Abstract
Infant vaccination using 2-dose priming at 3 and 5 mo of age with a booster at 11–12 mo of age was pioneered in Italy. The 3-5-11 schedule is now used in a growing number of European countries. Infanrix™ hexa (DTPa-HBV-IPV/Hib, GlaxoSmithKline Vaccines) was first licensed for use in 2000 and has been the only pediatric hexavalent vaccine available since 2005. We reviewed available clinical trial data describing the immunogenicity of DTPa-HBV-IPV/Hib when administered at 3, 5, and 11 mo of age, and conducted an analysis of safety using global and Italian post-marketing surveillance data. In Italy, DTPa-HBV-IPV/Hib has a demonstrated safety record extending over a decade of use, it has been associated with record levels of vaccine coverage, and with sustained disease control in vaccinated cohorts. Hexavalent vaccines will continue to contribute to high vaccine coverage in Italy and across Europe.
Introduction
Combination vaccines allow the administration of antigens that target multiple diseases in a single injection. The potential benefits of combination vaccines over separate administration of the component parts include increased patient and health care professional acceptance of the administration of multiple antigens at one visit; lower vaccination costs as a result of fewer required office visits; greatly reduced storage requirements for vaccines as well as reduced risk of administration errors and of missed doses. Studies from the United StatesCitation1-Citation3 and GermanyCitation4 show that the use of combination vaccines is associated with improved coverage of individual antigens and with more timely vaccination: this means that a higher percentage of children receive all their recommended vaccines at the recommended age.
Hexavalent vaccines targeting six diseases are the largest multi-component combination vaccines currently available. Infanrix™ hexa (DTPa-HBV-IPV/Hib, GlaxoSmithKline Vaccines) is a combined hexavalent vaccine containing 10 antigens (diphtheria toxoid, tetanus toxoid, pertussis toxin [PT], filamentous haemagglutinin [FHA], pertactin [PRN], recombinant hepatitis B surface antigen, inactivated poliovirus [IPV] types 1, 2, and 3, and Haemophilus influenzae type b [Hib] polysaccharide polyribosylribitol phosphate [PRP]). DTPa-HBV-IPV/Hib is designed to prevent disease due to diphtheria, tetanus, pertussis, hepatitis B (HBV), poliomyelitis, and Hib. DTPa-HBV-IPV/Hib was first licensed for use in Europe in 2000 and is currently licensed in at least 95 countries.Citation5 Since the end of 2012, more than 90 million doses of DTPa-HBV-IPV/Hib have been distributed globally, with nearly 15 million doses distributed in Italy. All components of the combined vaccine (DTPa, HBV, IPV, and Hib) are licensed separately for use in infants and young children as Infanrix™, Engerix™ B, Poliorix™, and Hiberix™, respectively, and as components of other combined vaccines.
DTPa-HBV-IPV/Hib is one of few acellular pertussis vaccines that contain PRN, which appears to be important in protection against pertussis disease.Citation6 PRN is a Bordetella pertussis surface protein thought to promote adhesion and colonisation of the respiratory tract.Citation7 While all pertussis vaccines contain PT and many contain FHA (so-called 1- or 2-component pertussis vaccines), not all contain PRN (3-, 4-, or 5-component vaccines).
DTPa-HBV-IPV/Hib is indicated for primary and booster vaccination of infants and toddlers,Citation5 and has been studied in a range of commonly used vaccination schedules.Citation8 Data have been generated in children between 6 weeks of age and less than 36 mo. Much of the clinical data during the development of DTPa-HBV-IPV/Hib were obtained in studies that employed a 3-dose primary vaccination schedule with a booster in the second year of life.Citation8 However, administration in a 2-dose primary vaccination at 3 and 5 mo of age, with a booster administered at 11–12 mo of age (3-5-11 schedule) is also specifically approved in the Summary of Product Characteristics.Citation5 Although the magnitude of the immune response to some antigens may be lower after two priming doses than after three doses,Citation9,Citation10 the percentage of subjects achieving seroprotection/seropositivity for each of the vaccine antigens is similar: ranging between 91.7% and 100% after 2 doses and between 96.4% and 100% after 3 doses.Citation5 Similarly, seroprotection/seropositivity rates are comparable after the booster dose in infants primed with 2 or 3 vaccine doses (range 99.2% to 100% and 98.4% to 100%, respectively).Citation5 The 3-5-11 schedule decreases the cost and complexity of the immunisation schedule, and promotes high rates of immunisation coverage. The 3-5-11 schedule was pioneered in Italy in the 1970s,Citation11 and is now also used in Austria, Denmark, Finland, Iceland, Norway, France, and Sweden, and is being considered in Poland and other countries. The 3-5-11 schedule is used in regions where infectious pressure is low and where high uptake of the booster dose is achieved. Alternative 3-dose primary schedules beginning earlier in life with/without neonatal hepatitis B vaccination are used in countries where infectious pressure is high, and where there is a higher risk of exposure to disease early in life.
DTPa-HBV-IPV/Hib has been available for use in Italy since 2001. The introduction of hexavalent vaccines to Italy was followed by increases in vaccine coverage rates, with coverage of 3 doses of Hib vaccine increasing from 54.7% in 2000 to 83.4% in 2002, and coverage of 3 doses of pertussis vaccine increasing from 87.3% in 2000 to 92.9% in 2002.Citation12 By 2011, vaccine coverage for three doses of DTPa, poliovirus, hepatitis B, and Hib in Italy was more than 95%.Citation12
This review summarizes the available immunogenicity, reactogenicity, safety, and effectiveness data after 12 y of experience using DTPa-HBV-IPV/Hib, focusing on the 3-5-11 schedule in the Italian context.
Clinical Studies that Evaluated DTPa-HBV-IPV/Hib Administered to Infants at 3, 5, and 11 Mo of Age
We identified seven clinical trials that evaluated the immunogenicity and/or safety of DTPa-HBV-IPV/Hib administered to infants according to the 3-5-11 schedule ().Citation13-Citation17,Citation19,Citation20 In three of the studies DTPa-HBV-IPV/Hib was co-administered with another vaccine: either meningococcal serogroup C conjugate vaccine (MenC-TT) or a pneumococcal conjugate vaccine (PCV).
Table 1. Studies of DTPa-HBV-IPV/Hib conducted using the 3-5-11 mo vaccination schedule
Immunogenicity and Efficacy
Well-established serological correlates of protection exist for antibodies against tetanus, diphtheria, hepatitis B, polio, and Hib. Therefore, for these antigens, efficacy of DTPa-HBV-IPV/Hib in preventing disease is extrapolated from the percentage of subjects who reach serological thresholds accepted as correlates of protection. The exception to this is pertussis, for which no correlate of protection currently exists.
Diphtheria and tetanus
In each study, antibodies against diphtheria and tetanus were measured by enzyme-linked immunosorbant assay (ELISA), with the assay cut-off of 0.1 IU/ml which is at least 10-times the generally accepted threshold for seroprotection.Citation21,Citation22In groups that received DTPa-HBV-IPV/Hib, the percentage of subjects that achieved seroprotective (≥0.1 IU/ml) antibody concentrations after the second primary vaccination dose was between 92.8% and 100% for diphtheria and between 92.5% and 100% for tetanus (). After the booster dose, the percentage increased to 100% for diphtheria and was 100% for tetanus in all studies except when co-administered with 7-valent PCV (PCV7) (93.8%) or 13-valent PCV (PCV13) (97.6).Citation19 Of note, in a field study in Italy in which DTPa-HBV-IPV/Hib was co-administered with PCV7, the anti-tetanus seroprotection rate after the booster dose was 100%,Citation20 suggesting that co-administration with PCV7 was not responsible for the somewhat lower seroprotection rates observed by Esposito et al.Citation19
Table 2. Seroprotection/seropositivity rates at each blood sampling time point (ATP immunogenicity cohorts*)
Pertussis
Antibodies against PT, FHA, PRN were measured by ELISA,Citation23,Citation24 with an assay cut-off of 5 EL.U/ml. In studies conducted using the 3-5-11 mo schedule, at least 93.1% of subjects were seropositive for antibodies for PT, FHA, or PRN after dose 2 (). After the booster dose, 100% of subjects were seropositive for PT and FHA and at least 98.6% were seropositive for PRN.
No serological correlate of protection against pertussis disease is currently generally accepted. However, efficacy of GlaxoSmithKline Vaccines’ pediatric pertussis vaccine (DTPa, Infanrix™) was demonstrated in a German household contact study in the 1990s. Efficacy against World Health Organization-defined typical pertussis after 3-dose primary vaccination was 88.7% (95% confidence interval [CI]: 76.6%; 94.6%).Citation25,Citation26 Efficacy was also confirmed in a large placebo-controlled prospective cohort study conducted in Italy under the auspices of the United States National Institute of Allergy and Infectious Diseases (Progetto Pertosse). In the Italian setting, efficacy against WHO-defined pertussis after 3-dose primary vaccination was 83.9% (95% CI: 75.8%; 89.4%).Citation27 Follow-up of subjects for up to 60 mo after vaccination showed no decrease in vaccine efficacy over this period, even though a booster dose was not administered in the second year of life.Citation28 The pertussis component of DTPa-HBV-IPV/Hib is identical to that of DTPa, and protection against pertussis disease is therefore likely to be of similar magnitude and duration following primary vaccination with either DTPa-HBV-IPV/Hib or DTPa.
Sweden re-introduced pertussis vaccination in 1996 after a 17-y pertussis-vaccine free period, with doses of DTPa administered at 3, 5, and 11 mo of age. Post-marketing data from Sweden attest to the effectiveness of the 3-5-11 schedule, with marked reductions in pertussis disease incidence rates observed in vaccinated cohorts.Citation29,Citation30 The highest incidences of pertussis disease in settings of high vaccine coverage such Sweden,Citation29 Italy,Citation31 as well as countries that use 3-dose primary vaccination schedules,Citation32 are now observed in infants too young to be fully vaccinated. Ways to reduce the disease burden in vulnerable infants include additional pertussis booster doses in childhood and adolescence, and pertussis vaccination of pregnant women and/or new parents/grandparents, and other adults in contact with newborn infants (the so-called “cocoon strategy”).Citation33 There is growing interest in applying the cocoon strategy in Italy, although a pilot study suggests that good communication with parents and engagement of health care professionals is needed to reach high coverage.Citation34,Citation35
Acellular pertussis vaccines prevent severe pertussis disease in young children but recent evidence suggests that immunity wanes rapidly over timeCitation36-Citation39 Maintenance of strong immunization programs that achieve high coverage for primary and booster doses remains important for control of pertussis in countries where acellular vaccines are used. The availability of low antigen-content acellular pertussis vaccines (combined with diphtheria and tetanus) allows for regular boosting against pertussis throughout life. Because the optimal period between booster doses is not known, most countries with adult pertussis booster policies recommend a single life-time dose. However, booster doses administered every 10 y to coincide with recommendations for decennial diphtheria-tetanus boosters have been explored and would improve coverage and require no additional injections.Citation40,Citation41
Hepatitis B
The HBV component of DTPa-HBV-IPV/Hib is identical to Engerix™ B, for which efficacy and persistence of immune memory for at least 20 y after vaccination has been demonstrated in endemic regions.Citation42,Citation43
An anti-hepatitis B surface antigen (anti-HBs) antibody level of 10 mIU/ml is generally accepted as indicative of protection.Citation44 In subjects vaccinated with DTPa-HBV-IPV/Hib at 3 and 5 mo of age, between 93.1% and 98.3% had anti-HBs antibodies ≥10 mIU/ml (). After the booster dose, the percentage of subjects with concentrations ≥10 mIU/ml was between 97.7% and 100%. In a key study conducted in Slovakia, the anti-HBs immune response following administration of DTPa-HBV-IPV/Hib at 3 and 5 mo of age was similar to monovalent HBV vaccine (96.4% ≥ 10 mIU/ml vs. 82.6%, respectively), with significantly higher antibody concentrations in the DTPa-HBV-IPV/Hib group.Citation13 After the booster dose, 98.6% of DTPa-HBV-IPV/Hib and HBV recipients had seroprotective anti-HBs antibody concentrations. Approximately 10 y after vaccination, administration of a booster dose of HBV vaccine demonstrated persisting anamnestic responses in more than 95% of subjects in both groups.Citation14 Together these data indicate that administration of DTPa-HBV-IPV/Hib in the 3-5-11 schedule induced long lasting immune memory against HBV that was similar to that induced by monovalent HBV vaccines, for which protective efficacy has been demonstrated. The data are consistent with results observed in children who received three primary DTPa-HBV-IPV/Hib doses or three monovalent HBV doses, and in whom long-term antibody persistence and immune memory was demonstrated.Citation45-Citation47
There have been two studies conducted in Italy that assessed long-term anti-HBs antibody persistence in children vaccinated with hexavalent vaccines.Citation48,Citation49 Hexavac™ (DTPa-HBV-IPV-Hib, Sanofi Pasteur), a fully liquid hexavalent vaccine, was suspended and withdrawn from the market in 2005 because of concerns about long-term protection afforded against hepatitis B.Citation50 Both studies demonstrated that up to 5 y after vaccination, significantly higher percentages of DTPa-HBV-IPV/Hib-GSK-vaccinated children than DTPa-HBV-IPV-Hib-SP-vaccinated children had persisting anti-HBs levels ≥10 mIU/ml (83.2% vs. 38.4% at year 5Citation49). In children boosted with monovalent HBV vaccine, anamnestic responses were observed in the majority of children, regardless of vaccine group.Citation48,Citation49 The successor product to DTPa-HBV-IPV-Hib-SP was recently approved for use in the European Union.Citation51
Poliomyelitis
Antibodies against poliovirus types 1, 2, and 3 were measured by a micro-neutralisation assay, with a 1:8 dilution (assay cut-off) considered seroprotective.Citation52 In subjects vaccinated at 3-5-11 mo of age, the percentage with seroprotective antibodies for each poliovirus was between 94.3% and 100% after dose 2 and between 97.9% and 100% after dose 3 (). Poliovirus titers, but not seroprotection rates, were observed to be significantly lower (nearly half as low) after vaccination with DTPa-HBV-IPV-Hib-SP compared with DTPa-HBV-IPV/Hib.Citation53 In Italy, a surveillance study confirmed that lower poliovirus titers (but not seroprotection rates) persisted in DTPa-HBV-IPV-Hib-SP vaccinees compared with DTPa-HBV-IPV/Hib-vaccinees 15 mo after dose 3.Citation54
Europe was declared free of poliovirus in 2002.Citation55 Nevertheless, importation of wild virus strains from areas in which poliovirus continue to circulate remains a theoretical risk, and requires maintenance of immunity throughout life. A recent review of poliovirus seroepidemiology in Italy showed waning immunity with age, supporting the introduction of an adolescent poliovirus booster dose in Italy.Citation56,Citation57 Thus, induction of high poliovirus titers by hexavalent vaccines may be relevant in children, as they are expected to confer protection until the next IPV booster which is scheduled to be given at pre-school age.
Hib
Antibodies against the Hib polysaccharide, PRP, were measured by ELISA, with a cut-off of 0.15μg/ml which is considered indicative of seroprotection.Citation58 The percentage of subjects with seroprotective anti-PRP antibody concentrations was between 87.0% and 95.5% after dose 2, increasing to between 98.2% and 100% after the booster dose ().
The effectiveness of hexavalent vaccines, including DTPa-HBV-IPV/Hib, in preventing invasive Hib disease was investigated through a nation-wide surveillance in Germany over a 5-year follow-up period, the effectiveness of the Hib components of 2 hexavalent vaccines, one of which was DTPa-HBV-IPV/Hib, was 90.4% for a full 3-dose primary series and 100% for a full primary series plus booster dose (irrespective of the Hib vaccine used for priming).Citation59
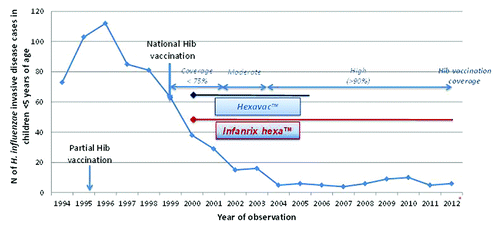
Long-term effectiveness of Hib vaccine administered in DTPa-based combinations in the 3-5-11 schedule has also been demonstrated in Sweden, where the incidence between 2005 and 2008 was estimated to be 0.4 per 100 000 children 0–4 y of age.Citation60 In Italy, the incidence of invasive H. influenzae disease began to decline when Hib vaccination was first introduced in 1998 ().Citation61 Since 2006, the incidence of invasive H. influenzae disease has remained at, or less than, 0.12 per 100 000, with around half of the cases identified as non-typeable and very few as type b.Citation62
Figure 1.Haemophilus influenzae invasive disease in children <5 y of age: Italy 1994–2012. Adapted from publically available data reports of Istituto Superiore di Sanità-Roma, accessed at http://www.simi.iss.it/files/Report_MBI.pdf and http://www.simi.iss.it/files/Report_meningiti_1994-2006.pdf April 2013). *partial data.
Reactogenicity and Safety
The reported reactogenicity profile of DTPa-HBV-IPV/Hib is similar to that of other inactivated vaccines, characterized by the early onset of local reactions and fever that are usually short lived and of minor clinical severity.Citation5
GlaxoSmithKline Vaccines receives reports of adverse reactions that occur after vaccination through spontaneous reporting from medical personnel, regulatory authorities, individuals, pharmacists, and literature sources. summarizes the ten most common adverse reactions reported worldwide and in Italy after vaccination with DTPa-HBV-IPV/Hib (primary and booster doses across all schedules) when co-administered with a PCV (since launch until the data lock point of 04 February 2013). Pyrexia after vaccination was the most frequently reported adverse reaction. This is consistent with the established safety profiles of DTPa-HBV-IPV/Hib and PCV7, for which a higher occurrence of fever has been reported when infants receive both vaccines at the same vaccination visit.Citation5 Adverse reaction values reported from Italy were higher to those of the rest of the world; however, in Italy vaccines undergo a national focused safety monitoring program where HCPs are requested to report all events, even those expected from SmPC such as fever.
Table 3. Overview of the 10 most frequently spontaneously reported events for DTPa-HBV-IPV/Hib when co-administered with a PCV, worldwide and in Italy (data from post-marketing passive surveillance reports received between 23 August 2005 and 4 February 2013).
More recently GlaxoSmithKline Vaccines performed an analysis to assess the occurrence of severe adverse events (fatalities, cardiorespiratory arrest, hypotonia/ hypotonic-hyporesponsive episode [HHE], and serious neurological disorders) occurring after vaccination with DTPa-HBV-IPV/Hib (co-administered with a PCV) during the period before and after the change from PCV7 to PCV13 in Italy (PCV7 reporting period 01-01-2008 until 31-04-2010; PCV13 reporting period 01-05-2012 until 18-07-2012). The reporting rate (number of reports per doses distributed) for fatalities in period when PCV7 co-administration was in use was 0.03 (95%CI 0.001; 0.14) compared with 0.11 (0.03; 0.29) when PCV13 was in use. The respective reporting rates for cardiorespiratory arrest were 0.31 (95% CI 0.16; 0.54) compared with 0.59 (0.36; 0.90), hypotonia/HHE 3.08 (2.55; 3.68), and 2.10 (1.65; 2.63) and for serious neurological events 4.05 (3.45; 4.74) and 4.56 (3.88; 5.31). The analysis did not show any major difference in the reporting rates of serious adverse events in Italy between the periods when DTPa-HBV-IPV/Hib was co-administered with PCV7 or PCV13.
Historical concerns about a potential temporal association between the occurrence of sudden unexpected death (SUD) and vaccination with hexavalent vaccines have been extensively investigated.Citation63 In Germany, a population-based evaluation demonstrated a possible safety signal for DTPa-HBV-IPV-Hib-SP but failed to show an imbalance between observed and expected SUD cases for DTPa-HBV-IPV/Hib.Citation64,Citation65 A large case series conducted in Italy was unable to confirm the presence of a safety signal, but noted that the power of the study was low due to small number of SUD cases that occurred.Citation66 In 2003 the European Medicines Agency concluded the absence of a cause-effect relationship and no change to the benefit-risk profile of the hexavalent vaccines then available.Citation67
Data in Pre-Term Infants
DTPa-HBV-IPV/Hib is specifically approved in the Summary of Product Characteristics for administration to premature infants.Citation5 In clinical trials DTPa-HBV-IPV/Hib is immunogenic when administered in a 3-dose primary schedule to premature infants (<37 weeks of gestation), with the majority of subjects achieving antibody concentrations consistent with seroprotection after vaccination.Citation68-Citation70 Furthermore, a booster dose of DTPa-HBV-IPV/Hib induced marked increases in antibody concentrations in toddlers who had been born prematurely, indicative of the development of immune memory and effective priming by DTPa-HBV-IPV/Hib.Citation71
In 2007 the Committee for Medicinal Products for Human Use reviewed cases of apnoea in prematurely born infants following vaccination with different vaccines, and concluded that the occurrence of apnoea following vaccination is increased in infants born very prematurely (≤28 weeks of gestation) due to immaturity of the immune system. This was considered to be an issue for all vaccines administered in this population. The recommendation of the Committee to monitor very premature infants closely for up to 48–72 h after vaccination was included in the Summary of Product Characteristics for DTPa-HBV-IPV/Hib.Citation5
Co-Administration
There is extensive clinical trial experience with DTPa-HBV-IPV/Hib co-administered with other routinely recommended pediatric vaccines, including PCVs,Citation19,Citation72 rotavirus,Citation73 meningococcal conjugate,Citation74-Citation76 measles, mumps, rubella, and varicellaCitation77 vaccines. In all studies the immune response was considered adequate. Specifically, clinical trials conducted in Italy showed that the immune response to DTPa-HBV-IPV/Hib antigens was similar when administered alone, or when co-administered with PCV7 or PCV13, with a clinically acceptable safety profile.Citation19,Citation20 Similarly, DTPa-HBV-IPV/Hib co-administered with a meningococcal serogroup C conjugate vaccine induced seroprotective antibody concentrations against all of the administered antigens, including meningococcal serogroup C, in the majority of subjects.Citation18 Thus, in line with current recommendations concerning inactivated vaccines,Citation78 DTPa-HBV-IPV/Hib can be co-administered with other live or inactivated vaccines without interference on the immune response.
Conclusion
DTPa-HBV-IPV/Hib has been licensed for use for 12 y and an extensive body of clinical trial and post-marketing experience attest to its safety and effectiveness in preventing diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, and invasive Hib disease when administered alone, or co-administered with other vaccines in infants and young children, including infants born prematurely. A safety record over 12 y provides confidence to health care providers about the acceptable safety profile of DTPa-HBV-IPV/Hib.
In Italy, DTPa-HBV-IPV/Hib has a demonstrated safety record extending over a decade of use and nearly 15 million doses distributed. The use of DTPa-HBV-IPV/Hib in Italy has been associated with record levels of vaccine coverage, and with sustained disease control in vaccinated cohorts. Hexavalent vaccines will continue to contribute to high vaccine coverage in Italy and across Europe.
| Abbreviations: | ||
| μg/ml | = | micrograms per milliliter |
| ATP | = | according to protocol |
| CI | = | confidence interval |
| DTPa-HBV-IPV/Hib | = | diphtheria-tetanus-acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine |
| ELISA | = | enzyme-linked immunosorbent assay |
| EL.U/ml | = | ELISA units per milliliter |
| FHA | = | filamentous haemagglutinin |
| GMC | = | geometric mean antibody concentration |
| IU/ml | = | international units per milliliter |
| mIU/ml | = | milli-international units per milliliter |
| PRN | = | pertactin |
| PRP | = | polyribosylribitol phosphate |
| PT | = | pertussis toxoid |
| SP | = | seroprotection |
| S+ | = | seropositivity |
| VR | = | vaccine response |
| WHO | = | World Health Organization |
Disclosure of Potential Conflicts of Interest
FM and MC are employees of GlaxoSmithKline group of companies and report ownership of stock options. During the last 5 years, BV and GG have received grants from GlaxoSmithKline Biologicals SA, Sanofi Pasteur MSD, Novartis, Crucell/Janssen, and Pfizer for taking part in advisory boards, expert meetings, being a speaker or an organizer of congresses/conferences, and acting as investigator in clinical trials. EF reports grants paid by Sanofi Pasteur MSD and Pfizer to her institution, and payments received from GlaxoSmithKline Biologicals SA, Pfizer, Crucell and Sanofi Pasteur MSD for travel and accommodations for meeting attendance. RP reports payments from Sanofi Pasteur MSD, Pfizer, GlaxoSmithKline Biologicals SA for Board membership; payments from Pfizer International Operations SAS for consultancy; payments from Pfizer for lectures including speakers bureaus and from Pfizer, GlaxoSmithKline Biologicals SA, Novartis for travel, accommodations, meeting expenses; grants received by her institution from Novartis, Pfizer and Wyeth Lederle. VF reports no conflict of interest.
Sources of Support
GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
Acknowledgments
The authors thank Daniel De Palmenaer and Céline Verheust for safety analysis, Dr Joanne Wolter (independent) for preparing the first draft of the manuscript and medical writing support, and Jérémie Dedessus le Moutier (Business and Decision Life Sciences on behalf of GlaxoSmithKline Vaccines) for manuscript co-ordination and editorial assistance.
Trademark
Infanrix, Engerix, Hiberix and Menitorix are trademarks of the GlaxoSmithKline group of companies. Hexavac is a trademark of Sanofi Pasteur. NeisVac-C is a trademark of Baxter. Prevenar is a trademark of Pfizer.
References
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007; 26:496 - 500; http://dx.doi.org/10.1097/INF.0b013e31805d7f17; PMID: 17529866
- Happe LE, Lunacsek OE, Kruzikas DT, Marshall GS. Impact of a pentavalent combination vaccine on immunization timeliness in a state Medicaid population. Pediatr Infect Dis J 2009; 28:98 - 101; http://dx.doi.org/10.1097/INF.0b013e318187d047; PMID: 19148039
- Happe LE, Lunacsek OE, Marshall GS, Lewis T, Spencer S. Combination vaccine use and vaccination quality in a managed care population. Am J Manag Care 2007; 13:506 - 12; PMID: 17803364
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt H-J, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25:507 - 12; http://dx.doi.org/10.1097/01.inf.0000222413.47344.23; PMID: 16732148
- Infanrix hexa product information. 2011 Sep 14 [cited 2013 Feb 18]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000296/human_med_000833.jsp&mid=WC0b01ac058001d124
- Poolman JT, Hallander HO. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev Vaccines 2007; 6:47 - 56; http://dx.doi.org/10.1586/14760584.6.1.47; PMID: 17280478
- Bassinet L, Gueirard P, Maitre B, Housset B, Gounon P, Guiso N. Role of adhesins and toxins in invasion of human tracheal epithelial cells by Bordetella pertussis.. Infect Immun 2000; 68:1934 - 41; http://dx.doi.org/10.1128/IAI.68.4.1934-1941.2000; PMID: 10722585
- Zepp F, Schmitt H-J, Cleerbout J, Verstraeten T, Schuerman L, Jacquet J-M. Review of 8 years of experience with Infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines 2009; 8:663 - 78; http://dx.doi.org/10.1586/erv.09.32; PMID: 19485747
- Carlsson RM, Claesson BA, Selstam U, Fagerlund E, Granström M, Blondeau C, Hoffenbach A. Safety and immunogenicity of a combined diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae type b vaccine administered at 2-4-6-13 or 3-5-12 months of age. Pediatr Infect Dis J 1998; 17:1026 - 33; http://dx.doi.org/10.1097/00006454-199811000-00013; PMID: 9849987
- Taranger J, Trollfors B, Knutsson N, Sundh V, Lagergård T, Ostergaard E. Vaccination of infants with a four-dose and a three-dose vaccination schedule. Vaccine 1999; 18:884 - 91; http://dx.doi.org/10.1016/S0264-410X(99)00341-2; PMID: 10580202
- Marino MG, Franco E. [Immunization in the 3rd, 5th and 11th month of age: an Italian intuition]. Ig Sanita Pubbl 2008; 64:391 - 401; PMID: 18936801
- Sintesi anni 2000-2011 - Ministero della Salute. Vaccinazioni dell’età pediatrica: coperture vaccinali* (per 100 abitanti) in Italia. 2012. Available from: http://www.salute.gov.it/imgs/C_17_pagineAree_811_listaFile_itemName_11_file.pdf
- Avdicová M, Prikazský V, Hudecková H, Schuerman L, Willems P. Immunogenicity and reactogenicity of a novel hexavalent DTPa-HBV-IPV/Hib vaccine compared to separate concomitant injections of DTPa-IPV/Hib and HBV vaccines, when administered according to a 3, 5 and 11 month vaccination schedule. Eur J Pediatr 2002; 161:581 - 7; http://dx.doi.org/10.1007/s00431-002-1079-5; PMID: 12424582
- Avdicová M, Crasta PD, Hardt K, Kovac M. Lasting immune memory against hepatitis B 10 years after the 2+1 primary vaccination schedule with DTPa-HBV-IPV/Hib or DTPa-IPV/Hib+HBV. 30th annual meeting of the European Society for Paediatric Infectious Diseases (ESPID). Thessalonika, Greece: 2012.
- Van Der Meeren O, Kuriyakose S, Kolhe D, Hardt K. Immunogenicity of Infanrix™ hexa administered at 3, 5 and 11 months of age. Vaccine 2012; 30:2710 - 4; http://dx.doi.org/10.1016/j.vaccine.2012.02.024; PMID: 22349525
- Gabutti G, Zepp F, Schuerman L, Dentico P, Bamfi F, Soncini R, Habermehl P, Knuf M, Crovari P, Cooperative Italian Group for the Study of Combined Vaccines. Evaluation of the immunogenicity and reactogenicity of a DTPa-HBV-IPV Combination vaccine co-administered with a Hib conjugate vaccine either as a single injection of a hexavalent combination or as two separate injections at 3, 5 and 11 months of age. Scand J Infect Dis 2004; 36:585 - 92; http://dx.doi.org/10.1080/00365540410017572; PMID: 15370670
- Kilpi TM, Silfverdal SA, Nilsson L, Syrjänen R, Belloni C, Desole M, Triban C, Storsaeter J, Soila M, Jacquet JM. Immunogenicity and reactogenicity of two diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio virus-Haemophilus influenzae type b vaccines administered at 3, 5 and 11-12 months of age. Hum Vaccin 2009; 5:18 - 25; http://dx.doi.org/10.4161/hv.5.1.6369; PMID: 18690013
- Vesikari T, Forstén A, Desole MG, Ferrera G, Caubet M, Mesaros N, Boutriau D. A combined Haemophilus influenzae type B Neisseria meningitidis serogroup C tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when coadministered with diphtheria, tetanus, acellular pertussis hepatitis B-inactivated poliovirus at 3, 5 and 11 months of age: results of an open, randomized, controlled study. Pediatr Infect Dis J 2013; 32:521 - 9; http://dx.doi.org/10.1097/INF.0b013e31827e22e3; PMID: 23190785
- Esposito S, Tansey S, Thompson A, Razmpour A, Liang J, Jones TR, Ferrera G, Maida A, Bona G, Sabatini C, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol 2010; 17:1017 - 26; http://dx.doi.org/10.1128/CVI.00062-10; PMID: 20427630
- Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, Marensi L, Giacchino R, Timitilli A, Carloni R, et al, Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine 2009; 27:3459 - 62; http://dx.doi.org/10.1016/j.vaccine.2009.01.052; PMID: 19200823
- Melville-Smith ME, Seagroatt VA, Watkins JT. A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand 1983; 11:137 - 44; http://dx.doi.org/10.1016/S0092-1157(83)80038-9; PMID: 6345548
- Camargo ME, Silveira L, Furuta JA, Oliveira EP, Germek OA. Immunoenzymatic assay of anti-diphtheric toxin antibodies in human serum. J Clin Microbiol 1984; 20:772 - 4; PMID: 6386879
- Granström M, Thorén M, Blennow M, Tiru M, Sato Y. Acellular pertussis vaccine in adults: adverse reactions and immune response. Eur J Clin Microbiol 1987; 6:18 - 21; http://dx.doi.org/10.1007/BF02097184; PMID: 2883004
- Karpinski KF, Hayward S, Tryphonas H. Statistical considerations in the quantitation of serum immunoglobulin levels using the enzyme-linked immunosorbent assay (ELISA). J Immunol Methods 1987; 103:189 - 94; http://dx.doi.org/10.1016/0022-1759(87)90289-4; PMID: 3668258
- Schmitt HJ, Schuind A, Knuf M, Beutel K, Schulte-Wissermann H, Gahr M, Schult R, Folkens J, Rauh W, Bogaerts H, et al. Clinical experience of a tricomponent acellular pertussis vaccine combined with diphtheria and tetanus toxoids for primary vaccination in 22,505 infants. J Pediatr 1996; 129:695 - 701; http://dx.doi.org/10.1016/S0022-3476(96)70152-X; PMID: 8917236
- Schmitt HJ, von König CH, Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H, Gahr M, Schult R, Folkens JU, Rauh W, et al. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA 1996; 275:37 - 41; http://dx.doi.org/10.1001/jama.1996.03530250041024; PMID: 8531284
- Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, Ciofi degli Atti ML, Giammanco A, Panei P, Blackwelder WC, et al, Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med 1996; 334:341 - 8; http://dx.doi.org/10.1056/NEJM199602083340601; PMID: 8538704
- Salmaso S, Mastrantonio P, Tozzi AE, Stefanelli P, Anemona A, Ciofi degli Atti ML, Giammanco A, Stage III Working Group. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics 2001; 108:E81; http://dx.doi.org/10.1542/peds.108.5.e81; PMID: 11694665
- Hallander HO, Gustafsson L. Efficacy and effectiveness of acellular pertussis vaccines: a 20-year Swedish experience. Expert Rev Vaccines 2009; 8:1303 - 7; http://dx.doi.org/10.1586/erv.09.88; PMID: 19803750
- Nilsson L, Lepp T, von Segebaden K, Hallander H, Gustafsson L. Pertussis vaccination in infancy lowers the incidence of pertussis disease and the rate of hospitalisation after one and two doses: analyses of 10 years of pertussis surveillance. Vaccine 2012; 30:3239 - 47; http://dx.doi.org/10.1016/j.vaccine.2011.10.089; PMID: 22094282
- Gabutti G, Rota MC, Bonato B, Pirani R, Turlà G, Cucchi A, Cavallaro A. Hospitalizations for pertussis in Italy, 1999-2009: analysis of the hospital discharge database. Eur J Pediatr 2012; 171:1651 - 5; http://dx.doi.org/10.1007/s00431-012-1791-8; PMID: 22790868
- Forsyth KD, Wirsing von Konig C-H, Tan T, Caro J, Plotkin S. Prevention of pertussis: recommendations derived from the second Global Pertussis Initiative roundtable meeting. Vaccine 2007; 25:2634 - 42; http://dx.doi.org/10.1016/j.vaccine.2006.12.017; PMID: 17280745
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131 - 5; PMID: 23425962
- Simonetti A, Martini I, Bonomo G, D’Avino R, Puggina P, Vairo U, Piscopo P, Marchetti F. Improving adherence rates to a cocooning program: A pilot experience in Italy. Hum Vaccin Immunother 2013; 9; Forthcoming http://dx.doi.org/10.4161/hv.23795; PMID: 23370334
- Prato R, Martinelli D, Marchetti F, Fortunato F, Tafuri S, Germinario CA. Feasibility of a cocoon strategy for the prevention of pertussis in Italy: a survey of prevention department healthcare providers. Pediatr Infect Dis J 2012; 31:1304 - 7; http://dx.doi.org/10.1097/INF.0b013e31826b7110; PMID: 22863911
- Misegades LK, Winter K, Harriman K, Talarico J, Messonnier NE, Clark TA, Martin SW. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA 2012; 308:2126 - 32; http://dx.doi.org/10.1001/jama.2012.14939; PMID: 23188029
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012; 367:1012 - 9; http://dx.doi.org/10.1056/NEJMoa1200850; PMID: 22970945
- Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 2013; 131:e1047 - 52; http://dx.doi.org/10.1542/peds.2012-1928; PMID: 23478868
- Quinn HE, McIntyre P. Pertussis vaccine effectiveness in Australia. 2011 [cited 21 June 2013]. Available from: http://www.ncirs.edu.au/news/past-news-events/Day%201/HQuinn-Australia-PertussisWS-25_26Aug11.pdf
- Booy R, Van der Meeren O, Ng S-P, Celzo F, Ramakrishnan G, Jacquet J-M. A decennial booster dose of reduced antigen content diphtheria, tetanus, acellular pertussis vaccine (Boostrix™) is immunogenic and well tolerated in adults. Vaccine 2010; 29:45 - 50; http://dx.doi.org/10.1016/j.vaccine.2010.10.025; PMID: 20974302
- Mertsola J, Van Der Meeren O, He Q, Linko-Parvinen A, Ramakrishnan G, Mannermaa L, Soila M, Pulkkinen M, Jacquet JM. Decennial administration of a reduced antigen content diphtheria and tetanus toxoids and acellular pertussis vaccine in young adults. Clin Infect Dis 2010; 51:656 - 62; http://dx.doi.org/10.1086/655825; PMID: 20704493
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, Jacquet JM. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat 2011; 18:369 - 75; http://dx.doi.org/10.1111/j.1365-2893.2010.01312.x; PMID: 20384962
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet J-M. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28:730 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.10.074; PMID: 19892043
- Mast E, Mahoney F, Kane M, Margolis H. Hepatitis B Vaccine In: PLotkin SA, Orenstein WA, eds. Vaccines. 4th edition. Philadelphia: Saunders Elsevier; 2004. p. 299–337.
- Zinke M, Kappes R, Kindler K, Paulus-Koschik A, Goering U, Disselhoff J, Soemantri P, Grunert D, Laakmann KH, Gunasekaran R, et al. Immune memory to hepatitis B virus in 4-9-year old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin 2009; 5:592 - 8; PMID: 19535920
- Heininger U, Sänger R, Jacquet J-M, Schuerman L, DTP-HBV-IPV-059 Study Group, DTP-HBV-IPV-096 Study Group. Booster immunization with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus vaccine and Haemophilus influenzae type b conjugate combination vaccine in the second year of life: safety, immunogenicity and persistence of antibody responses. Vaccine 2007; 25:1055 - 63; http://dx.doi.org/10.1016/j.vaccine.2006.09.060; PMID: 17049692
- Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet J-M, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis 2010; 10:9; http://dx.doi.org/10.1186/1471-2334-10-9; PMID: 20078876
- Giambi C, Bella A, Barale A, Montù D, Marchisio M, Oddone M, Zito S, Rapicetta M, Chionne P, Madonna E, et al. A cohort study to evaluate persistence of hepatitis B immunogenicity after administration of hexavalent vaccines. BMC Infect Dis 2008; 8:100; http://dx.doi.org/10.1186/1471-2334-8-100; PMID: 18662386
- Zanetti AR, Romanò L, Giambi C, Pavan A, Carnelli V, Baitelli G, Malchiodi G, Valerio E, Barale A, Marchisio MA, et al, study group. Hepatitis B immune memory in children primed with hexavalent vaccines and given monovalent booster vaccines: an open-label, randomised, controlled, multicentre study. Lancet Infect Dis 2010; 10:755 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70195-X; PMID: 20884297
- European Medicines Agency. Questions and answers on the suspension of Hexavac. Doc. Ref. EMEA/304888/200. 2005 Sep 20 [cited 2013 Feb 19];Available from: http://www.emea.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500017700.pdf
- European Medicines Agency. Summary of Opinion. Hexaxim. EMA/CHMP/409930/2012. 2012 Jun [cited 2013 July 31]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/06/WC500129095.pdf
- WHO Expanded Programme on Immunization. Guidelines for WHO/EPI collaborative studies on poliomyelitis: standard procedure for determining immunity to poliovirus using the microneutralization test. Geneva: World Health Organization. 1993. Available from: http://www.who.int/iris/handle/10665/70486#sthash.dQOk5dbw.dpuf
- Tichmann I, Preidel H, Grunert D, Habash S, Schult R, Maier R, Gildberg PK, Sengespeik HC, Meurice F, Sänger R. Comparison of the immunogenicity and reactogenicity of two commercially available hexavalent vaccines administered as a primary vaccination course at 2, 4 and 6 months of age. Vaccine 2005; 23:3272 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.01.087; PMID: 15837232
- Giambi C, Bella A, Barale A, Montù D, Marchisio M, Oddone M, Zito S, Rapicetta M, Chionne P, Madonna E, et al. A cohort study to evaluate persistence of hepatitis B immunogenicity after administration of hexavalent vaccines. BMC Infect Dis 2008; 8:100; http://dx.doi.org/10.1186/1471-2334-8-100; PMID: 18662386
- Certification of the Region’s polio-free status in 2002. [cited 2013 Feb 19];Available from: http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/poliomyelitis/activities/polio-certification-activities/certification-of-the-regions-polio-free-status-in-2002
- Baldo V, Baldovin T, Cocchio S, Lazzari R, Saracino E, Bertoncello C, Buja A, Trevisan A. Seroepidemiology of polioviruses among university students in northern Italy. Clin Vaccine Immunol 2012; 19:1292 - 5; http://dx.doi.org/10.1128/CVI.00054-12; PMID: 22739695
- Patti AM. [From the goal of the global polio eradication to the recent epidemics: reflections on the present epidemiological situation and future perspectives]. Ann Ig 2010; 22:521 - 37; PMID: 21425649
- Käyhty H, Peltola H, Karanko V, Mäkelä PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1983; 147:1100; http://dx.doi.org/10.1093/infdis/147.6.1100; PMID: 6602191
- Kalies H, Grote V, Siedler A, Gröndahl B, Schmitt H-J, von Kries R. Effectiveness of hexavalent vaccines against invasive Haemophilus influenzae type b disease: Germany’s experience after 5 years of licensure. Vaccine 2008; 26:2545 - 52; http://dx.doi.org/10.1016/j.vaccine.2008.03.001; PMID: 18403069
- Hallander HO, Lepp T, Ljungman M, Netterlid E, Andersson M. Do we need a booster of Hib vaccine after primary vaccination? A study on anti-Hib seroprevalence in Sweden 5 and 15 years after the introduction of universal Hib vaccination related to notifications of invasive disease. APMIS 2010; 118:878 - 87; http://dx.doi.org/10.1111/j.1600-0463.2010.02674.x; PMID: 20955461
- Cerquetti M. Haemophilus influenzae in epoca post-vaccinale. Rome: Workshop MIB; 2012. Available from: http://www.epicentro.iss.it/problemi/meningiti/wkshpdancona/web_Cerquetti_workshop_MIB_2012.pdf
- Giufrè M, Cardines R, Caporali MG, Accogli M, D’Ancona F, Cerquetti M. Ten years of Hib vaccination in Italy: prevalence of non-encapsulated Haemophilus influenzae among invasive isolates and the possible impact on antibiotic resistance. Vaccine 2011; 29:3857 - 62; http://dx.doi.org/10.1016/j.vaccine.2011.03.059; PMID: 21459175
- Kuhnert R, Schlaud M, Poethko-Müller C, Vennemann M, Fleming P, Blair PS, Mitchell E, Thompson J, Hecker H. Reanalyses of case-control studies examining the temporal association between sudden infant death syndrome and vaccination. Vaccine 2012; 30:2349 - 56; http://dx.doi.org/10.1016/j.vaccine.2012.01.043; PMID: 22289512
- von Kries R, Toschke AM, Strassburger K, Kundi M, Kalies H, Nennstiel U, Jorch G, Rosenbauer J, Giani G. Sudden and unexpected deaths after the administration of hexavalent vaccines (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, Haemophilius influenzae type b): is there a signal?. Eur J Pediatr 2005; 164:61 - 9; http://dx.doi.org/10.1007/s00431-004-1594-7; PMID: 15602672
- von Kries R. Comment on B. Zinka et al., Unexplained cases of sudden infant death shortly after hexavalent vaccination. Vaccine 2006; 24:5783 - 4, author reply 5785-6; http://dx.doi.org/10.1016/j.vaccine.2005.03.055; PMID: 16081190
- Traversa G, Spila-Alegiani S, Bianchi C, Ciofi degli Atti M, Frova L, Massari M, Raschetti R, Salmaso S, Scalia Tomba G, Hera Study Group. Sudden unexpected deaths and vaccinations during the first two years of life in Italy: a case series study. PLoS One 2011; 6:e16363; http://dx.doi.org/10.1371/journal.pone.0016363; PMID: 21298113
- European Medicines Agency. EMEA updatwe on hexavalent vaccines: Hexavac and Infanrix hexa. EMEA/CPMP/5889/03. London: 2003. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2010/01/WC500059303.pdf
- Omeñaca F, Garcia-Sicilia J, García-Corbeira P, Boceta R, Romero A, Lopez G, Dal-Ré R. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics 2005; 116:1292 - 8; http://dx.doi.org/10.1542/peds.2004-2336; PMID: 16322149
- Omeñaca F, Garcia-Sicilia J, García-Corbeira P, Boceta R, Torres V. Antipolyribosyl ribitol phosphate response of premature infants to primary and booster vaccination with a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio virus/Haemophilus influenzae type b vaccine. Pediatrics 2007; 119:e179 - 85; http://dx.doi.org/10.1542/peds.2005-2907; PMID: 17145903
- Omeñaca F, Garcia-Sicilia J, Boceta R, García-Corbeira P. Hepatitis B response of premature infants after primary and booster immunisation with a diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus/haemophilus influenzae type B vaccine. Infect Dis Obstet Gynecol 2010; 2010:802503; http://dx.doi.org/10.1155/2010/802503; PMID: 20396673
- Omeñaca F, Garcia-Sicilia J, Boceta R, Sistiaga-Hernando A, García-Corbeira P. Antibody persistence and booster vaccination during the second and fifth years of life in a cohort of children who were born prematurely. Pediatr Infect Dis J 2007; 26:824 - 9; http://dx.doi.org/10.1097/INF.0b013e318124a9c8; PMID: 17721379
- Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène J-P, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28:Suppl S66 - 76; http://dx.doi.org/10.1097/INF.0b013e318199f8ef; PMID: 19325449
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine 2010; 28:5272 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.05.057; PMID: 20538094
- Tejedor JC, Omeñaca F, García-Sicilia J, Verdaguer J, Van Esso D, Esporrín C, Molina V, Muro M, Marés J, Enrubia M, et al, Spanish DTPa-HBV-IPV/Hib-076 Study Group. Immunogenicity and reactogenicity of a three-dose primary vaccination course with a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-haemophilus influenzae type b vaccine coadministered with a meningococcal C conjugate vaccine. Pediatr Infect Dis J 2004; 23:1109 - 15; PMID: 15626947
- Tejedor JC, Moro M, Ruiz-Contreras J, Castro J, Gómez-Campderá JA, Navarro ML, Merino JM, Martín-Ancel A, Roca J, García-del-Río M, et al, Spanish DTaP-HBV-IPV-097 Study Group. Immunogenicity and reactogenicity of primary immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type B vaccine coadministered with two doses of a meningococcal C-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J 2006; 25:713 - 20; http://dx.doi.org/10.1097/01.inf.0000227725.61495.c4; PMID: 16874171
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine 2011; 29:4264 - 73; http://dx.doi.org/10.1016/j.vaccine.2011.03.009; PMID: 21420417
- Zepp F, Behre U, Kindler K, Laakmann K-H, Pankow-Culot H, Mannhardt-Laakmann W, Beckers F, Descamps D, Willems P. Immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine co-administered with a booster dose of a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine in healthy children aged 12-23 months. Eur J Pediatr 2007; 166:857 - 64; http://dx.doi.org/10.1007/s00431-007-0506-z; PMID: 17541639
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1 - 64; PMID: 21293327
