856 issued the 27th of October 2008), the vaccination program started on January 1, 2008. Cervarix® has ever since been offered to girls aged 12. One year later, free offer was expanded to girls aged 13–16 y, with girls aged 16 actively called for vaccination. The vaccination course consists of three doses over a period of six months: the recommended vaccination schedule is 0, 1, 6 mo.
The present study is part of a project called “Effective surveillance and impact of HPV vaccination on screening for cervical cancer in Tuscany” funded by the Tuscan Tumor Institute (ITT) in Florence, a randomized controlled trial offering vaccination at the time of the first call to the cervical cancer screening program, which is offered free of charge in some Italian regions to all women at 25 y old of age. Within the study, free anti-HPV vaccine was offered to a randomized sample of women undergoing Pap test screening for the first time.
The aim of the present study was to gather data on the safety of the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®) among women aged 25, evaluating the frequency and severity of adverse events reported after HPV vaccination of women participating in the study and to compare the results obtained with previously published data regarding a sample of Italian preadolescents.Citation13 The information obtained concerns the occurrence of local side effects (pain, pruritus, swelling, redness) and/or systemic effects (fever, including the use of anti-fever medications), as well as the requirement of medical intervention within the 14 d following each injection.
Results
A total of 271 participants was recruited in the study group; 221 of them received the first dose, 217 received the second dose and 213 received the third dose. Among the 50 women who did not receive the first dose after being recruited: 38 refused after randomization and the remaining 12 did not attend the appointment even after being solicited by phone for a second date. Among those who did not receive the second dose: 2 refused and 2 did not attend the appointment even after phone call for a second date. Among those who did not receive the third dose: 1 refused and 3 did not attend the appointment.
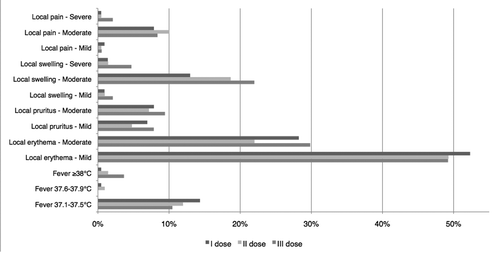
All three diary cards were completed and delivered by 186 subjects (85.7% of participants). In all, a total of 616 diary cards were collected: 216 after the 1st dose, 209 after the 2nd dose and 191 after the 3rd dose. No serious adverse events related to vaccination occurred. reports the numbers and the percentages of the local and general reactions elicited within 14 d of vaccination, regardless of the dose administered. The most frequently reported adverse reaction correlated with the administration of Cervarix proved to be pain at the site of injection (83.4% of doses), followed by local swelling (20.8%) and pyrexia (14.6%).
Table 1. Adverse reactions registered in the 14 d after immunization
shows data regarding the adverse reactions elicited within 14 d of vaccination, subdivided according to the dose administered. The local symptom most frequently reported after each dose was local pain, however, only 15 subjects after the first dose (6.9% of recipients), 10 subjects after the second dose (4.8%) and 15 subjects after the third dose (7.9%) suffered intense pain, which, even in these cases, was always temporary ().
Table 2. Adverse reactions registered in the 14 d following the administration of each dose
Local swelling was the second most frequently observed adverse reaction after each dose, reported as severe or moderate by only 5 subjects after the first dose (2.3%), 4 subjects after the second dose (1.9%) and 10 subjects after the third dose (5.2%) ().
With regards to the other local reactions evaluated, pruritus and erythema, no severe symptoms were registered. A moderate intensity was reported by only 2 subjects (pruritus) and by 1 subject (erythema) after the first dose (0.9% and 0.5% of recipients, respectively), by 2 subjects (pruritus) and by 1 subject (erythema) after the second dose (1% and 0.5% of recipients, respectively), by 4 subjects (pruritus) and by 4 subjects (erythema) after the third dose (2.1% and 2.1% of recipients in both cases).
An axillary temperature higher than 37.5°C was reported by only 2 subjects (0.9% of recipients) after the first dose, 5 subjects (2.4%) after the second dose and 7 subjects (3.7%) after the third dose (). The use of anti-fever medications was notified by 6 subjects following the administration of the first dose (2.8% of recipients), by 7 subjects following the administration of the second dose (3.3%) and by 8 subjects following the administration of the third dose (4.2%).
The intensity of the symptoms was such as to require medical attention only in 2 cases after the first dose (0.9% of recipients), in one case after the second dose (0.5%) and in 2 cases following the administration of the third dose (1.1%). Regarding the incidence of symptoms with respect to the dose administered, local pain was referred more frequently after the 1st and the 3rd dose than after the second; local swelling was significantly more frequent after the third dose than after the first dose. The incidence of all the other symptoms did not differ with each subsequent dose. The corresponding statistical analysis is shown in . The same analysis was performed with regards to the subset of 186 subjects who completed all doses and returned all three safety diary cards: the results did not differ from those seen in the entire data set ( and ).
Table 3. Assessment of difference in tolerability after each dose. Significant values (p < 0.05) are indicated in bold type
Table 4. Adverse reactions registered in the 14 d following the administration of each dose in the subset of 186 subjects who received all 3 doses of vaccine and completed and returned all three diary cards
Table 5. Assessment of difference in tolerability after each dose in the subset of 186 subjects who received all 3 doses of vaccine and completed and returned all three diary cards. Significant values (p < 0.05) are indicated in bold type
The proportion of women reporting at least one symptom other than those listed in the safety diary cards was 11.1% after the first dose, 5.3% after the second dose and 6.3% after the third dose. Among these symptoms, the most frequently reported was headache, reported by 11 subjects after the first dose (5.1%), 7 subjects after the second dose (3.4%) and 7 subjects after the third dose (3.7%). Gastrointestinal symptoms were recorded by 5 subjects after the first dose (2.3%), 3 subjects after the second dose (1.4%) and 2 subjects after the third dose (1.1%).
A vasovagal syncope was recorded by 2 subjects: one following the administration of the first dose, the other following the administration of the third dose.
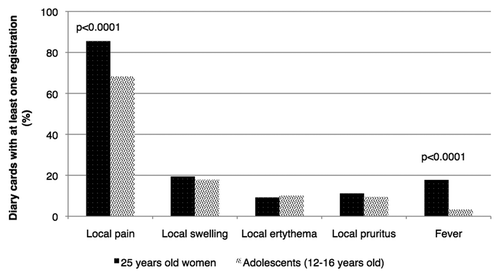
The results of the present study were compared with those of an independent study performed in Tuscany and Liguria, assessing the safety and tolerability of HPV bivalent vaccine on a sample of preadolescents women, in which more than 7100 questionnaires were collected.Citation13 A statistically significant difference in the frequency of symptoms reported was observed with regards to local pain and fever, both more frequently registered in the case of our sample of 25 y old women (83.4% against 68.3% and 14.6% against 3.3%, respectively), the value of Pearson χ2 being 61.2283 (p = 0.000) and 178.7089 (p = 0.000), respectively (). No statistically significant difference in the incidence of the other symptoms was noted.
Figure 2. Comparison between our findings and those previously published with regards to a sample of adolescent girls aged 12–16.Citation13
Discussion
Cervarix is a mixture of virus-like particles derived from the L1 capsid proteins of HPV types 16 and 18, formulated with the AS04 adjuvant system (aluminum hydroxide with 3-deacylated monophosphotyl lyped A).
Prior to licensure, efficacy and safety of Cervarix was extensively evaluated in clinical trials and assessed as safe:Citation14-Citation19 the vaccine appears to be generally well tolerated, being injection-site reactions the most frequently reported adverse events. General adverse effects, such as fever, fatigue, headache and myalgia can also be induced by the vaccine. Since Cervarix is not a live vaccine nor it contains HPV DNA, and aluminum-based adjuvant have been extensively used in other vaccines, there should be no reason to suspect serious vaccine safety problems a priori; nonetheless, post marketing monitoring is required in order to detect very rare but potentially important adverse reactions, that are difficult to identify in the pre-licensure assessment phase.
In July 2010, the Medicine and Healthcare products Regulatory Agency, the government agency responsible for regulating medicines and medical devices in the UK, issued the last Suspected Adverse Reaction Analysis on Cervarix:Citation20 according to this analysis, based on 4445 reports on 9673 suspected reactions following the administration of at least 4 million doses across the UK since September 2008, the vast majority of suspected adverse reactions are related to either the signs and symptoms of recognized side effects listed in the product information or are due to the injection process and not to the vaccine itself. For the isolated cases of other medical conditions reported, the analysis concludes indicating that the available evidence does not suggest that the vaccine caused the conditions and that those events may have been coincidental.
On-going post marketing monitoring is indeed needed in order to maintain confidence in vaccine safety. Our findings confirm the high safety and tolerability of the bivalent Human Papillomavirus vaccine.Citation21-Citation24 The safety and tolerability of the HPV-16/18 AS04-adjuvated vaccine in this sample of adult women aged 25 did not differ much from that previously observed in a sample of preadolescents Italian girls:Citation13 in both cases and in agreement with the data from the international scientific literature regarding both controlled clinical studies and post-licensure surveillance, the most frequently reported adverse reaction related with the administration of Cervarix proved to be pain at the site of injection and no unexpected or serious side effects with a causal relationship with the vaccination occurred following the administration of 616 doses. Fever and local pain were however more frequently registered in our sample of adult women than in the preadolescent girls group examined in the study cited.
All bivalent HPV vaccine related side effects registered for the present study were in general mild and transient, being pain at the site of injection the most frequently reported side effect, which only in 6.49% of the cases was perceived as severe by recipients. In two cases fainting was registered, but vasovagal syncope is a well-known recognized outcome after vaccination, for which observation of subjects for 15 min after administration is recommended. All solicited and unsolicited symptoms registered were transient and resolved spontaneously without sequelae.
The extension of the vaccination to adult women who are invited for the first time to participate in the cervical cancer screening, would allow to reach high levels of immunization coverage in a short time and would have a great impact on the disease, in the context of a comprehensive approach to the control of the disease. As a matter of fact, while offering vaccination to women invited to participate in the cancer screening for the first time would represent an excellent opportunity to reach women of childbearing age, the introduction of the vaccination in this subgroup, on the other hand, is likely to facilitate the compliance to the cancer screening itself, further contributing to the reduction of the burden of the illness, as most cases of cancer arise in individuals who are not willing to participate in screening programs. Obviously, the sensitivity and specificity of screening tests in immunized women would need to be reassessed: since both the incidence and the prevalence of the disease would be likely to decrease, it would be important to evaluate the data of the follow-up trials currently underway so as to assess the impact on the total number of cytological abnormalities and identify the best screening strategy in the vaccinated population (age of intervention, mode, HPV testing or cytology, and frequency of screenings in order to minimize unnecessary procedures and costs). Assessing the impact of vaccination on other genital and non-genital cancers associated with HPV would be equally important. In addition, in order to assist women in making informed decisions about the vaccination, appropriate communication procedures should be developed.
Materials and Methods
After receiving approval from the Ethics Committee of the Local Health Unit of Florence, a personalized invitation letter, comprising a brief and clear description of the study, was sent to every woman resident in the province of Florence targeted by the screening program i.e., all women aged 25, with the request to participate in a trial regarding the impact of vaccination on the screening program itself. All women complying with the invitation were asked to fill in the informed consent document. A code number was assigned to each participating subject, and a simple randomization, with a 1:2 allocation ratio, was performed at the Institute for the Study and Prevention of Cancer (ISPO), with the aim to include 300 subjects in the study arm and 600 in the control arm. Given the limited resources available, it was not possible to offer the free of charge vaccine to all women presenting to be screened and Cervarix was therefore offered solely to women randomized in the study arm, while women in the control arm received usual care.
Between April 2010 and December 2011, women in the vaccine group were invited to be vaccinated at the Meyer Children Hospital in Florence, where a room was hired on purpose. Two health professionals were involved in the project: a medical doctor, who performed vaccinations, and a health care assistant who was responsible for the personal and medical data collection.
A safety diary card was developed ad hoc and distributed to participants following each dose in order for them to register daily, and for 14 d post-vaccination, solicited local and systemic reactions, as well as unsolicited adverse events. Local side effects listed in the diary cards included local pain, injection site pruritus, (both local pain and pruritus were defined as “mild” in case of tolerable symptoms, “moderate” in case of symptoms interfering with normal activities, “severe” in case of symptoms preventing normal activities) local swelling, injection site erythema (both local swelling and erythema were defined as “mild” if large no more than 2.5 cm in diameter, “moderate” if between 2.5 cm and 5 cm, “severe” if greater than 5 cm). Women were asked to register their axillary temperature from day 0 (day of vaccine administration) until day 4, or day 14 in case of temperature ≥ 37.5°C. In addition, it was asked to participants to note down the possible use of anti-fever medications, and whether medical intervention was required. Space was left in the safety diary cards where participants could register any other reaction not included among those listed.
At the second dose, completed safety diary cards regarding the tolerability of the first dose were collected by the health care assistant; at the third dose, completed cards regarding the tolerability of the second dose were collected; diary cards related to the third dose were sent by participants to ISPO by fax or by e-mail.
The research group at the Department of Public Health of the University of Florence identified those participants who did not deliver the diary card, reached them telephonically and encouraged them to complete the diary card and return it by e-mail or fax. A specific database in Access was developed in order to collect the medical information on adverse reactions. Data analyses were conducted using STATA 11 SE statistical software. A descriptive analysis was performed for each adverse reaction to the vaccine. The null hypothesis of no difference in the frequency of symptoms with respect to the dose was tested by means of Pearson's chi-square (χ2) test.
In the final stage of the project, the results obtained were compared with previously published data with regards to a sample of 4643 Italian preadolescent girlsCitation13 by means of the χ2 test.
| Abbreviations: | ||
| HPV | = | human papillomavirus |
| ISPO | = | Institute for the Study and Prevention of Cancer |
| ITT | = | Tuscan Tumor Institute |
| AS04 | = | Adjuvant System 04 |
The HPV ScreeVacc Working Group
Livia Brandigi (Institute for Cancer Study and Prevention; Florence, Italy), Clementina Canessa (Department of Health Sciences; University of Florence; Florence, Italy), Carmelina di Pierro (Institute for Cancer Study and Prevention; Florence, Italy), Anna Iossa (Institute for Cancer Study and Prevention; Florence, Italy), Vieri Lastrucci (Specialization School for Hygiene and Preventive Medicine; University of Florence; Florence, Italy), Antonino Sala (Specialization School for Hygiene and Preventive Medicine; University of Florence; Florence, Italy), Tommaso Tanini (Specialization School for Hygiene and Preventive Medicine; University of Florence; Florence, Italy) and Emilia Tiscione(Department of Health Sciences; University of Florence; Florence, Italy).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Trottier H, Burchell AN. Epidemiology of mucosal human papillomavirus infection and associated diseases. Public Health Genomics 2009; 12:291 - 307; http://dx.doi.org/10.1159/000214920; PMID: 19684442
- Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM, ALTS Group. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis 2007; 195:1582 - 9; http://dx.doi.org/10.1086/516784; PMID: 17471427
- Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244 - 65; http://dx.doi.org/10.1136/jcp.55.4.244; PMID: 11919208
- Srodon M, Stoler MH, Baber GB, Kurman RJ. The distribution of low and high-risk HPV types in vulvar and vaginal intraepithelial neoplasia (VIN and VaIN). Am J Surg Pathol 2006; 30:1513 - 8; http://dx.doi.org/10.1097/01.pas.0000213291.96401.48; PMID: 17122506
- Kayes O, Ahmed HU, Arya M, Minhas S. Molecular and genetic pathways in penile cancer. Lancet Oncol 2007; 8:420 - 9; http://dx.doi.org/10.1016/S1470-2045(07)70137-7; PMID: 17466899
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med 2001; 345:1890 - 900; http://dx.doi.org/10.1056/NEJMra001375; PMID: 11756581
- Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, et al, IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772 - 83; http://dx.doi.org/10.1093/jnci/djg107; PMID: 14652239
- Cupp MR, Malek RS, Goellner JR, Espy MJ, Smith TF. Detection of human papillomavirus DNA in primary squamous cell carcinoma of the male urethra. Urology 1996; 48:551 - 5; http://dx.doi.org/10.1016/S0090-4295(96)00246-4; PMID: 8886059
- D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356:1944 - 56; http://dx.doi.org/10.1056/NEJMoa065497; PMID: 17494927
- Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol 2005 p; 6:204 - 204; http://dx.doi.org/10.1016/S1470-2045(05)70086-3; PMID: 15830458
- von Krogh G. Management of anogenital warts (condylomata acuminata). Eur J Dermatol 2001; 11:598 - 603, quiz 604; PMID: 11701422
- Ministero della Salute. Sessione XLVI, sessioni congiunte II e III. Seduta dell’11 Gennaio 2007.
- Gasparini R, Bonanni P, Levi M, Bechini A, Boccalini S, Tiscione E, et al. Safety and tolerability of bivalent HPV vaccine: an Italian post-licensure study. Hum Vaccin 2011; 7:Suppl 136 - 46; http://dx.doi.org/10.4161/hv.7.0.14576; PMID: 21266842
- Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, et al, GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 85; http://dx.doi.org/10.1016/S0140-6736(09)61567-1; PMID: 19962185
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al, HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 70; http://dx.doi.org/10.1016/S0140-6736(07)60946-5; PMID: 17602732
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, et al, HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/10.1016/S0140-6736(09)61248-4; PMID: 19586656
- Keam SJ, Harper DM. Human Papillomavirus Types 16 and Adjuvanted, Adsorbed) [ Cervarix TM ]. 2008;68(3):359–72.
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T. Safety of human papillomavirus (HPV) -16 / 18 AS04-adjuvanted vaccine for cervical cancer prevention. 2009;5(5):332–40.
- Verstraeten T, Descamps D, David M-P, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.09.049; PMID: 18845199
- MHRA. Suspected Adverse Reaction Analysis CERVARIX Human papillomavirus (HPV) vaccine 29 July 2010.
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al, HPV Study Group for Adult Women. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine 2009; 27:581 - 7; http://dx.doi.org/10.1016/j.vaccine.2008.10.088; PMID: 19022320
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al, HPV-010 Study Group. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705 - 19; http://dx.doi.org/10.4161/hv.5.10.9518; PMID: 19684472
- Petäjä T, Keränen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. J Adolesc Health 2009; 44:33 - 40; http://dx.doi.org/10.1016/j.jadohealth.2008.10.002; PMID: 19101456
- European Medicines Agency. Procedural steps taken and scientific information after the authorisation 2009.