Abstract
Enterovirus 71 (EV71) is one of the major causative agents for hand, foot and mouth disease (HFMD) in childhood. Nowadays, HFMD or EV71 infections have already become an important public health issue throughout the world. Vaccination may be the most effective measure to control the transmission of the virus. Therefore, to pave EV71 vaccine into human clinical trial, in the present study a comprehensive preclinical safety assessment of inactivated EV71 vaccine including single- and repeat-dose toxicity studies were conducted in rats and cynomolgus monkeys. No abnormal findings were observed in rats following single intramuscular administration with EV71 vaccine (640 U). The results also showed no obvious systemic toxicities from four repetitive intramuscular injections, with a 14-d interval, of two dosages of EV71 vaccine in the two animal species. Antinuclear antibody response was not detected after the repeated administrations. Histopathological examination demonstrated the minimal to severe inflammatory changes in muscle tissues of the injection sites in EV71 vaccine-injected animals and most of findings have been improved over time. Furthermore, test article could induce highly EV71-specfic neutralizing antibody response in both animal species. Taken together, these data suggested a favorable safety profile for inactivated EV71 vaccine and supported this product to enter human phase I clinical trial.
Introduction
Enterovirus 71 (EV71) is a non-enveloped single positive stranded RNA virus, belonging to the Picornaviridae family. Since it was first isolated in California in 1969,Citation1 EV71 has emerged as one of the major causes of hand, foot, and mouth disease (HFMD) in childhood.Citation2,Citation3 It is reported that EV71 infections are sometimes associated with severe neurological complications despite of the fact that most cases are asymptomatic and mild.Citation4-Citation6 In the past several decades, outbreaks of HFMD caused by EV71 infection have been reported throughout world.Citation2,Citation7-Citation12 The latest large outbreak of HFMD occurred in 2008 in Anhui Province, China and more than 6,000 cases and 22 deaths in children were reported.Citation9 In addition, regular epidemics occurred every 2–3 y in some countries. Therefore, concerns on HFMD or EV71 infections have become an important public health issue all over the world, especially in the Asia Pacific region.
Unfortunately, neither effective antiviral drugs nor established antiviral treatment is available for EV71 infection now. Since poliovirus was nearly eradicated by vaccine immunization, vaccination may be the most effective measure to control the transmission of the virus. Recently, several EV71 vaccine candidates, including inactivated whole virus,Citation13-Citation16 live-attenuated virus,Citation17 DNA vaccine,Citation18 virus-like particle,Citation19 have been developed. Given the genome variability of the picornavirus, its recombination characteristics in the natural environment, and the historical experience with poliovirus vaccine, an inactivated whole virus vaccine may be the preferred choice now.
Before any novel vaccines were approved the initiation of human clinical trials, a basis for their safety must be established for providing safety information to support the clinical development and licensure of the product.Citation20-Citation22 Therefore, in order to pave the inactivated whole virus vaccine into human phase I clinical trial, in the present study a comprehensive preclinical safety assessment of inactivated EV71 vaccine including single and repeat-dose toxicity studies were conducted in rats and cynomolgus monkeys.
Results
Single-dose toxicity study in rats
No abnormal findings were observed in rats treated with EV71 vaccine in the single-dose toxicity experiment. Compared with the control group, no abnormalities in animal routine diet, body weight, necropsy and histopathological analysis were found in the EV71 vaccine group. These results suggested that the maximum tolerated dose was greater than 640 U per rat when EV71 vaccine was injected intramuscularly.
Repeat-dose toxicity study in rats
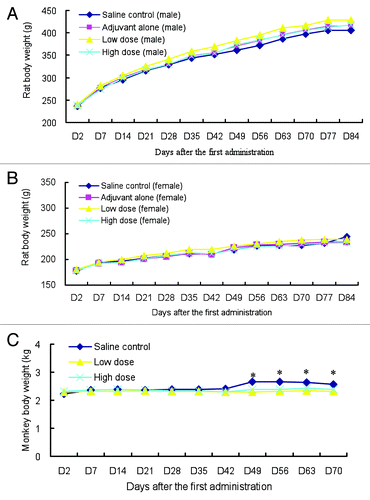
Intramuscular injection of EV71 vaccine at high and low dosages was well tolerated in rats with no obvious signs of systemic toxicity or abnormalities observed clinically when compared with the adjuvant and saline groups in whole study period. Animal body weights of all groups were increased and there were no significant differences on body weight between different groups at any time points (). The food consumption of female rats in the low-dose group was decreased significantly than that of the saline control group (12.7 ± 1.4 in low-dose group vs. 14.7 ± 0.9 in saline group, p < 0.05) on day 7 (Table S1), but no significant differences on food consumption were observed in other time points. It was considered to be incidental and not related to vaccination due to lacking the dose-response relationship and its observation only in one gender and one time point.
Figure 1. Group mean body weights of rats and cynomolgus monkeys after the repeated administrations. (A) Mean body weights of male rat; (B) Mean body weights of female rat; (C) Mean body weights of cynomolgus monkeys (n = 18 on study day 2, 13 on day 3–42 and 5 on day 43–84 in each sex of per group for rats; n = 8 on day 2–42, and 4 on day 43–70 in each group for cynomolgus monkeys except for the animal number of saline group were halved as described in Materials and Methods, *p < 0.05 vs. the saline control group)
Some transient significant changes on hematological parameters, such as the count of monocytes, neutrophiles or eosinophiles, were observed to be occurred equivalently in the adjuvant and treated groups at the dosing period (Table S2). At the end of recovery period, no significant difference for all hematological parameters had been found compared with the control group. Those changes may be related to the immune response to aluminum-containing vaccine. We also found out that the number of CD4+, CD8+ and the ratio of CD4+/CD8+ did not change in rats treated with EV71 vaccines or adjuvant alone (Table S2).
The clinical chemistry finding showed that only one parameter change (albumin/globulin ratio) was found in the EV71 treatment group (Table S3). Due to the fact that antibody is also a kind of globulin, it was considered that these changes of ratio were caused by the higher EV71-specific antibody levels elicited following vaccination.
At the second necropsy, a 24.9% increase in absolute prostate weight of low-dose male group was observed compared with the control group (0.652 ± 0.138 in low-dose group vs. 0.522 ± 0.050 in saline group, p < 0.05), but there was no corresponding histopathological finding. As a result, the prostate weight increase was considered to be unrelated to treatment. No treatment-related gross pathologic changes were found (Table S4).
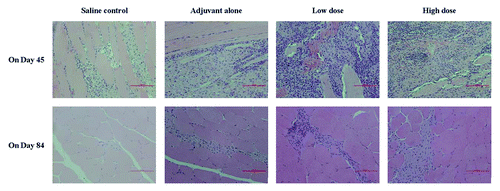
The histopathological examination showed that minimal to moderate necrosis of muscle fibers, minimal to moderate infiltration of inflammatory cells and minimal to severe formation of granulomas in muscle tissues at injection sites were found in adjuvant alone group as well as all EV71 vaccine-treated groups and the changes seem to be a dose-response relationship and they were believed to be related to the repeated dosing with EV71 vaccines (). It was also noted that minimal inflammatory cells infiltration were observed in saline control group. At the end of recovery period (on study day 84), these histopathological changes had been alleviated.
Repeat-dose toxicity study in cynomolgus monkeys
For the repeat-dose toxicity study in cynomolgus monkeys, no obvious clinical signs of systemic toxicity as well as significant differences on food consumption were found after administration. However, it was shown that body weights of cynomolgus monkeys in the low- and high-dose groups were lower than the control group at the recovery period (p < 0.05 for both) (). These changes may be caused by the small size of monkeys (n = 2) in the control group after the first necropsy and individual difference of monkey body weight. It was believed to be unrelated to the treatment.
It was showed that there were no significant differences on electrocardiogram (ECG) as well as clinical chemistry analysis on EV71 vaccine-treated groups compared with the control group throughout the study (Table S5). The hematological finding showed that some parameters changes were accidentally found in the treatment groups (Table S6). However, these changes were within the normal historical range and had no significant differences when compared with the corresponding results determined in the quarantine period (data not shown). Furthermore, no significant changes on hematological parameters were found at the end of recovery period.
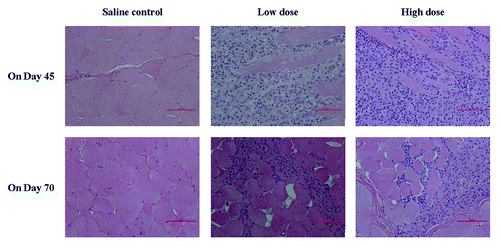
One female monkey of low-dose group with a diminished thymus size was found at the second necropsy, but with no corresponding histopathological change. Therefore, it was considered to be background or incidental changes, unrelated to treatment (Table S7). Histopathological finding demonstrated that minimal to mild necrosis of muscle fibers, minimal to mild infiltration of inflammatory cells and minimal to moderate formation of granulomas in muscle tissues at injection sites were also observed in cynomolgus monkeys of the low- and high-dose groups and there was a dose-response relationship at the dosing period (on study day 45). Moreover, these changes have also been improved greatly at the end of recovery ().
Neutralizing antibodies (NTAb) against EV71 and antinuclear antibodies (ANA)
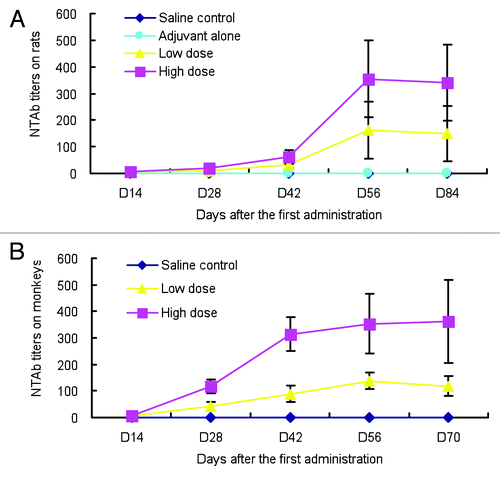
The results showed that NTAb to EV71 was noted on study day 14 after the first administration and the level continued to increase. All rats treated with low- and high- dose EV71 vaccines were seroconverted on day 28 and the highest peak was reached at day 56. An obvious dose-response relationship for NTAb level was noted (). No difference was observed of NTAb responses in males and females. In addition, it was revealed that no obvious antinuclear antibody was detected in rat at any time points. Similar to the finding in rats, no obvious ANA response was noted in cynomolgus monkeys on different time points. There was an obvious dose-dependent NTAb response and then both EV71 vaccines treated group were also seroconverted on day 28 at the dosing period (). Taken together, these results indicated that EV71 vaccine can elicit good immune response.
Figure 4. Kinetics of serum EV71-specific NTAb of rats and cynomolgus monkeys. Animals were injected with saline solution, adjuvant (only for rats) and EV71 vaccine on study days 0, 14, 28 and 42, respectively. The serum samples were collected on days 14, 28, 42, 56 and 84 (on study day 70 for cynomolgus monkey) after the first administration for detection of NTAb. The results were expressed as the mean ± SD (A) mean EV71-specific NTAb titers of rats, n = 8 rats in each group; (B) mean EV71-specific NTAb titers of cynomolgus monkeys, n = 8 cynomolgus monkeys in each group (n = 4 cynomolgus monkeys on days 56 and 70)
Discussion
Almost all prophylactic vaccines are administered to healthy individuals, most of them are children. Therefore, ensuring potency as well as safety is at the core of vaccine developments process.Citation20,Citation22 To identify signs of intrinsic toxicity or adverse effects of inactivated EV71 vaccine that could potentially affect humans, we conducted the preclinical comprehensive safety assessments in vivo and in vitro including single- and repeat-dose toxicity studies in rats and cynomolgus monkeys.
It is generally suggested that a repeated dose toxicity study in single related animal species is normally sufficient for vaccine product. The selection of animal species was critical for toxicity study. Ideally, the selected species should develop an immune response following vaccination and demonstrate a similar immunological effect to any adjuvant used.Citation22 To maximize the possibility of identifying responses that might predict human toxicity of inactivated EV71 vaccine, both Wistar rat and cynomolgus monkey were used in this preclinical study. First, the two animal species are well-characterized and versatile model in regulatory toxicology, for which there is a strong background of experience and of historical data.Citation23-Citation25 Second, previous studies also indicated that inactivated EV71 vaccine candidates could induce specific immune responses and protective effect in rats and monkeys.Citation13,Citation26 Furthermore, as expected, our observed high NTAb level against EV71 after administration in this study further supported the appropriateness of animal species used.
For the design of dose regime in the vaccine toxicity study, the WHO guideline suggested that one full human dose should be administered, not scaled for body weight or surface area.Citation20,Citation42 The proposed single full human dose of inactivated EV71 vaccine developed in this study is 320 U according to our previous immunogenicity and protection results (the unpublished data). The dose levels used in the single- and repeat-dose (monkey) studies were 2- and 4- fold higher than the proposed clinical dose, respectively. Despite the relatively high dose administered and the repeated inoculations (four consecutive doses in the repeated toxicity study), no “target” organs for toxicity were discovered and all animals survived to scheduled termination. Any clinical observations found were considered to be unrelated to vaccination or attribution to other causes due to their sporadic nature, equivalent occurrence of controls and treated animals, lack of correlation between genders and different time point. These results indicated that EV71 vaccine had a favorable safety profile in rats and monkeys.
Histological finding at the injection site were minimal to severe in the adjuvant and EV71 vaccine treated groups in both animal species, most of which were acute or subacute inflammatory responses and decreased over time. Furthermore, mild inflammatory infiltration of injection site was also found in rats with saline control. The changes may be caused by mechanical injury due to the repeated intramuscular administrations. Further analysis showed that animals with local inflammatory reaction often responded with higher antibody levels against EV71, suggesting an association between local inflammatory response and immune stimulation. Similar dose-response relationship of histological changes at the injection site was seen in previous study in animal injected intramuscularly of vaccine with adjuvant aluminum.Citation27-Citation29 These observations were deemed to result from the injection procedure as well as the intended immune response following the repeated administrations with aluminum-containing vaccines. Till now, aluminum adjuvant has been approved by regulatory authorities for more than 70 y and aluminum-containing vaccines have many years record of safety around the world. Serious adverse effects attributable to the aluminum adjuvant are rare despite of local reactions such as redness, swelling and/or tenderness at the injection site are not infrequent.Citation29 Now, the licensed viral vaccines such as hepatitis A, hepatitis B, human papillomavirus and rabies vaccines are formatted with aluminum adjuvant.Citation30,Citation31
Autoimmunity is one of the three main areas of immunotoxicology. There were only a few rare cases that autoimmune disease was resulted by consequence of vaccination, for example, a form associated with the 1976–77 vaccination campaign against swine influenza,Citation32 despite of the fact that mechanism actually involved is not clear. However, from a strictly regulatory point of view, the issues regarding autoimmunity and toxicity testing have to be addressed in the preclinical study for new vaccine products. Although no relationship could be firmly established between autoantibody levels and the development and/or severity of autoimmune diseases, serum autoantibodies have been widely considered the hallmarks of autoimmunity, especially for specific autoimmune diseases.Citation33,Citation34 In our study, no obvious ANA level were noted in rats and monkeys following the consecutive four vaccinations, suggesting the test article, EV71 vaccine, have a low risk for causing the autoimmune reaction.
According to previous poliovirus studies, the serum neutralizing antibody response played an important role for protection and lifelong immunity in humans.Citation35,Citation36 Similarly, it was reported that a close association were observed between EV71-neutralizing antibodies titers and protective efficacy against EV71 infection in animal model.Citation13,Citation15,Citation16,Citation19 Seroepidemiology also showed that age-specific pre-epidemic EV71 seroprevalence rates were inversely related to age-specific periepidemic mortality rates (r = -0.82) or severe case rates (r = -0.93).Citation37 It was found that genotype C4 of EV71 was often associated with some EV71 outbreaks in recent years and became the main genotype in China.Citation38,Citation39 Selection of the virus strain is key to the development of an inactivated vaccine. The strain H3TY, screened from epidemic genotype C4 strain, had been demonstrated to have high genetic stability, good immunogenicity and cross-protection against heterologous EV71 strains (the unpublished data).Citation40 In the study, all rats and monkeys treated with low- and high-dose EV71 vaccines were seroconverted following the second administration (on day 28), suggesting that inactivated EV71 vaccine developed in this study have good immunogenicity, even in low dosage (160 U). These data will contribute to the design of dose level and schedule in human phase I clinical trial of inactivated EV71 vaccine. Furthermore, it was also noted that EV71-neutralizing antibodies titers in rats seem to be lower than those in monkeys on day 28 and 42. It was speculated that these difference may be associated with different animal species.
Our study included multi-dose vaccination and both acute- and recovery-phase evaluations were conducted in two species in compliance with the Good Laboratory Practice (GLP), which provided sufficient safety data of inactivated EV71 vaccine intended for clinical use. Furthermore, the design of toxicology study can also serve as an example for the preclinical safety evaluation of other novel vaccine candidate product. However, although the study was designed according to the WHO guideline on nonclinical evaluation of vaccines,Citation42 the limitation is a relatively small group size of cynomolgus monkeys used. It is suggested that more animals should be used in the nonclinical assessment of new vaccines if non-human primates were included.
In conclusion, the preclinical safety assessment of inactivated EV71 vaccine in rats and cynomolgus monkeys described herein showed that EV71 vaccine were well tolerated with no obvious toxicity, and then elicited high NTAb response. These results support this product to enter human phase I clinical trial in the near future.
Materials and methods
Animals
7-week-old specific pathogen free (SPF) Wistar rats (body weight of 165.5–205.3 g for male and 148.9–177.4 g for female) were purchased from the animal center at the National Institutes for Food and Drug Control (Beijing, China). The rats were housed in polycarbonate cages (L × W × H: 460 × 315 × 210 mm) in an SPF barrier system, which was maintained at 20–25◦C, 40–70% relative humidity, a 12 h light-dark cycle and a room air exchange of 10–20 times per hour. The feeding density was 2 or 3 animals per cage after dosing. Animals had ad libitum access to the certified rodent diet and sterilized municipal tap water (Beijing) was given ad libitum via water bottles. Each rat was given a unique number and identified by ear tag and animal number.
Cynomolgus monkeys (body weight of 1.98–2.50 kg for male and 2.16–2.44 kg for female), at 2 to 3 y old of age, were purchased from Kangda Laboratory Animal Technology Co. Ltd. (Guangdong, China) and maintained in stainless steel cages (L × W × H: 800 × 700 × 750 mm) under condition of 16–26◦C, 40–70% relative humidity, a 12 h light-dark cycle and a room air exchange of 8–10 times per hour. Each monkey was provided with 300 g of standard monkey keeping diet, and fruits per day and sterilized municipal tap water (Beijing) was available ad libitum throughout the study. The monkey was individually housed and identified by tattoo and animal number.
Rats were quarantined for seven days and monkeys were quarantined for 50 d before the study was conducted. Animals were accepted for use on the study based on body weights and physical examination performed during the quarantine period. All animal experiment protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of National Center for Safety Evaluation of Drugs (NCSED), Beijing and all procedures conducted in this study were in accordance with GLP for nonclinical studies of drugs of China.
Test article
Test article EV71 vaccine was developed by Zhejiang Pukang Biotechnology Co. Ltd., China. EV71 strain H3TY (genotype C4) as vaccine virus seed was grown in Vero cell using serum-free medium. The cultivated virus was harvested, purified, and inactivated by formaldehyde. Adjuvant aluminum hydroxide was prepared by the reaction of aluminum chloride with sodium hydroxide and the pH of the mixture was adjusted to approximately pH 6.4. Finally, the above purified virus suspension was adsorbed on the aluminum hydroxide and the adsorption degree was more than 95% as determined using a double antibody sandwich ELISA assay. Each 0.5 mL vaccine dose contained 320 U antigen and 0.4 mg aluminum. The EV71 antigen contents were measured using national EV71 antigen standard (NIFDC20100023, China).Citation41 The proposed shelf-life of the EV71 vaccine was 24 mo when was stored at 2–8◦C according to the stability study results on the physical, chemical, and biological characteristics of the product. All test article was held at 2–8◦C before use. Saline solution (0.9% NaCl) was used as negative control.
Design of toxicology study
For the single-dose toxicity study, 20 rats were given 1 ml of EV71 vaccine (640 U) through single intramuscular injection (). Ten rats of control group were administrated equal volume of saline solution. All animals were examined for mortality, clinical signs and body weight for 14 d, and sacrificed on day 15 and given a complete necropsy.
Table 1. The study design for safety evaluation of inactivated EV71 vaccine
In order to evaluate comprehensively the safety of inactivated EV71 vaccine, additional group treated with adjuvant alone was also included in the repeat-dose toxicity study in rats. 176 rats were randomized into four groups and each group was composed of 22 male and 22 female rats (). Rats were administrated intramuscularly with 0.5 ml of saline solution, adjuvant aluminum (0.4 mg) alone, high (320 U) and low (160 U) dosages of EV71 vaccines on days 0, 14, 28, 42 and then followed by 42 d of treatment-free (recovery) period. 10, 16, 10 rats (female and male in half per time) of each group were sacrificed on study days 2, 42 and 84, respectively. The animals were given a full necropsy and histopathological examination. Before each necropsy, rats were anesthetized by sodium pentobarbital for the collection of blood samples. The blood samples were used for hematological and clinical chemistry analysis as well as CD4+ and CD8+ cell counting as described previously.Citation23
For the repeat-dose toxicity study in cynomolgus monkeys, total 20 cynomolgus monkeys were randomized into two EV71 vaccine groups (4 males and 4 females per group) and a control group of 2 males and 2 females (). Monkeys were injected intramuscularly on days 0, 14, 28, 42 with 0.5 ml of low dosage (320 U) or 2 ml of high dosage (1280 U) EV71 vaccines and then followed by 28 d recovery period. The control group was treated with 2 ml saline solution. Monkeys were sacrificed on days 45 and 70, respectively. The parameters for this study were basically the same as the rat study (). Additional parameters in this study were ECG and complement 3 and 4 for the clinical chemistry analysis.
Detection of NTAb against EV71 and ANA
In order to evaluate the immune response of EV71 vaccine in animal model, serum samples were collected from other eight rats of each group on days 14, 28, 42, 56 and 84, and used for detection of NTAb and ANA. NTAb against EV71 were determined using the cytopathogenic effect (CPE) assay as previously described.Citation40,Citation41 In brief, the serum samples were inactivated at 56◦C, diluted 2-fold serially and then mixed with equal volume (250 μl) of 100-TCID50 EV71 virus (strain FY-23K-B: genotype C4, provided by Zhejiang Pukang Biotechnology Co. Ltd.) suspension. After incubation at 37◦C for 1 h and at 5–8◦C for 20–24 h, 100 μl of the above mixture were added to a 96-well plate containing monolayer Vero cell and 5 replicate wells were used at each dilution. The cell plate was then incubated in 5% CO2 incubators for 5–7 d at 36◦C. NTAb titers were expressed as the reciprocal of the highest dilution capable of inhibiting 50% of the CPE. Cell control, virus control and serum positive control were included in each experiment. Seroconversion was defined as an NTAb titer of more than 4-fold when compared with the control group. Furthermore, ANA level in the sera were determined using indirect immunofluorescence assay kit (Euroimmun, Hangzhou, China) according to the recommendation of manufactures’ instruction. The screening dilution recommended by the manufacturer was 1:100. The fluorescence intensity classification was scored semi-quantitatively from 1+ to 4+ relative to the intensity of a negative and a positive control (4+) contained in each slide.
Similarly, serum samples were collected from the monkeys of each group on days 14, 28, 42, 56 and 70. NTAb and ANA levels in sera were determined as described above.
Statistical analyses
Group means and standard deviations were calculated for body weight, food consumption, body temperature, clinical chemistry, hematology, organ weights, percentages of CD4+ and CD8+ cells. These data were analyzed using the statistics software Toxstat 2006, NCSED. Bartlett test was performed to test for variance homogeneity. When the result showed no significance (p > 0.05) one-way analysis of variance (ANOVA) was used. When ANOVA showed significance (p < 0.05), Dunnett’s test was done for multiple comparisons (all dose groups vs. control group). The abnormal pathological changes were analyzed by Fisher’s exact test using SPSS 13.0 software. p < 0.05 was considered as indicative of significant difference.
| Abbreviations: | ||
| ANA | = | antinuclear antibodies |
| ANOVA | = | analysis of variance |
| CPE | = | cytopathogenic effect |
| ECG | = | electrocardiogram |
| EV71 | = | Enterovirus 71 |
| GLP | = | Good Laboratory Practice |
| HFMD | = | hand, foot, and mouth disease |
| IACUC | = | Institutional Animal Care and Use Committee |
| NCSED | = | National Center for Safety Evaluation of Drugs |
| NIFDC | = | National Institutes for Food and Drug Control |
| NTAb | = | neutralizing antibodies |
| SPF | = | specific pathogen free |
| WHO | = | World Health Organization |
Additional material
Download Zip (68.1 KB)Disclosure of Potential Conflicts of Interest
Yun-shui Jiang and Kang-fang Zhou are employees of Zhejiang Pukang Biotechnology Co. Ltd. The other authors declare no conflicts of interest.
Acknowledgments
We would like to thank Ming Li, Chao Wang, Ying Liu, Li Sun, Xin Li, Min Hong, Yufa Miao, Dongsheng Pan, Fang Liu, Yanwei Yang, Di Zhang, Ben Ma, Zhe Qu and Zhi Lin for excellent technical assistant in animal and pathological studies.
This work was supported by National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the Twelfth Five-year Plan Period” (No. 2012ZX09302001) from the Ministry of Science and Technology, People’s Republic of China.
References
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 1974; 129:304 - 9; http://dx.doi.org/10.1093/infdis/129.3.304; PMID: 4361245
- Brown BA, Oberste MS, Alexander JP Jr., Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol 1999; 73:9969 - 75; PMID: 10559310
- Chang LY, Hsia SH, Wu CT, Huang YC, Lin KL, Fang TY, et al. Outcome of enterovirus 71 infections with or without stage-based management: 1998 to 2002. Pediatr Infect Dis J 2004; 23:327 - 32; http://dx.doi.org/10.1097/00006454-200404000-00010; PMID: 15071287
- Chang LY, Huang LM, Gau SS, Wu YY, Hsia SH, Fan TY, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med 2007; 356:1226 - 34; http://dx.doi.org/10.1056/NEJMoa065954; PMID: 17377160
- Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child 1980; 55:583 - 8; http://dx.doi.org/10.1136/adc.55.8.583; PMID: 6254449
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778 - 90; http://dx.doi.org/10.1016/S1473-3099(10)70194-8; PMID: 20961813
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, et al, Taiwan Enterovirus Epidemic Working Group. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 1999; 341:929 - 35; http://dx.doi.org/10.1056/NEJM199909233411301; PMID: 10498487
- Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention. Enterovirus surveillance--United States, 1970-2005. MMWR Surveill Summ 2006; 55:1 - 20; PMID: 16971890
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J 2010; 7:94; http://dx.doi.org/10.1186/1743-422X-7-94; PMID: 20459851
- Schuffenecker I, Mirand A, Antona D, Henquell C, Chomel JJ, Archimbaud C, et al. Epidemiology of human enterovirus 71 infections in France, 2000-2009. J Clin Virol 2011; 50:50 - 6; http://dx.doi.org/10.1016/j.jcv.2010.09.019; PMID: 21035387
- McMinn P, Stratov I, Nagarajan L, Davis S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis 2001; 32:236 - 42; http://dx.doi.org/10.1086/318454; PMID: 11170913
- Ooi MH, Wong SC, Podin Y, Akin W, del Sel S, Mohan A, et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis 2007; 44:646 - 56; http://dx.doi.org/10.1086/511073; PMID: 17278054
- Dong C, Liu L, Zhao H, Wang J, Liao Y, Zhang X, et al. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine 2011; 29:6269 - 75; http://dx.doi.org/10.1016/j.vaccine.2011.06.044; PMID: 21722686
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, Wen SQ, et al. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine 2012; 30:3295 - 303; http://dx.doi.org/10.1016/j.vaccine.2012.03.010; PMID: 22426327
- Meng FY, Li JX, Li XL, Chu K, Zhang YT, Ji H, et al. Tolerability and immunogenicity of an inactivated enterovirus 71 vaccine in Chinese healthy adults and children: an open label, phase 1 clinical trial. Hum Vaccin Immunother 2012; 8:668 - 74; http://dx.doi.org/10.4161/hv.19521; PMID: 22634437
- Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001; 20:895 - 904; http://dx.doi.org/10.1016/S0264-410X(01)00385-1; PMID: 11738755
- Wu TC, Wang YF, Lee YP, Wang JR, Liu CC, Wang SM, et al. Immunity to avirulent enterovirus 71 and coxsackie A16 virus protects against enterovirus 71 infection in mice. J Virol 2007; 81:10310 - 5; http://dx.doi.org/10.1128/JVI.00372-07; PMID: 17626076
- Tung WS, Bakar SA, Sekawi Z, Rosli R. DNA vaccine constructs against enterovirus 71 elicit immune response in mice. Genet Vaccines Ther 2007; 5:6; http://dx.doi.org/10.1186/1479-0556-5-6; PMID: 17445254
- Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, et al. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008; 26:1855 - 62; http://dx.doi.org/10.1016/j.vaccine.2008.01.058; PMID: 18329759
- Forster R. Study designs for the nonclinical safety testing of new vaccine products. J Pharmacol Toxicol Methods 2012; 66:1 - 7; http://dx.doi.org/10.1016/j.vascn.2012.04.003; PMID: 22561062
- Sun Y, Gruber M, Matsumoto M. Overview of global regulatory toxicology requirements for vaccines and adjuvants. J Pharmacol Toxicol Methods 2012; 65:49 - 57; http://dx.doi.org/10.1016/j.vascn.2012.01.002; PMID: 22326278
- Wolf JJ, Kaplanski CV, Lebron JA. Nonclinical safety assessment of vaccines and adjuvants. Methods Mol Biol 2010; 626:29 - 40; http://dx.doi.org/10.1007/978-1-60761-585-9_3; PMID: 20099119
- Huo Y, Li B, Zhang Y, Wang S, Bao M, Gao X, et al. Pre-clinical safety evaluation of heat shock protein 65-MUC1 peptide fusion protein. Regul Toxicol Pharmacol 2007; 49:63 - 74; http://dx.doi.org/10.1016/j.yrtph.2007.05.005; PMID: 17600604
- Koszalka GW, Johnson NW, Good SS, Boyd L, Chamberlain SC, Townsend LB, et al. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 2002; 46:2373 - 80; http://dx.doi.org/10.1128/AAC.46.8.2373-2380.2002; PMID: 12121907
- Ryffel B, Weber M. Preclinical safety studies with recombinant human interleukin 6 (rhIL-6) in the primate Callithrix jacchus (marmoset): comparison with studies in rats. J Appl Toxicol 1995; 15:19 - 26; http://dx.doi.org/10.1002/jat.2550150106; PMID: 7538156
- Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ, Chang JY, et al. Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine 2011; 29:4362 - 72; http://dx.doi.org/10.1016/j.vaccine.2011.04.010; PMID: 21501643
- Keith LS, Jones DE, Chou CH. Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine 2002; 20:Suppl 3 S13 - 7; http://dx.doi.org/10.1016/S0264-410X(02)00165-2; PMID: 12184359
- Exley C, Burgess E, Day JP, Jeffery EH, Melethil S, Yokel RA. Aluminum toxicokinetics. J Toxicol Environ Health 1996; 48:569 - 84; http://dx.doi.org/10.1080/009841096161078; PMID: 8772799
- Hunter RL. Overview of vaccine adjuvants: present and future. Vaccine 2002; 20:Suppl 3 S7 - 12; http://dx.doi.org/10.1016/S0264-410X(02)00164-0; PMID: 12184369
- Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine 2007; 25:825 - 33; http://dx.doi.org/10.1016/j.vaccine.2006.09.039; PMID: 17014935
- Eickhoff TC, Myers M. Workshop summary. Aluminum in vaccines. Vaccine 2002; 20:Suppl 3 S1 - 4; http://dx.doi.org/10.1016/S0264-410X(02)00163-9; PMID: 12184358
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. Am J Epidemiol 1979; 110:105 - 23; PMID: 463869
- Verdier F, Patriarca C, Descotes J. Autoantibodies in conventional toxicity testing. Toxicology 1997; 119:51 - 8; http://dx.doi.org/10.1016/S0300-483X(96)03596-2; PMID: 9129195
- Descotes J. Autoimmunity and toxicity testing. Toxicol Lett 2000; 112-113:461 - 5; http://dx.doi.org/10.1016/S0378-4274(99)00234-9; PMID: 10720766
- Asturias EJ, Dueger EL, Omer SB, Melville A, Nates SV, Laassri M, et al. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants. J Infect Dis 2007; 196:692 - 8; http://dx.doi.org/10.1086/520546; PMID: 17674310
- Bonnet MC, Dutta A. World wide experience with inactivated poliovirus vaccine. Vaccine 2008; 26:4978 - 83; http://dx.doi.org/10.1016/j.vaccine.2008.07.026; PMID: 18680777
- Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88; http://dx.doi.org/10.1542/peds.109.6.e88; PMID: 12042582
- Guan D, van der Sanden S, Zeng H, Li W, Zheng H, Ma C, et al. Population dynamics and genetic diversity of C4 strains of human enterovirus 71 in Mainland China, 1998-2010. PLoS One 2012; 7:e44386; http://dx.doi.org/10.1371/journal.pone.0044386; PMID: 22984501
- Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol 2009; 44:262 - 7; http://dx.doi.org/10.1016/j.jcv.2009.02.002; PMID: 19269888
- Mao Q, Li N, Yu X, Yao X, Li F, Lu F, et al. Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol 2012; 157:37 - 41; http://dx.doi.org/10.1007/s00705-011-1136-3; PMID: 21984267
- Liang Z, Mao Q, Gao Q, Li X, Dong C, Yu X, et al. Establishing China’s national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine 2011; 29:9668 - 74; http://dx.doi.org/10.1016/j.vaccine.2011.10.018; PMID: 22015395
- World Health Organization Expert Committee on Biological Standardization. Guidelines on Nonclinical Evaluation of Vaccines. Geneva, Switzerland 17-21 November 2003. Available at: http://www.who.int/biologicals/publications/nonclinical_evaluation_vaccines_nov_2003.pdf [Last accessed March 1st, 2013]