Abstract
Domain III (DIII) of the dengue virus (DENV) envelope (E) protein induces strong neutralizing type-specific antibodies. In addition, a region near the fusion loop in domain II (DII) induces the production of cross-reactive antibodies with neutralizing potential. Thus, this study aimed to generate DENV-2 recombinant fusion proteins (i.e., rEII*EIII and rEII*EIII/NS1*) either alone or fused to 3 copies of P28, the minimum CR2-binding domain of the complement protein C3d. The 4 recombinant proteins were generated in a Drosophila melanogaster Schneider 2 (S2) cell system. The expression and secretion of the recombinant proteins were confirmed in vitro using immunofluorescence (IF) and western blot (WB) analyses. Human dengue immune serum samples recognized recombinant proteins. The immunogenicity of the 4 proteins in BALB/c mice was analyzed using ELISA, and the results revealed that the induced specific antibody response was higher in the groups of mice immunized with the P28 fusion proteins. Interestingly, although the 4 recombinant proteins were able to elicit high levels of neutralizing antibodies in BALB/c mice; no adjuvant effect was observed in terms of neutralizing antibodies in the groups immunized with proteins containing P28. Thus, ELISA and PRNT50 assays may evaluate different epitopes and responses, where ELISA showed a wider response that did not always correlate with neutralization. Furthermore, the elicited antibodies were able to recognize the immobilized E glycoprotein of DENV. All mice vaccinated with the DENV-2 recombinant proteins showed induction of higher levels of IgG1 antibodies than of IgG2a antibodies.
Introduction
Dengue disease remains an important public health problem in all tropical and subtropical regions of Asia and the Americas.Citation1,Citation2 Considerable efforts have been directed toward the development of a safe vaccine. Although several strategies have been tested on different expression systems,Citation3,Citation4 no effective vaccine is currently available.
The dengue envelope (E) protein is the most exposed structural glycoprotein in flaviviruses and is the principal target of neutralizing antibodies. Furthermore, it is considered the primary candidate target of subunit vaccines.Citation5-Citation7 The E protein folds into three domains. Monoclonal antibodies directed against domain II can neutralize the virus by inducing structural changes that affect the virus-cell membrane fusion. Moreover, cumulative evidence has shown that a natural infection with DENV or any other flavivirus induces cross-reactive antibodies directed toward the fusion peptide in domain II of the E protein. These antibodies have at minimum a weak neutralizing activity.Citation8-Citation10 Domain III contains multiple serotype-specific, conformation-dependent neutralizing epitopes and the antibody against this domain inhibits the virus binding to the host-cell.Citation11-Citation14
Conversely the non-structural protein 1 (NS1) is both expressed on the surface of the infected cells and secreted. NS1 elicits an immune protective response in natural infections and in experimental models,Citation15-Citation17 without the adverse effects that involve antibody-dependent enhancement.
The protein expression system in Drosophila melanogaster Schneider 2 (S2) cells has been successfully used to express flavivirus proteins.Citation18 Domain III of DENV-2 expressed in this system was able to elicit a protective response in mice and monkeys.Citation19-Citation21 Due to the low immunogenicity that the recombinant proteins in general possess, different strategies have been implemented to elicit a robust immune response against these antigens.Citation22-Citation24
Fearon et al.Citation25 provided the first evidence that the mammalian complement component C3d has an adjuvant effect and the number of copies of C3d fused with the antigens determines the magnitude of the immune response. C3d acts as an adjuvant in virtue of its interaction with the complement receptor (CR2 or CD21), which is primarily expressed in B and follicular dendritic cells (FDCs). C3d stimulates the antigen presentation, antibody secretions and cell memory against the co-ligated antigen.Citation26 Ross et al. demonstrated that the fusion of multimers of P28, a small peptide containing the minimum CR2-binding domain, was sufficient to potentiate the specific immune response.Citation27 Other vaccines containing the P28 have also been tested with other antigens, including those from West Nile virus (WNV).Citation28-Citation30
We developed four DENV-2 recombinant fusion proteins (i.e., rEII*EIII and rEII*EIII/NS1*) either alone or fused to three copies of P28 to increase the immune response. In the construction of these fusion proteins, we included only those fragments of the E protein located in domains II and III, which contain the regions that contribute to the induction of neutralizing antibodies. EII*, spanning the aminoacids (aa) 35–121 located in domain II, contains the regions that become exposed only under acid conditions into the endosome (fusogenic peptide).Citation31 The EIII region is constituted basically for the whole domain III and that contain the binding sequence to the cellular receptor.Citation32 NS1 was also included in these constructs. However, only the fragment responsible for protection (aa 57–130) was included, while its C-terminal region, involved in human cross-reactivity, was omitted.Citation33 These four recombinant proteins were each generated in a Drosophila S2 system. In this study we show that all of these fusion proteins induced a robust response to wild virus in BALB/c mouse model with a predominance of the IgG1 isotype. Furthermore, an effective neutralizing antibody response was observed similar to that elicited in the group immunized with DENV-2.
Results
Construction and expression of recombinant plasmids
The entire sequence of EII*EIII/NS1* amplified from the plasmid pcDNA-EII*EIII/NS1*, includes: Domain II (aa 35–121), Domain III (aa 268–397) and NS1* (aa 57–130) ().Citation34 shows each plasmid with its specific inserted sequence. The digestion of pD2EII*EIII generated the full cassette of 651 bp (), and the digestion of pD2EII*EIII (P28)3 generated a 1089-bp fragment (). The restriction digest (KpnI and XhoI) of the pEII*EIII/NS1* cassette, produced a 873-bp fragment (), while the digestion of plasmid pD2EII*EIII/NS1*(P28)3 with KpnI and ApaI generated a 1311-bp fragment (). The four constructs were sequenced, and all the elements of the cassettes were found in the same open reading frame.
Figure 1. Schematic representation of the fusion proteins. (A) Diagram of DENV-2 E and NS1 full-length sequences and the sequences used to construct the recombinant plasmid pD2EII*EIII/NS1*. The black arrow represents the EII* region, the gray arrow represents the EIII region, and the segmented arrow represents the NS1* region at the N-terminus of the NS1 protein. The cassettes were inserted into the pMTBiP/V5-HisA plasmid, and all the resulting constructs were digested with KpnI and ApaI. (B) rEII*EIII (C) rEII*EIII(P28)3 (D) rEII*EII/NS1* and (E) rEII*EIII/NS1*(P28)3. All constructs contained a linker composed of two repeats of four glycines and two serines [(G4S2)2]. Lane 1 was loaded with a molecular weight marker. The four sequences were cloned under the control of a transcriptional metallo-protease promoter placed in-frame with the V5 signal sequence, enabling antigen secretion from the plasmid pMTBiP/V5-HisA.
![Figure 1. Schematic representation of the fusion proteins. (A) Diagram of DENV-2 E and NS1 full-length sequences and the sequences used to construct the recombinant plasmid pD2EII*EIII/NS1*. The black arrow represents the EII* region, the gray arrow represents the EIII region, and the segmented arrow represents the NS1* region at the N-terminus of the NS1 protein. The cassettes were inserted into the pMTBiP/V5-HisA plasmid, and all the resulting constructs were digested with KpnI and ApaI. (B) rEII*EIII (C) rEII*EIII(P28)3 (D) rEII*EII/NS1* and (E) rEII*EIII/NS1*(P28)3. All constructs contained a linker composed of two repeats of four glycines and two serines [(G4S2)2]. Lane 1 was loaded with a molecular weight marker. The four sequences were cloned under the control of a transcriptional metallo-protease promoter placed in-frame with the V5 signal sequence, enabling antigen secretion from the plasmid pMTBiP/V5-HisA.](/cms/asset/8bbdcad3-7adc-4270-a825-016e81370e96/khvi_a_10925673_f0001.gif)
Then, the constructs were transiently transfected into S2 cells, and their expression was analyzed by WB using an anti-V5 epitope. The results of the WB analysis were consistent with the predicted sizes of the recombinant proteins: rEII*EIII/NS1*(P28)3, 58 kD; and rEII*EIII/NS1*, 43 kD (); rEII*EIII(P28)3, 55 kD; and rEII*EIII, 40 kD (). The cells transfected with the parental plasmid showed no reactivity with the same antibody.
Figure 2. Analysis of the in vitro expression of DENV-2 recombinant proteins. Secreted recombinant DENV-2 E products were assessed using SDS-PAGE and western blot analysis. The sizes of the molecular weight markers are indicated on the left in kDa. The estimated sizes of the recombinant proteins were as follows: (A) rEII*EIII/NS1*(P28)3, 58 kDa; and rEII*EIII/NS1*, 43 kDa, (B) rEII*EIII(P28)3, 55 kDa; and rEII*EIII, 40 kDa. The positions of the recombinant proteins are indicated with arrows, and the name of each protein is shown on the membrane. (C) Analysis of S2 cells that were transiently transfected with each plasmid (separately) (i.e., pD2EII*EIII/NS1*(P28)3, pD2EII*EIII/NS1*, pD2EII*EIII(P28)3, pD2EII*EIII. The transfected cells were treated with brefeldin A for 4 h prior to fixation and permeabilization. Indirect immunofluorescence was performed using a mix of human dengue immune serum and human normal serum samples (as described in Materials and Methods).
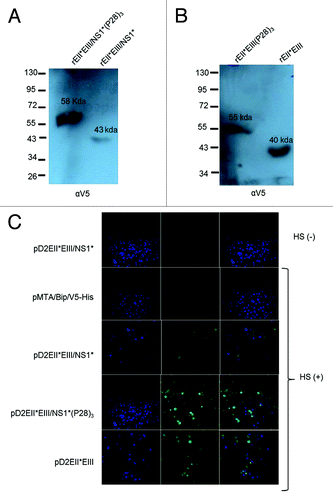
To establish whether the recombinant dengue proteins are recognized by human dengue immune serum, the constructs were transiently transfected into S2 cells, and their intracellular expression was analyzed using IF. A pool of human dengue immune serum samples and serum samples from healthy donors were used in the cell cultures previously treated with brefeldin A. The results () showed positive recognition by dengue immune serum samples, as demonstrated by immunofluorescence in S2 cells expressing the proteins rEII*EIII/NS1*, rEII*EIII/NS1*(P28)3, and rEII*EIII (green). No signal was observed in the un-transfected or parental-transfected S2 cells. In contrast, a mix of normal serum samples generated no positive signal. In any DENV constructs transfected cells.
The stable transfectant cells were grown in 2-L roller flasks. Then the supernatants were purified as described in the Materials and Method section. The day of maximum level of expression, the molecular weight and the isoelectric point of the four proteins are summarized in .
Table 1. Purification of the DENV-2 recombinant proteins expressed in S2 cells
The recombinant rEII*EIII/NS1* and rEII*EIII proteins elicited a specific antibody response after immunization, and P28 enhanced this response
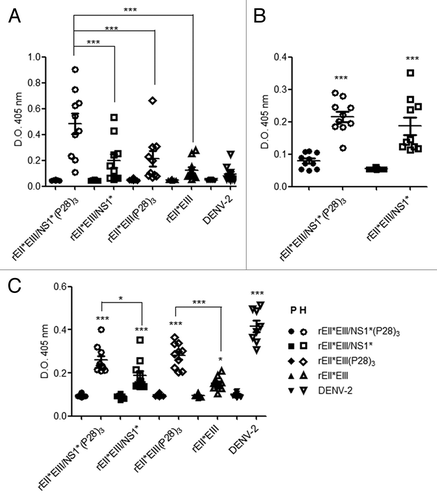
To analyze the specific antibody response to the E protein, the ELISA plates were coated with recombinant rEII*EIII/NS1* protein. Seroconversion was observed in 8/10 mice from the group immunized with rEII*EIII/NS1*(P28)3 and 6/10 from the group immunized with rEII*EIII/NS1*, in contrast to the pre-immune serum samples at the same dilution where no seroconversion was detected. Bonferroni analysis demonstrated significant differences between the immune and pre-immune groups. Moreover, the two proteins containing (P28)3, with or without the NS1* fragment, elicited responses greater than those not containing (P28)3 (p < 0.001). Interestingly, both groups (rEII*EIII/NS1* and rEII*EIII) elicited specific antibody responses similar to those observed in the group inoculated with the virus (). Moreover, the antibodies from DENV-immunized animals induced a very low specific immune response against the recombinant proteins. This finding suggests that the conformation of the viral protein and the recombinant protein may be slightly different.
Figure 3. Specific antibody response to the recombinant protein EIIEIII/NS1. (A) Groups of ten BALB/c mice were inoculated intradermally with 3 injections of rEII*EIII/NS1* (○) rEII*EIII/NS1*(P28)3 (□), rEII*EIII (Δ), rEII*EIII (P28)3 (⋄) and DENV-2 (∇) at 2-week intervals, and serum samples from the pre- and post-immunization were evaluated in ELISA plates coated with 6 μg/mL of rEII*EIII/NS1*. (B) Specific antibody response to NS1 was also evaluated using ELISA coated plates with 6 μg/mL of GST-NS1 recombinant protein. (C) In the trapping ELISA assay DEN viral antigen was immobilized with ConA and the total IgG response was measured. All sera collected from each group were diluted 1:200. Filled symbols represent the pre-immune serum samples, and open symbols represent the immune serum samples. The results are expressed as the OD at 405 nm. Each dot represents an individual mouse. The error bars denote the standard errors with a measurable titer. A 2-way un-matched ANOVA with a Bonferroni post-test was used to determine the significance of the differences between the groups; the significance is denoted by asterisks as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Furthermore, the expression of specific antibodies against the NS1 protein was evaluated in mice immunized with rEII*EIII/NS1* and rEII*EIII/NS1*(P28)3. Significant differences were observed between the pre-immune and immunized animals (p < 0.001). However no significant differences were observed between the groups inoculated with the rEII*EIII/NS1*(P28)3 and rEII*EIII/NS1* ().
A/NS1*, rEII*EIII/NS1*(P28)3.rEII*EIII, rEII*EIII(P28)3. In these experiments, the ELISA plates were coated with rEII*EIII/NS1*, prMEII* or an unrelated protein, which only share V5-HIS tag (CD38/V5-HIS). According to the results, the response against the full protein rEII*EIIINS1 was always very strong. In contrast, the response against prMEII* (which share EII* sequence with the full length EII*EIII/NS1*) was lower. The lowest response was against the unrelated protein, which contained V5 HYS. Some mice, produce high titer of antibodies against prMEII*, but never reached the magnitude of response obtained when the rEII*EIII/NS1* protein was used (). Thus, the antibodies raised by the recombinant proteins were mostly against the dengue domains, suggesting that V5 insertion neither contribute nor affect the natural antigenicity of the recombinant proteins.
Table 2. Antibody response against the different components of recombinant protein EIIIEII*/NS1*
P28 enhances the antibody response against the DENV-immobilized E protein
Trapping antibodies bound to the viral protein immobilized with ConA is an acceptable way to demonstrate that the antibody response elicited using a homologous recombinant protein is also able to react with the protein from wild-type virus. The mice immunized with rEII*EIII/NS1* and rEII*EIII proteins showed a clear antibody response against wild-type viral antigens immobilized with ConA. Five animals in the rEII*EIII/NS1* group showed a more pronounced response. Moreover, when mice were immunized with proteins fused to (P28)3, a stronger antibody response against the immobilized wild-type E protein was observed, with values similar to those observed in mice that were inoculated with DENV-2. These data are extremely important and demonstrate that in this assay the antibodies induced by a subunit fusion protein were able to interact with the DENV viral protein ().
Virus-neutralizing antibodies induced by the four different recombinant subunits proteins in mice
Although an increase in the titers of antibodies specific to NS1 and E protein was observed under the immunization regimen, the neutralizing antibodies played an important role in dengue infection in terms of protection. The neutralizing activity of the different groups was evaluated. Serum samples were collected 4 weeks after the last immunization and titrated for neutralizing antibodies (PRNT50). The results showed that mice vaccinated with any of the four recombinant proteins had high neutralizing titers after 4 immunizations. Although the average PRNT50 titer (1:885) of the group immunized with the virus was higher than those calculated for the mice immunized with rEII*EIII/NS1*(P28)3 (1:508.9), rEII*EIII/NS1* (1:562), rEII*EIII(P28)3 (1:449) and rEII*EIII (1:468), when the individual data were analyzed, some animals displayed neutralizing responses similar to those of mice vaccinated with DENV (). Nevertheless, P28 did not confer a significant increase in the levels of neutralizing antibodies. In contrast, the titers of pre-immune serum samples and the sera collected from mice inoculated with an unrelated protein were only < 10 (data not shown).
Table 3. Comparative analysis of neutralizing antibodies from the sera of mice immunized with DENV-2 subunit recombinant proteins
Recombinant proteins elicited mainly IgG1 isotype immune responses
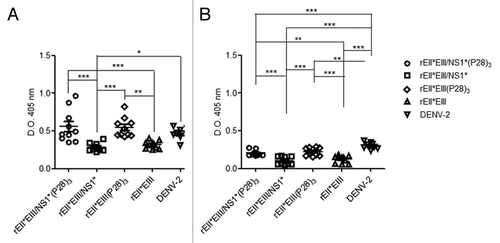
The serum samples were collected and analyzed at a dilution of 1:200 to determine the concentration of IgG1 () and IgG2a () antibodies specific to rEII*EIII/NS1*(P28).Citation3The results showed that the level of antibodies against the recombinant protein in all the groups increased considerably. The predominant isotype in all the groups was IgG1 (). Furthermore, the levels of IgG2a were significantly lower in all the groups (). Unexpectedly, mice immunized with DENV-2 showed higher levels of IgG1 than IgG2a in contrast with the previously reported when virus is used for the immunization. Once again, the recombinant proteins fused to P28 elicited a higher response higher (p < 0.05). These results indicated that the fusion of (P28)3 to both sequences induced a stronger immune response ().
Figure 4. IgG1 and IgG2a antibody subclass levels in mice immunized with DENV E protein bound to Con A. Groups of ten BALB/c mice immunized (four times) with rEII*EIII/NS1* (○) (rEII*EIII/NS1*(P28)3 (□), rEII*EIII (Δ), rEII*EIII (P28)3 (⋄) and DENV-2 (∇). The DENV E protein from serotype 2 derived from DENV-infected C6/36 cells was immobilized with ConA. The above serum samples from the immunized groups were evaluated, and the level of the response was determined using HRP-conjugated anti-mouse IgG1 (A) and IgG2a (B) antibodies. Pre-immune serum samples were used as negative controls (data not show). Each dot represents an individual mouse. The error bars denote the standard error of a measurable titer. Two-way un-matched ANOVA with a Bonferroni post-test was used to determine the significance of the differences between groups, denoted by asterisks as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Although significant progress has been made toward the development of an effective and safe vaccine against dengue virus, no approved vaccine for DEN is presently available.Citation35 Nevertheless, the use of recombinant proteins presents a feasible alternative that may avoid the issues associated with the use of an inactivated vaccine. All attempts to design a recombinant DENV vaccine have focused on the major structural envelope (E) protein.Citation36,Citation37 Indeed, utilization of E protein truncated at amino acid 395 expressed in the Drosophila S2 system has provided important advances toward the generation of a safe and effective tetravalent dengue subunit vaccine.Citation19,Citation21
To reduce the risk of cross-reactive antibodies, some studies have focused on domain III of the E protein.Citation19,Citation20 However, during the course of natural infection, antibodies against domain II are elicited, and these antibodies induce the production of cross reactive antibodies with a low neutralizing activity.Citation8-Citation10,Citation38 Therefore, vaccines composed of the relevant sequence of domain II and the whole domain III may trigger a more effective immune response.
The selection of molecules that may increase the specific immune response for the antigens is a real challenge in the design of vaccines. However, we were able to increase the immune response against DENV in a mouse model by targeting the domain III sequence to DEC-205 receptor of dendritic cells.Citation39 In parallel, we built 4 constructs based on the EIII, EII*, and NS1 antigens (Reported by Mellado-Sanchez et al.Citation33). Two of these constructs were fused with P28 and were expressed in a Drosophila S2 cell system. This experiment revealed that the response (evaluated by ELISA) of mice immunized with recombinant proteins was strikingly higher than of that of mice immunized with DENV. These data suggest that although the recombinant proteins are good antigens in the ELISA plate, they may assume structural conformations different from the wild-type E protein with higher responses that cannot be detected. Thus, using immobilized viral antigens provided insights into the antibody response against the wild antigens. The results of trapping ELISA experiments revealed that antibodies raised against the recombinant proteins were clearly able to recognize the wild-type dengue envelope protein. Thus, our study demonstrated that the antibody response to dengue protein subunits can be increased either by fusion to P28 or by the use of the eukaryotic expression systems that permit the post-translational modification of the recombinant proteins. These events likely facilitate the generation of proteins that are similar to the wild-type proteins.Citation18,Citation40
Cumulative studies have shown that both full-length C3d and the minimal P28 domain can be used as molecular adjuvants when fused to an antigen. Although our results did not demonstrate a significant difference in the neutralizing antibody response when recombinant proteins were fused to P28, the total level of antibodies against the native E protein increased (p < 0.01). These data indicate that proteins generate differential responses when fused with P28. This evidence suggests that C3d does not always function as expected, i.e., the conjugation of certain antigens to C3d does not guarantee an adjuvant effect.Citation41
Experimental data suggest that the interaction of P28 with CR2 is complex and involves more than one mechanism, including electrostatic interactions. Owing to the small size of P28, the mechanisms by which it interacts with CR2 may be limited.Citation42 Moreover, the fusion of C3d to other antigens may inhibit the humoral immune response.Citation43,Citation44
Previously, we showed in a mice model that immunization with a DNA vaccine pcDNA-EII*EIII/NS1* from DENV2 stimulated low but long-lasting antigen-specific antibody responses.Citation34 As shown here, this response is improved by expression in the S2 system and fusion with recombinant proteins to P28. Although it is well known that neutralizing antibodies are undoubtedly the main effectors arm of protection during infection by flaviviruses, it is clear that the neutralizing activity of antibodies is related to their avidity in the process of affinity maturation and production of high levels of antibody production. It is generally accepted that 50% neutralization titers of 1:10 or 1:20 are evidence of immunity. From this perspective, the peak titers for the 4 groups of animals vaccinated with rEII*EIII/NS1* ranged from 271 to 980. High virus-neutralizing antibody titers (e.g., > 200) are generally associated with protection from viraemia, when measured using a plaque assay.Citation45,Citation46 Despite our encouraging results, the increase in antibody levels (detected by ELISA) did not reflect the level of neutralizing activity. Interestingly, this observation may provide a clue to the availability of the epitopes that crosslink BCR with the CD21/CD19/CD81 complex (the explanation of the adjuvanticity is in the constructions). It is possible that epitopes involved in neutralization do not have the appropriate orientation to stimulate specific clones of B cells, while other epitopes, not involved in neutralization, may be more exposed and enable this co-stimulatory effect.
On other the hand, the above data may be explained also by the general conventional PRNT assay that, in this case, measures only the neutralizing antibodies against domain III, which are antibodies of high affinity in contrast with the antibodies directed to the domain II (low affinity).Citation47,Citation48 Despite that, those antibodies participate in neutralizing the virus. Thus, ELISA and PRN50 evaluate different epitopes and responses, where ELISA shows a wider response that does not always correlate with neutralization.
If we consider the total amount of the immune response elicited for the constructs according with the above discussed; C3d as a vaccine adjuvant is still very attractive strategy because of the small size of the molecule, which, in our experimental model, showed very low immunogenicity (data do not shown) and relative simplicity of fusion construct design; however, the nature of the antigen itself appears to be critical for the success of C3d-conjugated vaccine. Once this issue is overcome, our strategy may provide a potential application and contribute greatly to the development of therapies and vaccines not only for DENV but also for other human diseases
In addition, the recombinant proteins elicited a predominant IgG1 response, which is typically associated with Th2-type immunity. The IgG2a isotype response was low in all the groups, including mice immunized with the virus, which should theoretically induce a higher level of IgG2a. This unexpected result suggests that the inoculated virus was partially inactivated and may act simply as an antigen. However, this hypothesis requires further experimental validation.
In conclusion, a recombinant DENV-2 protein containing relevant wild-type sequences fused to 3 copies of P28 induces an effective Th2 response based on neutralizing antibody.
Materials and Methods
Mice and antibodies
BALB/c mice (H-2d), 6 to 8 weeks old, were housed and handled in accordance with the CINVESTAV’s guidelines. The protocol procedures were approved by the “Animal Use Ethical Committee” (protocol approval: 118).
Anti-V5-HRP, anti-V5-FITC, (Invitrogen), anti-human IgG H+L, anti-mouse IgG1, and IgG2a (Zymed) antibodies were used. DENV positive Human serum samples were obtained from Veracruz, Gulf of Mexico.
Serum samples description
A single serum sample was collected from each patient 5–7 d after the onset of symptoms. Patients ranged from 6 to 60 y old, and they were defined as secondary infection. These dengue immune serum samples were collected as part of studies approved the State Commission of investigation bioethics and biosafety from the Health Ministry of Veracruz State and for the Committee of Ethics of the medical biological Institute of Medical research Veracruzana University (Protocol number 18/2010). Written informed consent was obtained from all subjects after they were informed of the nature and possible risks of the study.
Cells and viruses
C6/36 cells from Aedesalbopictus and baby hamster kidney (BHK-21) cells were grown in MEM at 34°C. S2 Drosophila melanogaster cells were grown in Schneider’s Drosophila medium (Invitrogen) at 28°C or room temperature without CO2. All cells were supplemented with 10% fetal bovine serum (FBS) and 0.29 mg∙mL−1 glutamine, 200 U∙mL−1 penicillin, and 0.2 mg∙mL−1 streptomycin (Gibco).
The DENV-2 clinical isolate stock was prepared and stored as previously described. The virus titers and plaque reduction neutralization test (PRNT50) were performed as previously described.Citation49,Citation50
Construction of the pD2EII*EIII/NS1* recombinant plasmid
The previously reported DENV antigen was modified to yield four constructs that were expressed in the S2 Drosophila system. The EII*EIII/NS1* sequence was amplified by PCR using the plasmid pEII*EIII/NS1*Citation34 as template and the following primers: (forward) 5′ (KpnI) GGGGTACCGA TGGCAAAAAA CAND (reverse) 3′ (XhoI) TGCCGCTCGA GGTTATGTGC CGCTCGAGGT TATGAGACT. This construct contained the nucleotide sequences of the E protein from position 943 to 1203 (261 aa) corresponding to domain II, and from 1639 to 2032 (394 aa) corresponding to domain III. Both fragments were linked by a Gly-Asn-Ser linker sequence followed by Gly-Ile-Ser and a NS1* sequence comprising residues 2494 to 2716 (222 aa). The PCR product and the plasmid pMTBiP/V5-HisA (Invitrogen) were digested with KpnI and XhoI, purified, and then ligated into the corresponding restriction sites of the parental vector. The recombinant plasmid, named pD2EII*EIII/NS1* (under the Methallothionein promoter) was sequenced to verify the construct. Afterwards, the NS1* sequence was removed and the plasmid re-ligated to obtain the second recombinant plasmid pD2EII*EIII. A second set of plasmids was constructed by adding a (Gly4-Ser2)Citation2 linker and three copies of P28 (aa1187–1214: KFLTTAKDKN RWEDPGKQLY NVEATSYA) at the 3′ end of the EIII domain. The recombinant plasmids directed the synthesis of the corresponding recombinant proteins rEII*EIII/NS1*, rEII*EIII/NS1*(P28)3, rEII*EIII and rEII*EIII(P28)3. All the constructs were fused with a V5 epitope and contained a histidine tag. The constructs were verified using automated sequencing.
Recombinant protein expression in S2 cells
The plasmids rEII*EIII/NS1*, rEII*EIII/NS1*(P28)3, rEII*EIII and rEII*EIII(P28)3 were transiently and then stably transfected into Drosophila S2 cells (supplemented with 10% FBS)using calcium phosphate according to the manufacturer’s instructions. The cells were incubated at 28°C, then CuSO4 at 500 mM final concentration for 2 d prior to the addition of copper sulfate at a final concentration of 500 µM. After 24 h, the cells were subjected to immunofluorescence analysis as described previously.Citation34 Briefly, the cells were treated with brefeldin A (BFA) for 6 h to disrupt intracellular traffic. Subsequently, the cells were fixed with 4% paraformaldehyde (PFA) in PBS for 20 min at room temperature, permeabilized with 0.1% Triton in PBS and blocked with 10% normal goat serum (NGS). The cell monolayers then were stained for 60 min with a mix of dengue immune serum samples antibodies diluted 1:500. Irrelevant isotype-matched monoclonal antibodies were used as negative controls.
Characterization of the recombinant DENV proteins by western blotting
The stable transfectant S2 cells were treated with BFA before SDS-PAGE and western blotting analysis (WB).Citation34 For the WB, the membranes were incubated with anti-V5 antibodies (Zymed) and then treated with antibodies conjugated to horseradish peroxidase (HRP) for 45 min at 37°C. The proteins were visualized using a Femto Chemiluminescence Kit (Pierce) and photographic film (Kodak).
Preparation of recombinant fusion proteins
The secreted recombinant DEN subunits proteins were clarified culture medium by immunoaffinity chromatography supernatants of stably pD2EII*EIII/NS1*, pD2EII*EIII/NS1*(P28)3 pD2EII*EIII and pD2EII*EIII(P28)3 transfected S2 cells were harvested on days 21, 28, 30, and 25, respectively, concentrated using an ultra-filter column (10 000 MWCO) (Merck Millipore) and dialyzed. Nickel columns (Invitrogen) were prepared for the purification of the recombinant proteins in native conditions. Cells (6 × 106 /m) were maintained in 2-L roller flasks containing DMEM medium supplemented with 10% FBS.
Immunization of mice
Ten BALB/c mice for each recombinant protein were injected 3 times with 25 µg of protein diluted 1:1 (v/v) in incomplete Freund’s adjuvant (Sigma) at 15 d intervals. The negative control group was inoculated with PBS, and the positive control group was immunized with DENV-2 (4 × 106 PFU). Each immunization was administered in a final volume of 100 µL (50 µL intraperitoneal and 25 µL in each rear footpad). Blood samples were collected at time 0 and at day 7 after each inoculation, and the serum was stored at -20 °C.
Antibody detection of specific antibodies against recombinant proteins
Specific antibodies against the DENV-2 E protein and NS1 protein were measured by ELISA in 96-well polyvinyl plates (Nunc) coated with either purified recombinant rEII*EIII/NS1* and GST-NS1 proteins (4 μg/mL) or GST protein alone as a control. Then, 2-fold serial dilutions of test sera were incubated at 4 °C overnight in duplicate. After incubation with 0.33 μg/mL−1 goat anti-mouse immunoglobulin peroxidase-conjugated secondary antibody anti-IgG (Zymed) or IgG1 (Zymed) or IgG2b (Invitrogen), H2O2, and 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich) were added as substrates. The absorbance values were determined at 405 nm. Negative and positive sera were included in each assay.Citation34
Additionally, since V5-HIS tag was not eliminated, an ELISA experiment was performed where 5 serum samples from each group were assayed. The 96-well polyvinyl plates (Nunc) were coated with 4 μg/mL of purified recombinant proteins rEII*EIII/NS1*, rprMeII* or rCD38-V5HIS (unrelated protein). All pre-immune and immune serum samples were then used at 1:200 dilution. Then all the procedure was completed as described above
Antibody detection of wild-type E protein
The dengue E glycoprotein isolated from infected C6/36 cells was immobilized in wells coated with Concanavalin A (Con A), which binds the glycoproteins of enveloped viruses.Citation14 Briefly, 96-well plates (Costar) were coated with 100 µL per well of Con A (Sigma-Aldrich) at 50 µg/mL in 0.01 M HEPES (Gibco) for 1 h. The wells were washed and incubated with solubilized DENV-2 (prepared in serum-free medium) for 1 h. After washing with PBS containing 0.1% (v/v) Triton-X 100, the unbound ConA binding sites were blocked with RPMI medium 1640 containing 10% FBS for 30 min. Dilutions of the serum samples from the immunized mice were incubated for 1 h at room temperature. Human immune serum containing DENV-2 was used as a positive control. Pre-immune serum samples were used as negative controls. After the wells were washed again and they were incubated with 100 µL of peroxidase-conjugated secondary antibody anti-IgG (Zymed) as described above.
Neutralization test
Neutralizing antibodies were titrated as described by Morens et al. Serial dilutions of the test sera were mixed with the DENV-2 virus. Briefly, BHK-21 cells were plated at a density of 1.5 × 105 cells per well in 24-well plates, and then, 30 PFUs of dengue virus mixed with heat-inactivated immune mouse serum (diluted at different ratios in 2% FBS in Hanks’ medium) were added in each well. The plates were incubated for 2 h at 37 °C under 5% CO2, and then, 500 µL of overlay medium were added to each well. After incubation for 4–5 d under the same conditions, the supernatant was discarded, the cells were washed with water by immersion and stained with naphthol blue-black dye. Subsequently, the plates were extensively washed with water and air-dried. The virus titer was estimated using the 50% plaque reduction neutralization test (PRNT50). Pre-immune mouse serum was added to the virus-mouse serum mixture to establish the basal virus titer, and the results were expressed as previously described.Citation50
Statistical analysis
ANOVA analysis with one-way Dunnett's Multiple Comparison tests or Bonferroni test was used to compare the pre-immune and hyper-immune results. All analyses were performed with the GraphPad Prism 5.01
Acknowledgments
We thank Dr T. Ross for providing the plasmid encoding CR2-binding domain of murine complement C3d (P28).The authors would like to thank Julio Garcia Cordero for his technical assistance. This work was supported by the National Council for Science and Technology (CONACyTGrant FONSEC0115401). Additionally, JGM and MLG received fellowships from CONACyT. LCB, LSA, NVS, are members of the National System of Researchers, SNI.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- PAHO Dengue regional information: Number of cases. http://new.paho.org/hq/index.php?option=com_content&task=view&id=264&Itemid=363. PAHO (2011).
- Añez G, Morales-Betoulle ME, Rios M. Circulation of different lineages of dengue virus type 2 in Central America, their evolutionary time-scale and selection pressure analysis. PLoS One 2011; 6:e27459; http://dx.doi.org/10.1371/journal.pone.0027459; PMID: 22076162
- Durbin AP, Whitehead SS. Next-generation dengue vaccines: novel strategies currently under development. Viruses 2011; 3:1800 - 14; http://dx.doi.org/10.3390/v3101800; PMID: 22069516
- Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine 2012; 30:4301 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.09.114; PMID: 22682286
- Megret F, Hugnot JP, Falconar A, Gentry MK, Morens DM, Murray JM, et al. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology 1992; 187:480 - 91; http://dx.doi.org/10.1016/0042-6822(92)90450-4; PMID: 1372140
- Beasley DW, Barrett AD. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol 2002; 76:13097 - 100; http://dx.doi.org/10.1128/JVI.76.24.13097-13100.2002; PMID: 12438639
- Heinz FX, Stiasny K. Flaviviruses and their antigenic structure. J ClinVirol 2012; 55:289 - 95; http://dx.doi.org/10.1016/j.jcv.2012.08.024; PMID: 22999801
- Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010; 8:271 - 83; http://dx.doi.org/10.1016/j.chom.2010.08.007; PMID: 20833378
- Goncalvez AP, Purcell RH, Lai CJ. Epitope determinants of a chimpanzee Fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J Virol 2004; 78:12919 - 28; http://dx.doi.org/10.1128/JVI.78.23.12919-12928.2004; PMID: 15542644
- Gollins SW, Porterfield JS. A new mechanism for the neutralization of enveloped viruses by antiviral antibody. Nature 1986; 321:244 - 6; http://dx.doi.org/10.1038/321244a0; PMID: 3713806
- Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 2001; 75:7769 - 73; http://dx.doi.org/10.1128/JVI.75.16.7769-7773.2001; PMID: 11462053
- Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002; 108:717 - 25; http://dx.doi.org/10.1016/S0092-8674(02)00660-8; PMID: 11893341
- Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 2005; 79:1223 - 31; http://dx.doi.org/10.1128/JVI.79.2.1223-1231.2005; PMID: 15613349
- Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, et al. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol J 2010; 7:28; http://dx.doi.org/10.1186/1743-422X-7-28; PMID: 20132551
- Amorim JH, Diniz MO, Cariri FA, Rodrigues JF, Bizerra RS, Gonçalves AJ, et al. Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine 2012; 30:837 - 45; http://dx.doi.org/10.1016/j.vaccine.2011.12.034; PMID: 22178517
- Costa SM, Paes MV, Barreto DF, Pinhão AT, Barth OM, Queiroz JL, et al. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 2006; 24:195 - 205; http://dx.doi.org/10.1016/j.vaccine.2005.07.059; PMID: 16122850
- Lu H, Xu XF, Gao N, Fan DY, Wang J, An J. Preliminary evaluation of DNA vaccine candidates encoding dengue-2 prM/E and NS1: their immunity and protective efficacy in mice. MolImmunol 2013; 54:109 - 14; http://dx.doi.org/10.1016/j.molimm.2012.11.007; PMID: 23270684
- Zhang F, Ma W, Zhang L, Aasa-Chapman M, Zhang H. Expression of particulate-form of Japanese encephalitis virus envelope protein in a stably transfected Drosophila cell line. Virol J 2007; 4:17; http://dx.doi.org/10.1186/1743-422X-4-17; PMID: 17324254
- Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine 2010; 28:2705 - 15; http://dx.doi.org/10.1016/j.vaccine.2010.01.022; PMID: 20097152
- Coller BA, Clements DE, Bett AJ, Sagar SL, TerMeulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 2011; 29:7267 - 75; http://dx.doi.org/10.1016/j.vaccine.2011.07.021; PMID: 21777637
- Block OK, Rodrigo WW, Quinn M, Jin X, Rose RC, Schlesinger JJ. A tetravalent recombinant dengue domain III protein vaccine stimulates neutralizing and enhancing antibodies in mice. Vaccine 2010; 28:8085 - 94; http://dx.doi.org/10.1016/j.vaccine.2010.10.004; PMID: 20959154
- Hermida L, Rodríguez R, Lazo L, Silva R, Zulueta A, Chinea G, et al. A dengue-2 Envelope fragment inserted within the structure of the P64k meningococcal protein carrier enables a functional immune response against the virus in mice. J Virol Methods 2004; 115:41 - 9; http://dx.doi.org/10.1016/j.jviromet.2003.09.024; PMID: 14656459
- Srivastava AK, Putnak JR, Warren RL, Hoke CH Jr.. Mice immunized with a dengue type 2 virus E and NS1 fusion protein made in Escherichia coli are protected against lethal dengue virus infection. Vaccine 1995; 13:1251 - 8; http://dx.doi.org/10.1016/0264-410X(94)00059-V; PMID: 8578812
- Chen HW, Liu SJ, Li YS, Liu HH, Tsai JP, Chiang CY, et al. A consensus envelope protein domain III can induce neutralizing antibody responses against serotype 2 of dengue virus in non-human primates. Arch Virol 2013; 158:1523 - 31; http://dx.doi.org/10.1007/s00705-013-1639-1; PMID: 23456422
- Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol 1995; 13:127 - 49; http://dx.doi.org/10.1146/annurev.iy.13.040195.001015; PMID: 7542009
- Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity 2012; 37:199 - 207; http://dx.doi.org/10.1016/j.immuni.2012.08.002; PMID: 22921118
- Ross TM, Xu Y, Green TD, Montefiori DC, Robinson HL. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res Hum Retroviruses 2001; 17:829 - 35; http://dx.doi.org/10.1089/088922201750252025; PMID: 11429124
- Bower JF, Ross TM. A minimum CR2 binding domain of C3d enhances immunity following vaccination. AdvExp Med Biol 2006; 586:249 - 64; http://dx.doi.org/10.1007/0-387-34134-X_17; PMID: 16893077
- Liu D, Wang J, Niu ZX. Contribution of Chinese Pekin duck complement component C3d-P29 repeats to enhancement of Th2-biased immune responses against NDV F gene induced by DNA immunization. ImmunopharmacolImmunotoxicol 2010; 32:297 - 306; http://dx.doi.org/10.3109/08923970903311802; PMID: 20148704
- Dunn MD, Rossi SL, Carter DM, Vogt MR, Mehlhop E, Diamond MS, et al. Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice. Virol J 2010; 7:95; http://dx.doi.org/10.1186/1743-422X-7-95; PMID: 20462412
- Roehrig JT. Antigenic structure of flavivirus proteins. Adv Virus Res 2003; 59:141 - 75; http://dx.doi.org/10.1016/S0065-3527(03)59005-4; PMID: 14696329
- Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol 1996; 70:8765 - 72; PMID: 8971005
- Chen MC, Lin CF, Lei HY, Lin SC, Liu HS, Yeh TM, et al. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J Immunol 2009; 183:1797 - 803; http://dx.doi.org/10.4049/jimmunol.0800672; PMID: 19592650
- Mellado-Sánchez G, García-Machorro J, Sandoval-Montes C, Gutiérrez-Castañeda B, Rojo-Domínguez A, García-Cordero J, et al. A plasmid encoding parts of the dengue virus E and NS1 proteins induces an immune response in a mouse model. Arch Virol 2010; 155:847 - 56; http://dx.doi.org/10.1007/s00705-010-0652-x; PMID: 20390312
- Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011; 29:7229 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.06.094; PMID: 21745521
- Robert Putnak J, Coller BA, Voss G, Vaughn DW, Clements D, Peters I, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine 2005; 23:4442 - 52; http://dx.doi.org/10.1016/j.vaccine.2005.03.042; PMID: 16005749
- Zheng Q, Fan D, Gao N, Chen H, Wang J, Ming Y, et al. Evaluation of a DNA vaccine candidate expressing prM-E-NS1 antigens of dengue virus serotype 1 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in immunogenicity and protection. Vaccine 2011; 29:763 - 71; http://dx.doi.org/10.1016/j.vaccine.2010.11.014; PMID: 21095256
- Hughes HR, Crill WD, Chang GJ. Manipulation of immunodominant dengue virus E protein epitopes reduces potential antibody-dependent enhancement. Virol J 2012; 9:115; http://dx.doi.org/10.1186/1743-422X-9-115; PMID: 22709350
- Coconi-Linares N, Ortega-Dávila E, López-González M, García-Machorro J, García-Cordero J, Steinman RM, Cedillo-Barrón L, Gómez-Lim MA. Targeting of envelope domain III protein of DENV type 2 to DEC-205 receptor elicits neutralizing antibodies in mice. Vaccine 2013; 31:2366 - 71; http://dx.doi.org/10.1016/j.vaccine.2013.03.009; PMID: 23499580
- Augusto EF, Moraes AM, Piccoli RA, Barral MF, Suazo CA, Tonso A, et al. Nomenclature and guideline to express the amount of a membrane protein synthesized in animal cells in view of bioprocess optimization and production monitoring. Biologicals 2010; 38:105 - 12; http://dx.doi.org/10.1016/j.biologicals.2009.07.005; PMID: 19699109
- Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, et al. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol 2005; 175:8011 - 23; PMID: 16339538
- Haspel N, Ricklin D, Geisbrecht BV, Kavraki LE, Lambris JD. Electrostatic contributions drive the interaction between Staphylococcus aureus protein Efb-C and its complement target C3d. Protein Sci 2008; 17:1894 - 906; http://dx.doi.org/10.1110/ps.036624.108; PMID: 18687868
- Gor DO, Ding X, Li Q, Greenspan NS. Genetic fusion of three tandem copies of murine C3d sequences to diphtheria toxin fragment B elicits a decreased fragment B-specific antibody response. ImmunolLett 2006; 102:38 - 49; http://dx.doi.org/10.1016/j.imlet.2005.06.020; PMID: 16105692
- Suradhat S, Braun RP, Lewis PJ, Babiuk LA, van DrunenLittel-van den Hurk S, Griebel PJ, et al. Fusion of C3d molecule with bovine rotavirus VP7 or bovine herpesvirus type 1 glycoprotein D inhibits immune responses following DNA immunization. Vet ImmunolImmunopathol 2001; 83:79 - 92; http://dx.doi.org/10.1016/S0165-2427(01)00369-5; PMID: 11604163
- da Silva Voorham JM, Rodenhuis-Zybert IA, Ayala Nuñez NV, Colpitts TM, van der Ende-Metselaar H, Fikrig E, et al. Antibodies against the envelope glycoprotein promote infectivity of immature dengue virus serotype 2. PLoS One 2012; 7:e29957; http://dx.doi.org/10.1371/journal.pone.0029957; PMID: 22431958
- de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. ProcNatlAcadSci U S A 2012; 109:7439 - 44; http://dx.doi.org/10.1073/pnas.1200566109; PMID: 22499787
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol 2006; 80:12149 - 59; http://dx.doi.org/10.1128/JVI.01732-06; PMID: 17035317
- Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, et al. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 2013; 87:52 - 66; http://dx.doi.org/10.1128/JVI.02273-12; PMID: 23077306
- Bustos-Arriaga J, García-Machorro J, León-Juárez M, García-Cordero J, Santos-Argumedo L, Flores-Romo L, et al. Activation of the innate immune response against DENV in normal non-transformed human fibroblasts. PLoSNegl Trop Dis 2011; 5:e1420; http://dx.doi.org/10.1371/journal.pntd.0001420; PMID: 22206025
- Morens DM, Halstead SB, Repik PM, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J ClinMicrobiol 1985; 22:250 - 4; PMID: 4031038