Abstract
The trivalent inactivated influenza vaccine Fluarix™ is licensed in the US for adults and children from 3 years old. This randomized observer-blind study (NCT00764790) evaluated Fluarix™ at two doses; 0.25 ml (Flu-25) and 0.5 ml (Flu-50) in children aged 6–35 months. The primary objective was to demonstrate immunogenic non-inferiority vs. a control vaccine (Fluzone®; 0.25 ml). Children received Flu-25 (n = 1107), Flu-50 (n = 1106) or control vaccine (n = 1104) at Day 0 and for un-primed children, also on Day 28. Serum hemagglutination-inhibition titers were determined pre-vaccination and at Day 28 (primed) or Day 56 (un-primed). Non-inferiority was assessed by post-vaccination geometric mean titer (GMT) ratio, (upper 95% confidence interval [CI] ≤ 1.5) and difference in seroconversion rate (upper 95% CI ≤ 10%). Reactogenicity/safety was monitored. The immune response to Flu-50 met all regulatory criteria. Indicated by adjusted GMT ratios [with 95% CI], the criteria for non-inferiority of Flu-50 vs. control vaccine were reached for the B/Florida strain (1.13 [1.01–1.25]) but not for the A/Brisbane/H1N1 (1.74 [1.54–1.98]) or A/Uruguay/H3N2 (1.72 [1.57–1.89]) strains. In children aged 18–35 months similar immune responses were observed for Flu-50 and the control vaccine. Flu-50 induced a higher response than Flu-25 for all strains. Temperature (≥ 37.5°C) was reported in 6.2%, 6.4%, and 6.6% of the Flu-25, Flu-50, and control group, respectively. Reactogenicity/safety endpoints were within the same range for all vaccines.
In children aged 6–35 months, immune responses with Flu-50 fulfilled regulatory criteria but did not meet the pre-defined criteria for non-inferiority vs. control. This appeared to be due to differences in immunogenicity in children aged < 18 months.
Introduction
Influenza vaccination has been used for many years to prevent influenza and its complications. Although influenza affects people of all ages, in the past, vaccination was mainly targeted toward the elderly or younger adults and children with underlying medical disorders. Following recognition of the substantial burden of influenza disease in the pediatric population and the importance of young children for transmission,Citation1-Citation5 there has been a shift in focus toward universal vaccination of healthy children. In the US, seasonal influenza vaccination is recommended for all children aged 6 months to 18 years and in Canada for children aged 6–23 months, whereas vaccination of healthy children is currently recommended in only a few European countries.Citation6-Citation9
To achieve adequate antibody titers in children aged 6 months to 8 years who are vaccine-naïve (un-primed), first time vaccination should comprise two doses of TIV given about a month apart.Citation6 In children aged >3 years, the recommended dose per TIV injection is 0.5 ml (the adult dose), yet dosing in children aged 6–35 months varies, with some countries such as Canada, the UK and Finland recommending the full ‘adult’ dose, and others, including the US, recommending a half ‘pediatric’ dose of 0.25 ml per injection.Citation6,Citation8,Citation10,Citation11 The rationale for using 0.25 ml rather than 0.5 ml is based on concerns about increased reactogenicity with the higher dose, according to historic studies of whole cell vaccines.Citation12 More recent studies however have shown that TIV doses of 0.5 ml may improve immune responses in very young children compared with the 0.25 ml dose without increasing reactogenicity.Citation13,Citation14
This study evaluated the use of the trivalent inactivated influenza vaccine (TIV) Fluarix™ in children aged 6–35 months, the age range with the highest incidence of influenza.Citation2 Fluarix™ has been manufactured in Germany since 1987 and since 1992 has been licensed in over 100 countries including indications for children over 6 months of age. In the US, Fluarix™ was licensed for adults in 2005 and for children from 3 years of age in 2009.Citation15,Citation16 The aim of the study was to assess the immunogenicity of Fluarix™ at two different dose levels in relation to an established control vaccine Fluzone®, which is licensed in the US for use in children from 6 mo of age. Previously, Fluarix™ was shown to be as immunogenic as Fluzone® in adults and in children over 3 y of age.Citation17,Citation18 Fluarix™ was however less immunogenic than Fluzone® in children aged ≤ 3 y old.Citation17 The current study therefore focused on children aged ≤ 3 y old and evaluated Fluarix™ at both the standard recommended TIV dose for young children in the US (0.25 ml) and also at double this dose (0.5 ml).
Results
Study Population
A total of 3318 children aged 6 to 35 months were enrolled into the study and 3317 were vaccinated including 1107 children with the study vaccine at 0.25 ml dose (Flu-25), 1106 children with the study vaccine at 0.5 ml dose (Flu-50) and 1104 children with the control vaccine. There were 109 children who did not complete the study (38 in the Flu-25 group, 41 in the Flu-50 group and 30 in the control group). Most were lost to follow-up and there were no withdrawals due to adverse events. Most children (71.1% of the Flu-25 group, 71.3% of the Flu-50 group and 71.6% of the control group) were un-primed (i.e., had not received a two-dose priming of influenza vaccine in any prior year) and so were administered two doses of study vaccine. The percentages of children who had a history of influenza vaccination (at least one dose) within the last three seasons prior to the study were 42.5% (Flu-25), 43.0% (Flu-50) and 42.7% (control). shows the trial profile and exclusions from the immunogenicity assessment.
Figure 1. Study flowchart showing number of children enrolled, random allocation into groups and exclusion from analyses. *ATP, according to protocol; **, one subject was administered the vaccine incorrectly and a second subject experienced an SAE considered by the investigator to be related to vaccination.
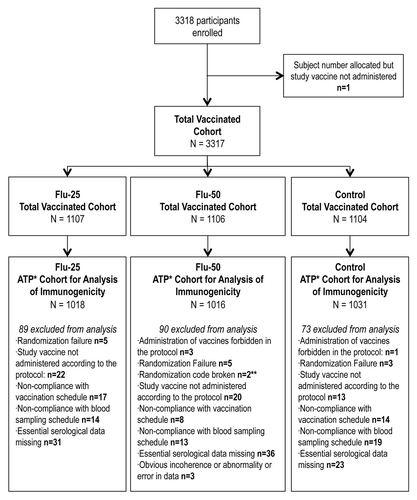
The demographic profiles of the three vaccine groups for the according to protocol (ATP) cohort for immunogenicity were comparable with respect to mean age, gender and racial distribution (). When stratified by priming status the mean ages were lower for un-primed subjects (19.2 months [Flu-25], 19.3 months [Flu-50], and 19.2 months [control] with minimum age of 6 months) than for primed subjects (25.6 months [Flu-25], 25.6 months [Flu-50], and 25.7 months [control] with minimum age of 12–14 months). The percentages of subjects above 18 months of age were 67% (Flu-25), 65% (Flu-50) and 68% (control) in the three groups.
Table 1. Baseline characteristics of study participants (according to protocol cohort for immunogenicity)
Immune response
The outcome of the analysis of the primary objective of non-inferiority is presented in which shows that in terms of hemagglutination-inhibition (HI) Geometric Mean Titer (GMT) ratios and seroconversion rate (SCR) differences, the criteria for non-inferiority of Flu-50 vs. the control were reached for the B/Florida strain, but not for the A/Brisbane (H1N1) or A/Uruguay (H3N2) strains. As indicated in the use of the B/Brisbane strain as antigen in the HI assay confirmed non-inferiority for the B strain. As the criteria for non-inferiority for the first of the sequential objectives were not met for all strains, non-inferiority of Flu-25 vs. the control could not be statistically assessed. An evaluation of interactions potentially confounding the non-inferiority analysis was performed and this showed that, for the A/Brisbane (H1N1) and A/Uruguay (H3N2) strains, there was evidence of an interaction (p-value < 0.0001) between the pre-vaccination HI titer and vaccine group on the post-vaccination titer and on SCR. Investigations of the interaction suggested that Flu-50 was less immunogenic than the control vaccine in children with low baseline titers, but tended to be as immunogenic as the control in children with higher baseline titers for A/H1N1 and A/H3N2. Although the randomization system ensured that the vaccine groups were balanced, about two-thirds of the population was un-primed by previous vaccination, and this population drove the conclusions of the non-inferiority analysis. The non-inferiority analysis was based on an ANCOVA model which assumed the treatment effect would not depend on pre-vaccination HI titer. Exploratory analyses were hence conducted in a descriptive way to better characterize the immunogenicity in the different subpopulations and are described below.
Table 2. Non-inferiority of Flu-50 vs. control vaccine for each vaccine strain (according to protocol cohort for immunogenicity)
The outcome for all immunogenicity endpoints is detailed in . For all three vaccine groups and all three strains, all US regulatory acceptance criteria (for subjects < 65 years including pediatric subjects) and all European criteria (for adults aged 18 to 60 years) were met except for the seroprotection rate (SPR) for the A/Brisbane (H1N1) strain in the Flu-25 group (68.7% with a 95% CI lower limit of 65.7%). Flu-50 vaccination induced a higher antibody response as compared with Flu-25 vaccination for all strains as the lower limit of the two-sided 95% confidence interval (CI) of the adjusted GMT ratios (Flu-50/Flu-25) was > 1 for all three strains: A/Brisbane (H1N1) 1.21 (1.04–1.40), A/Uruguay (H3N2) 1.34(1.20–1.49) and B/Florida 1.40 (1.26–1.56).
Table 3. Immune response in children aged 6 to 35 mo and 18 to 35 mo (according to protocol cohort for immunogenicity)
also details a post hoc analysis of immunogenicity for the 18 to 35 months age strata for Flu-50 and the control vaccine which indicates that in children in this age range, Flu-50 induced similar responses to the control vaccine. The 95% CI upper limits of the GMT ratio of the control group over the Flu-50 group for the 18 to 35 months age strata were 1.23, 1.33 and 0.98 respectively for the A/Brisbane (H1N1), A/Uruguay (H3N2) and B/Florida strains. The 95% CI upper limits for the difference in SCRs (control minus Flu-50) for the 18 to 35 months age strata were 10.48%, 9.02% and -0.43% respectively for the A/Brisbane (H1N1), A/Uruguay (H3N2) and B/Florida strains.
The analysis of immune response by vaccine priming status is presented in . In all vaccine groups the GMT ratios for A/Brisbane and A/Uruguay strains were higher following one dose in vaccine-primed subjects than following two doses in un-primed subjects. The reverse trend was observed for the B/Florida strain. In un-primed subjects the immune responses to all three strains was higher in the control group than in the Flu-50 group and were lowest in the Flu-25 group while in primed subjects the response was similar for all three groups.
Table 4. Immune response in primed vs. un-primed children (according to protocol cohort for immunogenicity)
Safety and reactogenicity
The reactogenicity profiles in terms of the percentages of children reporting any adverse event (solicited or unsolicited) during the study or solicited adverse events within 4 days after each vaccination (Day 0 to Day 3) were similar between the three vaccine groups (). For children who received two doses of vaccine; the second dose was not more reactogenic than the first (data not shown).
Table 5. Adverse Events (Total vaccinated cohort)
Injection site pain was the most frequently reported solicited local adverse event across vaccine groups (37.3% Flu-25; 37.4% Flu-50; 33.3% control). Grade 3 injection site pain and redness/swelling > 50mm were infrequently reported. Most injection site adverse events occurred for no longer than two days. Irritability was the most frequently reported solicited adverse event across the three vaccine groups (35.7% Flu-25; 35.6% Flu-50; 34.4% control). Most general adverse events were considered as related to vaccination by the investigators but lasted no longer than two days. Grade 3 solicited general adverse events were infrequently reported in all vaccine groups. The rates of increased temperature were relatively low in all three vaccine groups for all (≥ 37.5°C) fever (6.2% Flu-25; 6.4% Flu-50; 6.6% control), and fever ≥ 38.5°C (2.6% Flu-25; 3.4% Flu-50; 3.6% control), and Grade 3 fever (> 39°C) was infrequent in all vaccine groups (1.2% Flu-25; 2.0% Flu-50; 2.0% control).
The percentage of children who experienced at least one unsolicited adverse event was similar between the vaccine groups (51.0% Flu-25; 48.9% Flu-50; 50.9% control). Few events were considered as related to vaccination (4.3% Flu-25; 4.0% Flu-50; 3.9% control) and Grade 3 events were infrequent (5.0% Flu-25; 4.2% Flu-50; 3.6% control). There was one report of a febrile seizure occurring on day 6 following a first dose of Flu-50, this event was not considered to be related to the study vaccination by the investigator and had resolved within 2 d.
Overall, 95 children (35 children Flu-25; 29 children Flu-50; 31 children control) experienced 133 serious adverse events (SAEs). All events resolved. One subject had two SAEs which were considered by the investigator to be possibly related to vaccination. An 8- months old female concomitantly experienced apnea and cyanosis 17 day after the second vaccination with Flu-50. The subject was hospitalized and the events resolved on the same day as they occurred. Monitoring of rare events in children who received Flu-50 or Flu-25 identified four events with an incidence above 1/300 (0.3%). These were bronchiolitis (0.4% for Flu-25), gastroenteritis (0.5% for Flu-25 and 0.4% for Flu-50), pneumonia (0.4% for both Flu-25 and Flu-50), and upper respiratory tract infection (0.6% for Flu-25 group and 0.4% for Flu-50). New onset chronic illnesses were reported for 10 children in the Flu-25 group, 8 children in the Flu-50 group and 9 children in the control group. The most frequently reported new onset chronic illness was asthma (9 children overall), followed by anemia and allergic rhinitis (reported overall for 4 subjects each). No children were withdrawn from the study due to adverse events.
Discussion
The TIV study vaccine was immunogenic and well tolerated at a 0.5 ml dose (Flu-50) and 0.25 ml dose (Flu-25). The TIV vaccine at a dose of 0.5 ml induced an immune response which fulfilled all US regulatory criteria for immunogenicity in children aged 6–35 months. However, the immunogenic non-inferiority of Flu-50 (and therefore Flu-25) vs. the control vaccine was not demonstrated in children aged 6–35 months against the two vaccine influenza A strains.
The failure to show the non-inferiority of Flu-50 (FluarixTM; 0.5 ml) and the control vaccine (Fluzone®; 0.25 ml) appeared to have been attributed to differences in immunogenicity in children aged less than 18 months and vaccine un-primed children, as responses were similar for Flu-50 and the control vaccine in children aged 18–35 months and in primed children (who also tended to be in the older range). So although doubling the dose of the study TIV vaccine to 0.5 ml improved immunogenicity it was not sufficient to eliminate the difference relative to the control TIV in children aged below 18 months. These results suggest that there is a difference between the study vaccine and the control vaccine which influences the HI immune response only in younger influenza vaccine-naïve children with limited immunologic priming from prior infection or vaccination. This is consistent with a previous study which failed to show the non-inferiority of FluarixTM vs. Fluzone® in children 6 months to < 5 years, which was driven by the difference in immunogenicity between the vaccine groups in children aged <3 years.Citation17
Although TIVs produced by similar egg-based processes are generally considered to provide similar immunogenicity, it has been suggested that small differences in the production and purification processes between the vaccines may underlie the differences in immunogenicity. The manufacture of both TIVs in our study is based on disruption of influenza virus particles (splitting) and purification but variations in the manufacturing process may result in differences with respect to the degree of virus splitting and inclusion of virus or host cell components. Whereas the hemagglutinin surface glycoprotein provides the main antigenic stimulus, the presence of other viral components in the vaccine, such as internal proteins or RNA, could potentiate the priming of naïve immune cells. This is suggested by studies performed about 30 years ago with more highly purified subunit influenza vaccines which showed that addition of whole virus enhanced responses to subunit vaccines in children and un-primed young adults whereas addition of whole virus provided no additional benefit for primed subjects.Citation19-Citation22
TIVs are reported to have modest efficacy in older children, and in a Cochrane review of influenza vaccines in children including studies published up to 2004, in the limited number of studies in children < 2 years, efficacy was reported to be no better than placebo.Citation23 Poor immunogenicity of TIVs in vaccine un-primed children with limited exposure to natural viruses is thought to underlie the limited efficacy in children < 2 years, and therefore, two doses of vaccine given about a month apart is recommended in the US for first time vaccination to provide adequate antibody responses.Citation6 However, given the burden of influenza-disease in young children, various studies have evaluated whether 0.25 ml per injection is appropriate or whether this should be increased to 0.5 ml. Most recently, studies have reported that increasing the ‘pediatric’ half-dose (0.25 ml) to the ‘adult’ full-dose (0.5 ml) may improve antibody responses in children aged 6–35 mo. In one randomized controlled study of TIV (Flulaval™) in children 6–35 months, seroprotection rates in the full-dose group were 63.6–92.4%, and in the half-dose group were 53.4–84.7%, yet despite a modest increase in immune responses with the full- vs. half-dose, the GMTs were not significantly different.Citation24 Another study, which assessed full- and half-doses of TIV (Vaxigrip®, Sanofi-Pasteur), showed that although the seroprotection rate was 10% higher in the full- vs. half-dose group in children aged 6–11 months, and was superior for the A/H3N2 and B strain, superiority between the different dose groups was not shown in the overall population of children aged 6–23 months.Citation14 In a further study, Esposito et al. (2012) hypothesized that the logistical difficulty of administering two 0.25 ml doses in un-primed children may result in poor compliance and reduced immunogenicity, and as such, evaluated two 0.25 ml doses vs. one 0.5 ml dose of virosomal subunit influenza vaccine (Inflexal® V, Crucell). The results showed that in children aged 6–35 months, a single full-dose provided immunogenicity which fulfilled EU and US regulatory criteria for immunogenicity, with antibody responses similar to those observed with two half-doses.Citation25
Our study now provides further insights into the immunogenicity of TIVs according to dosage, age range, and vaccine-priming status in very young children. In the population overall who received Flu-50 or Flu-25, GMTs with the full-dose were significantly better than with the half-dose for all vaccine strains. However, consistent with previous reports, the immunogenicity of Flu-50 was lower in the overall population aged 6–35 months than in the older stratum aged 18–35 months against influenza A strains (seroprotection rates: 74.2–83.3% and 90.3–93.5%, respectively), but not against the vaccine B strain (90.6% and 90.3%, respectively). Nevertheless, apart from SPRs against A/H1N1 in the Flu-25 group overall, all US licensure criteria were fulfilled in both study vaccine groups in the overall population and in the older age stratum.
From our results it is not possible to draw conclusion about which dose is optimum as although antibody responses were clearly higher for the full-dose group, both doses provided immunogenicity considered ‘protective’ according to regulatory criteria. However, it should be noted that there is on-going debate about the validity of current HI immune response thresholds as correlates of protection, and furthermore, HI surrogates are based on studies in adults and there are no accepted correlates specific for children.Citation26-Citation29 Indeed, a study conducted by Black et al. (2011), showed that the titer for seroprotection (1:40) that is generally recognized as corresponding to a 50% reduction in the risk of influenza in adults, was insufficient to achieve this level of protection in children aged < 6 years.Citation30 In children aged 6–72 months, a cut-off of 1:110 was proposed as predicting a 50% protection rate, and 1:330 predicted a 80% protective level, which may be a more appropriate threshold given the vulnerability of very young children to serious influenza-related complications.Citation30
Regarding the optimum dose in un-primed children, we observed better antibody responses for the Flu-50 vs. the Flu-25 group (seroprotection rates: 64.9–90.2% and 57.5–87.5%, respectively). However, in both study vaccine groups in un-primed children, all licensure criteria were fulfilled apart from SPRs against A/H1N1. An observation of note was that whereas the antibody response was higher for the influenza A strains following one dose of vaccine in primed children than following two doses in un-primed children, the reverse trend was observed for the B strain in all three vaccine groups. This is most likely due to the existence of two distinct lineages of circulating B viruses only one of which is selected annually for inclusion in the trivalent vaccine. The B/Florida/4/2006-like vaccine B strain for the Northern Hemisphere in the 2008–2009 influenza season belonged to the B/Yamagata lineage whereas the vaccine B strains for the preceding two influenza seasons belonged to the unrelated B/Victoria lineage. This means that any of the children participating in the study who were vaccinated in the previous two years were not primed for the B/Yamagata lineage. Quadrivalent influenza vaccines containing B strains from both lineages have recently been approved so this should address the absence of priming for one specific influenza B strain when there is a mismatch between the vaccine and the circulating strains.Citation31-Citation34
As previously observed in children reactogenicity and safety endpoints were within the same range for the study and control vaccines.Citation17 Furthermore the reactogenicity and safety profile of the study vaccine did not appear to be affected by doubling the dose. Other studies have also shown that TIV at double the standard dose was also well tolerated in that pediatric population,Citation10,Citation24and another study with a virosomal-adjuvanted influenza vaccine in children aged < 3 years also found that the vaccine dose could be doubled without any increase in the incidence of local or systemic adverse events.Citation25 However, there has been concern about an increased frequency of febrile reactions in young children in Australia, but this was only observed with the 2010 Southern Hemisphere vaccine.Citation6 There was however another report from surveillance for US-licensed influenza vaccines during the 2010–2011 season which subsequently detected safety signals for febrile seizures in young children after TIV administration. Further assessment determined that the increased risk was in children aged 6 months to 4 years on the day of vaccination to the day after (the 0–1 day risk window). The risk was higher when children received concomitant PCV13 (i.e., when the two vaccines are administered at the same health-care visit) and peaked at approximately 16 months of age. The magnitude of the increased risk for febrile seizures in young children in the US (< 1 per 1000 children vaccinated) was substantially lower than the risk observed in Australia in 2010. Surveillance data on febrile seizures in young children after administration of the influenza vaccine for the 2011–2012 influenza season (same vaccine formulation as 2010–2011) were consistent with those from the 2010–2011 influenza season (CDC, unpublished data, 2012). In the current study population of over 3000 children there was one single report of a febrile seizure which occurred 6 days after vaccination and was not considered to be vaccine-related.
One limitation of the study was that the non-inferiority analysis was confounded by an interaction between the pre-vaccination HI titer and vaccine group and the immune response. Investigations of the interaction suggested that Flu-50 was less immunogenic than the control vaccine in children with low baseline titers, but tended to be as immunogenic as the control in children with higher baseline titers for A/H1N1 and A/H3N2. Although the randomization system ensured that the vaccine groups were balanced, about two-thirds of the population was un-primed by previous vaccination, and this population drove the conclusions of the non-inferiority analysis. A higher proportion of vaccine-primed children might have therefore influenced the outcome of the analysis. A further limitation was the choice of control vaccine. Fluzone® was selected as it is the only TIV licensed for children aged 6–35 months in the US, and it was administered at the recommended dose in this age group (0.25 ml) in the US.Citation6 However, the inclusion of a control vaccine group at the higher dose (0.5 ml) would have enabled a better evaluation of the effect of dose on immune responses. Finally, the study did not have sufficient statistical power to evaluate immune responses according to age strata or by previous vaccination history, both of which are known to influence immunogenicity.
In conclusion, the study TIV vaccine at a dose of 0.5 ml induced an immune response which satisfied all US regulatory criteria for immunogenicity in children aged 6–35 months. However the antibody response was lower than the control vaccine and this appeared to be mainly due to differences in immunogenicity in children aged below 18 months. There was no evidence that doubling the dose of the study vaccine had any impact on the reactogenicity and safety profile.
Materials and Methods
Study Design and Participants
This was a randomized, observer blind study conducted in the US, Hong Kong, Mexico, Thailand and Taiwan between October 2008 and March 2009. The protocol, its amendments and other relevant study documentation were approved by the appropriate Ethics Committees and the study was conducted in accordance with good clinical practice guidelines, the Declaration of Helsinki and all applicable regulatory requirements. Eligible participants were children aged 6–35 months at the time of first vaccination, without acute illness at the time of enrollment and who had not been vaccinated during the 2008–2009 influenza season. Administration of influenza vaccine in a previous season was not however an exclusion criteria. Informed consent was obtained from each child’s parent/guardian at study entry.
The primary objective of the study was to demonstrate the immunogenic non-inferiority of the study trivalent inactivated split virion influenza vaccine at 0.5 ml dose (Flu-50) or 0.25 ml dose (Flu-25) vs. the control trivalent inactivated split virion influenza vaccine at a dose of 0.25 ml (control). Secondary objectives were comparison of the immunogenicity of Flu-50 to Flu-25 and to the control, comparison of safety/reactogenicity in children vaccinated with Flu-50 to Flu-25 or the control and documentation of rare events, in children administered with Flu-25 or Flu-50.
Randomization into the three study groups (ratio 1:1:1) was performed by the sponsor. At the time of vaccination, the responsible on-site personnel accessed the internet randomization system that used a minimisation procedure accounting for center, age and prior influenza vaccination. Minimization factors had equal weight in the minimization algorithm. As the appearance of the vaccines was different, the study was observer-blind with vaccinations performed by specific study personnel not involved in the assessment of immunogenicity or safety/reactogenicity.
Vaccines and vaccinations
The study vaccines (Fluarix™ (thiomersal free) from GlaxoSmithKline, Dresden, Germany) contained hemagglutinin (HA) from the three influenza strains A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2) and B/Brisbane/3/2007 (15µg HA per strain for Flu-50, 7.5µg HA per strain for Flu-25). The control vaccine (Fluzone® from Sanofi-Pasteur –Swiftwater, PA, USA) contained HA from the three strains (A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2) and B/Florida/4/2006 (7.5µg HA per strain). The two different B strains in the study and control vaccines were both recommended by the WHO for the Northern Hemisphere 2008–2009 influenza season as B/Florida/4/2006-like strains.
Both vaccines were administered by intramuscular injection into the right deltoid muscle or anterolateral thigh at study entry (Day 0) and a second dose (Day 28) for unprimed children only.
Serological assessments
Blood samples were collected on Day 0 (pre-vaccination) and either Day 28 (post-vaccination 1; for primed children) or Day 56 (post-vaccination 2; for unprimed children). Sera were analyzed in a validated micro-titer hemagglutination-inhibition (HI) assay as described previously with the virus strains present in the two vaccines used as antigens.Citation35 The serum titer was expressed as the reciprocal of the highest dilution that showed complete inhibition of hemagglutination.
Assessment of safety and reactogenicity
Parents/guardians were provided with diary cards to record the occurrence and intensity of injection site solicited adverse events (pain, redness and swelling) and general adverse events (drowsiness, irritability, loss of appetite and fever) experienced during the first 4 days after vaccination. In addition to solicited events, data were also collected on the occurrence and intensity of any unsolicited adverse events that occurred within 28 days after each vaccination. The diameters of any injection site redness and swelling, and daily body temperature were recorded. The intensities of other adverse events were recorded according to a standard three grade scale: An assessment of causality was made by the investigator for solicited general and unsolicited adverse events. Data on SAEs and new onset of chronic illnesses were collected during the entire study period.
Statistical analysis
The immunological endpoints (with 95% CI) at each time point were the standard HI test endpoints of GMT, SCR, SPR, GMFR defined according to regulatory criteria used for evaluation of influenza vaccines by the US Center for Biologics Evaluation and Control and the European Medicines Agency.Citation36
The study criteria to demonstrate non-inferiority of Flu-50 or Flu-25 vs. the control vaccine (sequential primary objectives) were to show that at Day 28 (for primed subjects) or Day 56 (for un-primed subjects) the upper limit of the two-sided 95% CI of the GMT ratio (control / Flu-50 or Flu-25) did not exceed 1.50, as well as to show that the upper limit of the two-sided 95% CI for the difference (control minus Flu-50 or Flu-25) in SCRs did not exceed 10% for each vaccine strain. The reference values to calculate the sample size to reach these two criteria of non-inferiority were taken from the 6–35 months old age stratum in a previous study comparing Flu-25 and the control vaccine.Citation17 It was calculated that 779 evaluable subjects per group would give 90% global power to show non-inferiority in terms of GMT ratios and SCR for all three vaccine strains.
The adjusted GMT ratio of HI antibodies at post-vaccination for each vaccine, the GMT ratio and the two-sided 95% CI on each GMT ratio was computed after fitting an ANCOVA model on the logarithm10 transformation of the reciprocals of the titers, including the vaccine group as fixed effect and the pre-vaccination titer as covariate. The SCR of each vaccine, the difference in SCRs and the two-sided 95% CI of the SCR differences was computed after fitting a logistic regression on the seroconversion response, including the vaccine group as fixed effect and the pre-vaccination titer as covariate. Both ANCOVA and logistic regression models assume that the treatment effect does not depend on the pre-vaccination serological level. This assumption was checked and additional analyses were to be performed in case of evidence of interaction. Hence, exploratory analyses of the immune response by priming status (planned) and by age (post hoc) were performed.
The safety endpoints (percentage of subjects and 95% CI) were solicited injection site and general and unsolicited adverse events. All SAEs, rare events (defined as SAEs with an occurrence rate above 1/300) and cases of new onset of chronic illnesses occurring during the entire 6 months study period were described.
| Abbreviations: | ||
| ATP | = | according to protocol |
| A/Brisbane (H1N1) | = | A/Brisbane/59/2007 (H1N1) |
| A/Uruguay (H3N2) | = | A/Uruguay/716/2007 (H3N2) |
| B/Brisbane | = | B/Brisbane/3/2007 |
| B/Florida | = | B/Florida/4/2006 |
| CI | = | confidence interval |
| GMT | = | geometric mean titer |
| HA | = | hemagglutinin |
| HI | = | hemagglutination inhibition |
| SAEs | = | serious adverse events |
| SCR | = | seroconversion rate |
| GMFR | = | geometric mean fold rise (also known as Seroconversion Factor) |
| SPR | = | seroprotection rate |
| TIV | = | trivalent inactivated influenza vaccine |
Acknowledgments
• All authors participated in the implementation of the study including substantial contributions to conception and design and/or the gathering of the data, or analysis and interpretation of the data. All authors were involved in the drafting of the article or revising it critically for important intellectual content, and final approval of the manuscript. All authors had full access to the data and had final responsibility to submit for publication.
• We thank all the participating study volunteers and their parents, study doctors and trial nurses, and laboratory technicians at the study site. In particular we thank J Hedrick, B Essink, M Leonardi, M Schear, L Chu, L Harris-Ford, C Ashley, M Cox, W Daly, E Franklin, E Goldblatt, D Hurley, W Johnston, M Lauret, K Zollo, T Chotpitayasunondh, NC Chiu, C Ying-Hsiang and N Dominguez.
• We are grateful to all teams of GlaxoSmithKline Vaccines for their contribution to this study: L Ray (4 Clinics on behalf of GlaxoSmithKline Vaccines) for preparation of the study report; I Naeije for global study management; A. Bastidas, Clinical Safety Representative.
• We thank M. Hynes and A. Moon (Freelance writers on behalf of GlaxoSmithKline Vaccines) for providing medical writing services, and J. Dedessus le Moutier and B. Dumont (Business & Decision Life Sciences on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Financial disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Conflict of interest
V. Jain, M. El Idrissi, Y. Feng, B. Innis, M. Peeters, J.M. Devaster are employee of GlaxoSmithKline group of companies. V. Jain, B. Innis, M. Peeters, J.M. Devaster report ownership of stock options. L.M. Huang reports payment for consulting fee or honorarium for Pneumococcus advisory board, lectures, other advisory board, consultancy for vaccine use in Taiwan, pneumonia etiology study from GlaxoSmithKline group of companies and grants to his institution for clinical trials from GlaxoSmithKline group of companies. Y.L. Lau reports grants received for travel support and for other clinical trials received from GlaxoSmithKline group of companies. E.A.S. Nelson reports grants to his institution for performing this study from GlaxoSmithKline group of companies, for participating in diarrheal and respiratory disease surveillance studies from Merck and Pfizer (Wyeth), and for vaccine clinical trials from Baxter, GlaxoSmithKline group of companies, MedImmune and Pfizer (Wyeth). E.A.S. Nelson reports having received payments for travel, accommodation and honorarium for a lecture on respiratory disease surveillance and pneumococcal vaccine. E.A.S. Nelson is a member of Hong Kong's Scientific Committee on Vaccine Preventable Diseases, a member of a data Safety Monitoring Board for a Japanese Encephalitis vaccine study and a member of the ROTA Council. E.A.S. Nelson has been a technical advisor to the SIVAC (Supporting Independent Vaccine Advisory Committees) Initiative (2008–2011) and a member of WHO Quantitative Immunization and Vaccines Related Research Expert Advisory Group (2007–2012). M. Blatter reports payment received for board membership, lectures including service on speakers bureau from GlaxoSmithKline group of companies, and money paid to his institution for grants/grants pending, support for travel to meetings from GlaxoSmithKline group of companies. R. Jeanfreau reports payment for travel/accommodations/meeting expenses from GlaxoSmithKline group of companies. N. Pavia-Ruz reports payment for board membership received from Tibotec and payment to his institution for grants from GlaxoSmithKline group of companies and Bristol Myers Squibb. M.A.R. Weber, P. Silas, P. Qaqundah, P. Lei and A. Kerdpanich report no conflict of interest.
Trademark
Fluarix™ is a trademark of the GlaxoSmithKline group of companies. Fluzone® is a registered trademark of Sanofi Pasteur. Inflexal® V is a registered trademark of Crucell. Vaxigrip® is a registered trademark of Sanofi Pasteur
References
- Viboud C, Boëlle PY, Cauchemez S, Lavenu A, Valleron AJ, Flahault A, et al. Risk factors of influenza transmission in households. Br J Gen Pract 2004; 54:684 - 9; PMID: 15353055
- Heikkinen T, Silvennoinen H, Peltola V, Ziegler T, Vainionpaa R, Vuorinen T, et al. Burden of influenza in children in the community. J Infect Dis 2004; 190:1369 - 73; http://dx.doi.org/10.1086/424527; PMID: 15378427
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232 - 9; http://dx.doi.org/10.1056/NEJM200001273420402; PMID: 10648764
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr., Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342:225 - 31; http://dx.doi.org/10.1056/NEJM200001273420401; PMID: 10648763
- Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis 2002; 185:147 - 52; http://dx.doi.org/10.1086/338363; PMID: 11807687
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep 2012; 61:613 - 8; PMID: 22895385
- Mereckiene J, Cotter S, Nicoll A, Levy-Bruhl D, Ferro A, Tridente G, et al. National seasonal influenza vaccination survey in Europe, 2008. Euro Surveill 2008; 13:19017; PMID: 18947524
- Public Health Agency of Canada National Advisory Committee on Immunization. Statement on seasonal influenza vaccine for 2011-2012. 2011; 37 http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/11vol37/acs-dcc-5/ (Accessed 30 April 2013)
- Usonis V, Anca I, André F, Chlibek R, Ivaskeviciene I, Mangarov A, et al, Central European Vaccination Advisory Group. Central European Vaccination Advisory Group (CEVAG) guidance statement on recommendations for influenza vaccination in children. BMC Infect Dis 2010; 10:168; http://dx.doi.org/10.1186/1471-2334-10-168; PMID: 20546586
- Steinhoff MC. Influenza in young children: burden, immunisation, and policy. Lancet Infect Dis 2011; 11:2 - 3; http://dx.doi.org/10.1016/S1473-3099(10)70263-2; PMID: 21106444
- UK Department of Health. Influenza. Green Book 2012; 19-v4_71 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/147958/Green-Book-Chapter-19-v4_71.pdf (Accessed 30 April 2013)
- Wright PF, Dolin R, La Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines--II. J Infect Dis 1976; 134:633 - 8; http://dx.doi.org/10.1093/infdis/134.6.633; PMID: 794423
- Langley JM, Scheifele DW, Quach C, Vanderkooi OG, Ward B, McNeil S, et al. Safety and immunogenicity of 2010-2011 H1N12009-containing trivalent inactivated influenza vaccine in children 12-59 months of age previously given AS03-adjuvanted H1N12009 pandemic vaccine: a PHAC/CIHR Influenza Research Network (PCIRN) study. Vaccine 2012; 30:3389 - 94; http://dx.doi.org/10.1016/j.vaccine.2012.03.046; PMID: 22469860
- Skowronski DM, Hottes TS, Chong M, De Serres G, Scheifele DW, Ward BJ, et al. Randomized controlled trial of dose response to influenza vaccine in children aged 6 to 23 months. Pediatrics 2011; 128:e276 - 89; http://dx.doi.org/10.1542/peds.2010-2777; PMID: 21768314
- FDA News Release. FDA approves seasonal influenza vaccine Fluarix for pediatric use; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187156.htm (Accessed 30 April 2013)
- Treanor JJ, Campbell JD, Brady RC, Keitel WA, Drame M, Jain VK, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin 2005; 1:239 - 44; http://dx.doi.org/10.4161/hv.1.6.2376; PMID: 17012883
- Baxter R, Jeanfreau R, Block SL, Blatter M, Pichichero M, Jain VK, et al. A Phase III evaluation of immunogenicity and safety of two trivalent inactivated seasonal influenza vaccines in US children. Pediatr Infect Dis J 2010; 29:924 - 30; http://dx.doi.org/10.1097/INF.0b013e3181e075be; PMID: 20431425
- Campbell JD, Chambers CV, Brady RC, Caldwell MC, Bennett NL, Fourneau MA, et al. Immunologic non-inferiority of a newly licensed inactivated trivalent influenza vaccine versus an established vaccine: a randomized study in US adults. Hum Vaccin 2011; 7:81 - 8; http://dx.doi.org/10.4161/hv.7.1.13553; PMID: 21285532
- Frank AL, Webster RG, Glezen WP, Cate TR. Immunogenicity of influenza A/USSR (H1N1) subunit vaccine in unprimed young adults. J Med Virol 1981; 7:135 - 42; http://dx.doi.org/10.1002/jmv.1890070207; PMID: 7264612
- Glezen WP, Kasel JA, Webster RG, Taber LH. Alternative approaches to immunization of children with inactivated influenza virus vaccines. J Infect Dis 1977; 136:Suppl S677 - 82; http://dx.doi.org/10.1093/infdis/136.Supplement_3.S677; PMID: 606791
- Webster RG, Glezen WP, Hannoun C, Laver WG. Potentiation of the immune response to influenza virus subunit vaccines. J Immunol 1977; 119:2073 - 7; PMID: 915292
- Webster RG, Kasel JA, Couch RB, Laver WG. Influenza virus subunit vaccines. II. Immunogenicity and original antigenic sin in humans. J Infect Dis 1976; 134:48 - 58; http://dx.doi.org/10.1093/infdis/134.1.48; PMID: 59787
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; 16:CD004879
- Langley JM, Vanderkooi OG, Garfield HA, Hebert J, Chandrasekaran V, Jain VK, et al. Immunogenicity and Safety of 2 Dose Levels of a Thimerosal-Free Trivalent Seasonal Influenza Vaccine in Children Aged 6-35 Months: A Randomized, Controlled Trial. J Pediatric Infect Dis Soc 2012; 1:55 - 63; http://dx.doi.org/10.1093/jpids/pis012; PMID: 23687572
- Esposito S, Marchisio P, Montinaro V, Bianchini S, Weverling GJ, Pariani E, et al. The immunogenicity and safety of a single 0.5 mL dose of virosomal subunit influenza vaccine administered to unprimed children aged ≥6 to <36 months: data from a randomized, Phase III study. Vaccine 2012; 30:7005 - 12; http://dx.doi.org/10.1016/j.vaccine.2012.09.069; PMID: 23059357
- Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011; 204:1879 - 85; http://dx.doi.org/10.1093/infdis/jir661; PMID: 21998477
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767 - 77; http://dx.doi.org/10.1017/S0022172400022610; PMID: 4509641
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18; http://dx.doi.org/10.1186/1471-2288-10-18; PMID: 20210985
- Rimmelzwaan GF, McElhaney JE. Correlates of protection: novel generations of influenza vaccines. Vaccine 2008; 26:Suppl 4 D41 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.07.043; PMID: 19230158
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081 - 5; http://dx.doi.org/10.1097/INF.0b013e3182367662; PMID: 21983214
- Block SL, Falloon J, Hirschfield JA, Krilov LR, Dubovsky F, Yi T, et al. Immunogenicity and safety of a quadrivalent live attenuated influenza vaccine in children. Pediatr Infect Dis J 2012; 31:745 - 51; http://dx.doi.org/10.1097/INF.0b013e31825687b0; PMID: 22466322
- U.S. Food and Drug Administration. FDA approves first quadrivalent vaccine to prevent seasonal influenza. February 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm294057.htm (Accessed 30 April 2013)
- Domachowske JB, Pankow-Culot H, Bautista M, Feng Y, Claeys C, Peeters M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis 2013; 207:1878 - 87; http://dx.doi.org/10.1093/infdis/jit091; PMID: 23470848
- U.S. Department of Health and Human Services FaDA. Approval Letter- Fluarix Quadrivalent. December 14, 2012http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm332484.htm (Accessed 30 April 2013)
- Hehme NW, Künzel W, Petschke F, Türk G, Raderecht C, van Hoecke C, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Investig 2002; 22:751 - 69; http://dx.doi.org/10.2165/00044011-200222110-00004
- US Food and Drug Administration. Guidance for industry. Clinical data needed to support the licensure of seasonal inactivated influenza vaccines 30th April 2009. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074794.htm (Accessed 30 April 2013)