Abstract
Introduction: The aim of the present study is to review the economic burden of varicella disease and the benefit of universal varicella vaccination in different settings pending its implementation in all Italian regions.
Materials and Methods: Research was conducted using PubMed, Scopus and ISI databases. Score quality and data extraction were performed for all included studies.
Results: Twenty-three articles met the criteria: 15 cost-effectiveness, 8 cost-benefit and one cost-utility analysis. Varicella vaccination could save the society from €637,762 (infant strategy) to 53 million annually (combined infant and adolescent strategy). The median and the mean quality scores resulted in 91.8% and 85.4% respectively; 11 studies were considered of high quality and 12 of low quality.
Discussion: The studies are favorable to the introduction of universal varicella vaccination in Italy, being cost saving and having a positive impact on morbidity. The quality score of the studies varied greatly: recent analyses were of comparable quality to older studies.
Introduction
Varicella or chickenpox is a common childhood exanthematic disease caused by the Varicella zoster virus (VZV) -a member of the herpesvirus family- which in most cases affects 5–10 y old children. Although benign, is highly contagious, pandemic and causes several complications that are frequently underestimated.Citation1
Vaccination is one of the most effective and safe Public Health interventions available for the primary prevention of infectious diseases. This practice brings benefits not only to the vaccinated subjects, but also indirectly by inducing protection to non-vaccinated: herd immunity.
Since 1992 a varicella vaccine, consisting of live attenuated virus, is available and recommended for all children in the second year of life. The efficacy of varicella vaccination is about 95% in the prevention of moderate or severe cases and between 70–85% in the prevention of mild forms. The vaccine is safe, well tolerated and protection appears to be of long duration; it is now recommended as a two-dose schedule starting after the first year of life (second dose 1–3 mo apart or administered at 4–6 y).
The trend of varicella in Italy shows a series of fluctuation and basically increased until the mid-nineties. The maximum number of cases was reported in 2004 and amounted to more than 126.000 infected subjects. Following that peak, there was a decrease in cases, especially in 2005. Generally, the trend of varicella in Italy shows a decrement from 1996 to 2006 due to the introduction of the vaccine in some Regions (104.216 vs 97.634), but the number is still high.Citation1
The reform of the V Title of the Constitution (Constitutional Law n.3/2001) endows all 21 Italian Regions with the responsibility of organizing and delivering health services while the State determines the essential health care interventions that all Regions have to offer to the population.Citation2 Both mandatory (diphtheria, tetanus, poliomyelitis, hepatitis B) and non-mandatory vaccinations (pertussis, Hemophilus influenzae type b, measles, mumps and rubella) are included in essential health services. Nevertheless, vaccination policies in Italian Regions have been extremely heterogeneous in the last few years, with the same vaccine offered free of charge to all newborns and susceptible adolescents in seven regions, and only to people at risk in the remaining 14 Regions. There are even differences within the same region, related to the disparate vaccination strategies of Local Health Units.
In several countries routine varicella vaccination for all children has been introduced into the national immunisation schedule, e.g., Germany,Citation3 Greece,Citation4 Australia,Citation5 Canada,Citation6 Republic of Korea,Citation7 Saudi ArabiaCitation8 and the United States of America (USA).Citation9 In Spain, as in Italy, it’s only at regional level.Citation10
Along with the adoption of routine vaccination program, a two dose schedule has been recommended in Greece and by the Spanish Association of Pediatrics.Citation11 In Germany, the combined MMRV (measles-mumps-rubella-varicella) vaccine can be used, in place of separate injections of measles mumps- rubella (MMR) and varicella vaccines, with the two doses administered close together (at least 1 mo apart). A short interval between the doses enhanced compliance and vaccine coverage. Also, a good immune response has been obtained when the MMRV vaccine was given to children with an interval of 1 to 6 y, as in Finland.Citation12 In other European countries (e.g., Belgium, Norway), MMR vaccines are administered with a longer schedule (7 to 12 y apart). A long interval for the second dose of varicella-containing vaccine is associated with higher risk of breakthrough disease between doses and could have negative impact on vaccine coverage.Citation13
In the USA, a two-dose schedule for varicella vaccination has already been implemented, and a decrease in the number of patients with varicella -including those with breakthrough disease- has been observed.Citation14,Citation15 The Advisory Committee on Immunization Practices (ACIP) recommends the first dose of vaccine administered at 12 to 15 mo and the second dose at 4 to 6 y or at an earlier age, provided the interval between the two doses is 3 mo.Citation9 Furthermore, the American Academy of Pediatrics recommends either MMR and varicella vaccines separately, or the MMRV be used for the first dose. Generally, the use of MMRV for dose 2 is preferred over separate injections.Citation16
Regarding varicella vaccination coverage, data are not routinely collected by all Italian Regions; hence, the national coverage is assessed every 5 y by survey method. The survey conducted in 2008, among children aged 12–24 mo, showed a rate of 17%.Citation17 The rate is very low compared with other developed countries with routine varicella vaccination program, such as the USA (90.8% among children aged 19–35 mo in 2011)Citation18 and Australia (83% among children aged 24 mo in 2011).Citation19 The Italian coverage rate is also lower than in Japan, which has a voluntary varicella vaccination program (90% for children up to 4 y old and 35% among those 1 y of age, in 2011).Citation20
The Italian National Immunization Plan 2012–2014 (NIP), approved in February 2012, has the primary objective to harmonize vaccine strategies, and to ensure an active offer and free vaccination priority for the general population. In particular, the Plan states that varicella vaccination should become universally recommended in all Regions, albeit from 2015, after obtaining the results of the pilot programs activated in eight regions (Basilicata, Calabria, Puglia, Sardinia, Sicily, Tuscany, Veneto and the Province of Bolzano).Citation21
The aim of the present study is to evaluate the economic burden of varicella disease and the benefit of universal varicella vaccination pending its implementation in all Italian regions.
Results
Identification of relevant research
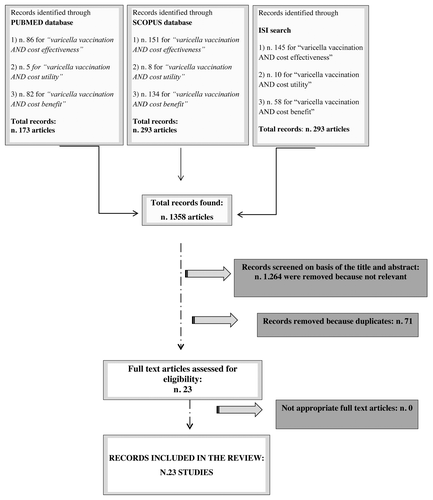
Using the aforementioned inclusion criteria we found (see ):
- 173 articles for Pubmed search;
- 293 articles for Scopus search;
- 213 articles for ISI search.
A total of 1358 articles were found for all strings, of which 1264 were removed because they were not relevant and 71 articles were duplicates in two or all search engines. At the end of the evaluation, 23 articles met the pre-determined criteria described above.Citation22-Citation44
Quality Assessment
The 23 articles reviewed, with the assigned score quality, are shown in . The maximum quality score of 113/113 (100%) was obtained by Coudeville L. et al. 2004Citation36 and Brisson M. et al. 2002,Citation26 and the lowest score was 71/107 (66.3%) assigned to Jean-Jasmin LM et al. 2004.Citation35 The median score resulted in a value of 91.8% ().
Table 1. Score quality of the included studies by year of publication (according to the Drummond’s checklist modified by La Torre et al.)
Considering the median score of the 23 articles, we found that 11 were of high quality (articles with assigned score over the median value), while the remaining 12 were of low quality (articles with assigned score under the median value). The mean score quality for all 23 articles considered was 85.41%.
The review evidenced that the quality score of the studies varied over years, even in the same year and in analyses conducted by the same authors: Scuffham et al.Citation22 vs. Scuffham et al.Citation23 got 90.3% and 75.6% respectively; Coudeville L. et al. in 1999,Citation25 in 2004,Citation36 and 2005,Citation38 obtained respectively 91.5%, 100% and 94.8%. The differences are especially evident in the 3rd section - analysis and interpretation of results - of the Drummond’s checklist. No difference is evident in the quality of the examined studies along the years.
Type of Economic evaluation of the included studies
The classification of the studies is based on the type of economic evaluation:
- CEA (cost-effectiveness analysis) was performed in 15 economic evaluation studies;Citation22,Citation23,Citation26-Citation28,Citation31-Citation34,Citation37-Citation39,Citation41,Citation42,Citation44
- CBA (cost-benefit analysis) was evaluated by 8 studies;Citation24,Citation25,Citation29,Citation35-Citation37,Citation40,Citation43
- CUA (cost-utility analysis) was considered in the study performed by Brisson M. et al. 2003.Citation38
Among the CEA, nine studies demonstrate the cost-effectiveness of childhood varicella vaccination compared with no vaccination.Citation26,Citation27,Citation31,Citation34,Citation38,Citation39,Citation41,Citation42,Citation44 The results of Scuffham et al.Citation22,Citation23 varied in different environments: in 1999 they affirmed that the introduction of varicella program is cost saving for the New Zealand society, while in Australia, the childhood vaccine program is still preferred but has greater costs than the no vaccination program. Furthermore, according to Getsios et. al.,Citation28 the childhood vaccination program is not cost-effective for the Canadian healthcare system.
Thiry et al.Citation33 underlined the economic savings of an adolescent program in Italy, while Hanslik T. et al.Citation32 concluded that targeted vaccination of non-immune adolescents and adults could reduce medical and financial burden of varicella in countries where no routine childhood immunization is implemented.
The only CUA included in the review (Brisson M. et al.Citation30) does not consider the childhood program as a cost saving strategy compared with the adolescent one.
Finally, all the CBA substantially reported that varicella vaccine can reduce the number of cases, complications and hospitalizations.
The population and the countries considered were different in the studies: 14 analyses were conducted in Europe, 3 in America, 4 in Asia and 2 in Oceania.
Discussion
The present review includes 23 studies on varicella vaccination, which highlight the importance of varicella vaccine and the economic burden of the disease both from the societal and the healthcare perspective. Generally, varicella vaccination is cost saving from the societal perspective, infact BCR ranges between €637,762 (childhood strategy)Citation22 and 53 million annually (combined childhood and adolescent strategy).Citation27
The three studies conducted in ItalyCitation33,Citation36,Citation43 support a routine varicella vaccination program, being cost saving and having a positive impact on morbidity. For recommended vaccination of adolescents to be cost saving for the NHS - up to €292.134 savings in direct treatment costs- the overall price of the vaccine would have to decrease below €25.10. The society will definitely benefit from a catch-up program, targeting children aged 2–11 y old, which can prevent the greatest number of varicella outcomes and avoid 45% of cases.Citation33
The analysis by Coudeville et al.Citation36 states that a routine childhood immunization represents a good option for Italy. Similarly, the study by Bonanni et al.Citation43 demonstrates that the introduction of universal varicella vaccination in young children (with or without an adolescent catch-up program) would be very effective in reducing the high disease burden in Italy. They also clarified that significant net savings are obtained from the societal perspective, while universal vaccination is not cost-saving to the Italian National Health System (NHS), but the amount needed to avoid a case or gain a year of life is very low and favorable all the same.
The introduction of MMRV vaccines given in a two-dose schedule could influence the cost-effectiveness of varicella vaccination in different settings. The analysis performed in Germany, demonstrated that two doses of MMRV vaccine are cost-saving from a societal and a health system perspective (BCR of 2.56 and 1.08, respectively) compared with an adolescent immunisation strategy with one dose of varicella vaccine.Citation40 Furthermore, the study conducted in the USA highlighted that, compared with no intervention, the two-dose regimen is cost-saving from a societal perspective (BCR = 2.73). However, compared with 1-dose program, the incremental second dose was not cost saving from a societal perspective (BCR = 0.56).Citation42
In addition, the MMRV vaccine provides other benefits by increasing the level of protection and, thus, limiting the risk of breakthrough cases. It also reduces the number of injections to complete vaccination, leading to major vaccine acceptance and improvement of the vaccination coverage rateCitation13,Citation40,Citation41. Consequently, adding varicella vaccination to the routine vaccination program and implementation of the two-dose schedule with MMRV should be taken into account in countries where it has not been implemented.
In conclusion, the scenarios presented by the 23 articles analyzed are heterogeneous but are also undoubtedly favorable to the introduction of universal varicella vaccination. The results should be handled in consideration of the studies quality. Fortunately for the Italian context, the analysis by Coudeville L. et al.Citation36 obtained the highest quality score (100%), followed by Thiry et al.Citation33 (97.4%) and Bonanni et al.Citation43 (91.5%). In 2003, Bonanni P.Citation45 affirmed that compulsory varicella vaccination will not be introduced in Italy, confirmed by Thiry et al.Citation33 in 2004, since the ultimate aim of the Italian NHS is the abolition of compulsory vaccination. It is foreseen, by the National Vaccination Plan 2012–2014, that from 2015 childhood routine vaccination will be offered free of charge during the second year of life in all Italian Regions. Further public health interventions will be therefore necessary to inform and educate the general population and to avoid a drastic drop of vaccination coverage in Italy; healthcare workers will surely have a key role in this process.
On the basis of the review results, it’s possible to conclude that the implementation of universal vaccination in all Italian regions by 2015 will be cost-saving from the societal perspective, and will imply a favorable cost-effectiveness profile from the NHS perspective.
Materials and Methods
Identification of relevant studies
A literature review was conducted using three electronic medical journal databases: Scopus, PubMed and ISI engines for published studies on economic evaluations of varicella vaccination programs. The keywords used were “vaccination”, “varicella”, “cost effectiveness,” “cost utility,” and “cost benefit”. Combined searches were performed for: “varicella vaccination AND cost effectiveness,” “varicella vaccination AND cost utility”, “varicella vaccination AND cost benefit.” Search criteria are summarized in .
The selection was limited to articles published in English and Italian language and we applied date restrictions from 1999 to 2011 included. We selected all studies focused on the economic evaluation of varicella vaccination programs without limit of population and country.
All the review process, including search and selection (identification, screening, eligibility of included studies) was performed according to the PRISMA criteriaCitation46 ().
In the selection process, abstracts were initially read independently by two researchers to identify potentially eligible full text papers which were then retrieved and assessed in order to decide on the final inclusion.
Articles were examined and were excluded if: (1) the research was based on modeling the impact of a combined varicella and zoster vaccination program on the epidemiology of varicella zoster virus; (2) studies were not pertaining to varicella vaccination; (3) the full text was not available. When Medline outcomes overlapped, all duplicate articles were eliminated.
Quality assessment and data extraction
For each selected study, two researchers (RS and BU) independently assessed the quality according to the original Drummond’s checklistCitation47 () modified by la Torre et al.,Citation48 weighting-median score for each item by different experts.
Discrepancies between the two investigators were solved by oral discussion and consensus with a senior investigator (GLT). Each item was assigned with the median weight attributed by the consensus, if applicable. Finally, the global score was computed summing up weights of each item. To compare different studies, global scores were referred in percentage, to the highest score achievable with the weighted Drummond’s checklist.
Drummond’s checklist is composed of 35 items divided into 3 sections: study design, data collection and analysis and interpretation of results. To weight the items, a group of experts was asked to attribute a score according to their importance. The weighted scores assigned by the consensus to study design, data collection and analysis and interpretation of results were 26, 45 and 48, respectively. For each item section, the maximum achievable score was as follows: (1) Study design (7 items): Maximum global score = 26; (2) Data collection (14 items): Maximum global score = 45; (3) Analysis and interpretation of results (14 items): Maximum global score = 48. When the item was not applicable to the study we reduced the Maximum global score from the relative weighted score item.
Two reviewers used a data collection form to independently abstract data from the studies. The information extracted was: references, publication year and type of analyses, alternatives, nation/ perspective, sample, efficacy measures/cost measure and results. The reviewers discussed any discrepancies in their results to reach an agreement. The characteristics of each study are shown in .
Table 2. Characteristics of the selected studies by year of publication and types of economic analysis
| Abbreviations: | ||
| VZV | = | Varicella zoster virus |
| NIP | = | National Immunization Plan |
| CEA | = | cost-effectiveness analysis |
| CBA | = | cost-benefit analysis |
| CUA | = | cost-utility analysis |
| NHS | = | National Health System |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Epicentro: Il portale dell'epidemiologia per la sanità pubblica. Centro Nazionale di Epidemiologia, Sorveglianza e Promozione della Salute. Available at: http://www.epicentro.iss.it/problemi/varicella/varicella.asp; last access 19.11.12.
- Presidente del Consiglio dei Ministri. D.P.C.M. Definizione dei livelli essenziali di assistenza. G.U.8 Febbraio 2002, n.33, S.
- Institut RK. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: Juli 2006. Epidemiol Bull 2006; 30:235 - 54
- Childhood immunisation calendar, Greece. Available at: http://www.mohaw.gr/gr/thefuture/anakoinoseis/Timetable%202008.xls
- Macartney KK, Burgess MA. Varicella vaccination in Australia and New Zealand. J Infect Dis 2008; 197:Suppl 2 S191 - 5; http://dx.doi.org/10.1086/522157; PMID: 18419396
- National Advisory Committee on Immunization. National Advisory Committee on Immunization (NACI) update on varicella. Can Commun Dis Rep 2004; 30:1 - 26; PMID: 14964716
- Sadzot-Delvaux C, Rentier B, Wutzler P, Asano Y, Suga S, Yoshikawa T, et al. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis 2008; 197:Suppl 2 S185 - 90; http://dx.doi.org/10.1086/522163; PMID: 18419395
- WHO vaccine preventable disease monitoring system: Immunization schedules by antigen, selection centre. Available at: http/www.who.int/immunization_monitoring/en/globalsummary/scheduleselect.cfm
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:RR-4 1 - 40; PMID: 17585291
- Alfonsi V, D’Ancona F, Giambi C, Nacca G, Rota MC, Regional Coordinators for Infectious Diseases and Vaccinations. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176 - 83; http://dx.doi.org/10.1016/j.healthpol.2011.10.002; PMID: 22030308
- Bernaola Iturbe E, Giménez Sánchez F, Baca Cots M, de Juan Martín F, Díez Domingo J, Garcés Sánchez M, et al, Spanish Association of Pediatrics. [Vaccination schedule of the Spanish Association of Pediatrics: recommendations 2007]. An Pediatr (Barc) 2007; 66:62 - 9; PMID: 17266854
- Vesikari T, Baer M, Willems P. Immunogenicity and safety of a second dose of measles-mumps-rubella-varicella vaccine in healthy children aged 5 to 6 years. Pediatr Infect Dis J 2007; 26:153 - 8; http://dx.doi.org/10.1097/01.inf.0000250689.09396.21; PMID: 17259879
- Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med 2009; 7:26; http://dx.doi.org/10.1186/1741-7015-7-26; PMID: 19476611
- Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis 2011; 203:312 - 5; http://dx.doi.org/10.1093/infdis/jiq052; PMID: 21208922
- Kattan JA, Sosa LE, Bohnwagner HD, Hadler JL. Impact of 2-dose vaccination on varicella epidemiology: Connecticut--2005-2008. J Infect Dis 2011; 203:509 - 12; http://dx.doi.org/10.1093/infdis/jiq081; PMID: 21199882
- Committee on Infectious Diseases. Policy statement—Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. Pediatrics 2011; 128:630 - 2; http://dx.doi.org/10.1542/peds.2011-1968; PMID: 21873692
- Gruppo di lavoro ICONA. ICONA 2008: Indagine di Copertura vaccinale Nazionale nei bambini e negli adolescenti. Rapporti ISTISAN 09/29. Istituto Superiore di Sanità, Roma; 2009.
- Centers for Disease Control and Prevention (CDC). National, state, and local area vaccination coverage among children aged 19-35 months--United States, 2011. MMWR Morb Mortal Wkly Rep 2012; 61:689 - 96; PMID: 22951450
- Ward K, Dey A, Hull B, Quinn HE, Macartney K, Menzies R. Evaluation of Australia’s varicella vaccination program for children and adolescents. Vaccine 2013; 31:1413 - 9; http://dx.doi.org/10.1016/j.vaccine.2012.12.052; PMID: 23290837
- Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother 2013; 19:188 - 95; http://dx.doi.org/10.1007/s10156-013-0577-x; PMID: 23483311
- Piano Nazionale Prevenzione vaccinale 2012-2014. Ministero della salute. Available at: http://www.salute.gov.it/dettaglio/phPrimoPianoNew.jsp?id=339; last access 19.11.12.
- Scuffham P, Devlin N, Eberhart-Phillips J, Wilson-Salt R. The cost-effectiveness of introducing a varicella vaccine to the New Zealand immunisation schedule. Soc Sci Med 1999; 49:763 - 79; http://dx.doi.org/10.1016/S0277-9536(99)00115-X; PMID: 10459888
- Scuffham PA, Lowin AV, Burgess MA. The cost-effectiveness of varicella vaccine programs for Australia. Vaccine 1999; 18:407 - 15; http://dx.doi.org/10.1016/S0264-410X(99)00261-3; PMID: 10519929
- Díez Domingo J, Ridao M, Latour J, Ballester A, Morant A. A cost benefit analysis of routine varicella vaccination in Spain. Vaccine 1999; 17:1306 - 11; http://dx.doi.org/10.1016/S0264-410X(98)00394-6; PMID: 10195765
- Coudeville L, Paree F, Lebrun T, Sailly JC. The value of varicella vaccination in healthy children: cost-benefit analysis of the situation in France. Vaccine 1999; 17:142 - 51; http://dx.doi.org/10.1016/S0264-410X(98)00161-3; PMID: 9987148
- Brisson M, Edmunds WJ. The cost-effectiveness of varicella vaccination in Canada. Vaccine 2002; 20:1113 - 25; http://dx.doi.org/10.1016/S0264-410X(01)00437-6; PMID: 11803072
- Wutzler P, Neiss A, Banz K, Goertz A, Bisanz H. Can varicella be eliminated by vaccination? Potential clinical and economic effects of universal childhood varicella immunisation in Germany. Med Microbiol Immunol 2002; 191:89 - 96; http://dx.doi.org/10.1007/s00430-002-0123-4; PMID: 12410347
- Getsios D, Caro JJ, Caro G, De Wals P, Law BJ, Robert Y, et al. Instituting a routine varicella vaccination program in Canada: an economic evaluation. Pediatr Infect Dis J 2002; 21:542 - 7; http://dx.doi.org/10.1097/00006454-200206000-00012; PMID: 12182379
- Hsu HC, Lin RS, Tung TH, Chen TH. Cost-benefit analysis of routine childhood vaccination against chickenpox in Taiwan: decision from different perspectives. Vaccine 2003; 21:3982 - 7; http://dx.doi.org/10.1016/S0264-410X(03)00270-6; PMID: 12922134
- Brisson M, Edmunds WJ. Varicella vaccination in England and Wales: cost-utility analysis. Arch Dis Child 2003; 88:862 - 9; http://dx.doi.org/10.1136/adc.88.10.862; PMID: 14500303
- Banz K, Wagenpfeil S, Neiss A, Goertz A, Staginnus U, Vollmar J, et al. The cost-effectiveness of routine childhood varicella vaccination in Germany. Vaccine 2003; 21:1256 - 67; http://dx.doi.org/10.1016/S0264-410X(02)00431-0; PMID: 12559807
- Hanslik T, Boëlle PY, Schwarzinger M, Carrat F, Freedberg KA, Valleron AJ, et al. Varicella in French adolescents and adults: individual risk assessment and cost-effectiveness of routine vaccination. Vaccine 2003; 21:3614 - 22; http://dx.doi.org/10.1016/S0264-410X(03)00405-5; PMID: 12922090
- Thiry N, Beutels P, Tancredi F, Romanò L, Zanetti A, Bonanni P, et al. An economic evaluation of varicella vaccination in Italian adolescents. Vaccine 2004; 22:3546 - 62; http://dx.doi.org/10.1016/j.vaccine.2004.03.043; PMID: 15315834
- Ginsberg GM, Somekh E. Cost containment analysis of childhood vaccination against varicella in Israel. J Infect 2004; 48:119 - 33; http://dx.doi.org/10.1016/S0163-4453(03)00079-3; PMID: 14720487
- Jean-Jasmin LM, Lynette SP, Stefan M, Kai CS, Chew FT, Wah LB. Economic burden of varicella in Singapore--a cost benefit estimate of implementation of a routine varicella vaccination. Southeast Asian J Trop Med Public Health 2004; 35:693 - 6; PMID: 15689089
- Coudeville L, Brunot A, Giaquinto C, Lucioni C, Dervaux B. Varicella vaccination in Italy : an economic evaluation of different scenarios. Pharmacoeconomics 2004; 22:839 - 55; http://dx.doi.org/10.2165/00019053-200422130-00003; PMID: 15329030
- Tseng HF, Tan HF, Chang CK. Varicella epidemiology and cost-effectiveness analysis of universal varicella vaccination program in Taiwan. Southeast Asian J Trop Med Public Health 2005; 36:1450 - 8; PMID: 16610647
- Coudeville L, Brunot A, Szucs TD, Dervaux B. The economic value of childhood varicella vaccination in France and Germany. Value Health 2005; 8:209 - 22; http://dx.doi.org/10.1111/j.1524-4733.2005.04005.x; PMID: 15877593
- Lenne X, Diez Domingo J, Gil A, Ridao M, Lluch JA, Dervaux B. Economic evaluation of varicella vaccination in Spain: results from a dynamic model. Vaccine 2006; 24:6980 - 9; http://dx.doi.org/10.1016/j.vaccine.2006.04.051; PMID: 16860909
- Hammerschmidt T, Bisanz H, Wutzler P. Universal mass vaccination against varicella in Germany using an MMRV combination vaccine with a two-dose schedule: an economic analysis. Vaccine 2007; 25:7307 - 12; http://dx.doi.org/10.1016/j.vaccine.2007.08.017; PMID: 17881097
- Valentim J, Sartori AMC, de Soárez PC, Amaku M, Azevedo RS, Novaes HM. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine 2008; 26:6281 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.07.021; PMID: 18674582
- Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis 2008; 197:Suppl 2 S156 - 64; http://dx.doi.org/10.1086/522135; PMID: 18419391
- Bonanni P, Boccalini S, Bechini A, Banz K. Economic evaluation of varicella vaccination in Italian children and adolescents according to different intervention strategies: the burden of uncomplicated hospitalised cases. Vaccine 2008; 26:5619 - 26; http://dx.doi.org/10.1016/j.vaccine.2008.07.096; PMID: 18723062
- Banz K, Iseli A, Aebi C, Brunner M, Schmutz AM, Heininger U. Economic evaluation of varicella vaccination in Swiss children and adolescents. Hum Vaccin 2009; 5:847 - 57; PMID: 19829048
- Bonanni P. Italie-la politique de vaccination. Vax Info 2003; 36:6 - 8
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Int J Public Health 2009; 4:94 - 131
- Drummond MF, Jefferson TO, The BMJ Economic Evaluation Working Party. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996; 313:275 - 83; http://dx.doi.org/10.1136/bmj.313.7052.275; PMID: 8704542
- La Torre G, Nicolotti N, de Waure C, Ricciardi W. Development of a weighted scale to assess the quality of cost-effectiveness studies and an application to the economic evaluations of tetravalent HPV vaccine. Int J Public Health 2011; 19:103 - 11; http://dx.doi.org/10.1007/s10389-010-0377-z