Abstract
Pneumococcal pneumonia has a high clinical burden in terms of morbidity, mortality and hospitalization rate, with heavy implications for worldwide health systems. In particular, higher incidence and mortality rates of community-acquired pneumonia (CAP) cases, with related costs, are registered among elderly. This study aimed to an economic evaluation about the immunization with PCV13 in the adult population in Campania region, South Italy. For this purpose we performed, considering a period of 5 y, a budget impact analysis (BIA) and a cost-effectiveness analysis which considered 2 scenarios of immunization compared with lack of immunization for 2 targeted cohorts: first, the high risk subjects aged 50–79 y, and second the high risk individuals aged 50–64 y, together with all those aged 65 y. Regarding the first group, the decrease of pneumonia could give savings equal to €29 005 660, while the immunization of the second cohort could allow savings equal to €10 006 017.
The economic evaluation of pneumococcal vaccine for adult groups represents an essential instrument to support health policies. This study showed that both hypothesized immunization strategies could produce savings. Obtained results support the use of pneumococcal conjugate vaccine for adults. This strategy could represent a sustainable and savings-producer health policy.
Introduction
Streptococcus pneumoniae (Spn) may cause a wide spectrum of diseases ranging from otitis and pneumonia to invasive forms such as meningitis or sepsis.Citation1-Citation3 Among pneumococcal diseases, pneumonia has the higher clinical burden in terms of morbidity, mortality and hospitalization rate, with heavy implications for worldwide health systems. In particular, higher incidence and mortality rates of pneumococcal community-acquired pneumonia (CAP) cases, with related costs, are registered among elderly.Citation4,Citation5 Incidence values vary among different countries and surveys, but generally mortality rate increases with age and comorbidities.Citation5-Citation9 In the future, this will lead to an inevitable increase of hospitalizations, with an economic burden that should not be underestimated.Citation1,Citation3,Citation10-Citation12
Therefore, pneumococcal vaccination is extensively recommended for subjects aged ≥65 y and for those aged 50–64 y at high risk (HR) for specific health conditions, such as diabetes, cardiovascular diseases, nephropathies, hypertension and chronic obstructive pulmonary disease (COPD). In Italy, the Ministry of Health recommends the use of conjugated 13-valent vaccine (PCV13) for children aged <2 y, while the immunization with 23-valent polysaccharide vaccine (PPV23) is recommended for adults aged ≥65 y and for HR subjects aged ≥2 y.Citation13-Citation15 However, the levels of immunization coverage among adults are so far insufficient, and probably this depends on the doubts about polysaccharide vaccine efficacy. In fact, clinical studies showed that this formulation is unable to induce an adequate and durable immune response, especially in HR individuals and against non-invasive pneumococcal diseases.Citation4,Citation16-Citation19
The 13-valent conjugate vaccine, recently indicated for the immunization of all the ages, has showed adequate safety and immunogenicity levels and seems to induce a durable protection against pneumococcal diseases, both invasive and not.Citation20 Several studies suggest that immunological properties of PCV are higher than those of PPV23, while safety and tolerability are comparable.Citation21,Citation22
This study aimed to realize an economic evaluation about the immunization with PCV13 in the adult population in Campania region, South Italy. For this purpose we performed, considering a period of 5 y, a budget impact analysis (BIA) and a cost-effectiveness analysis which considered 2 immunization targeted cohorts.
Results
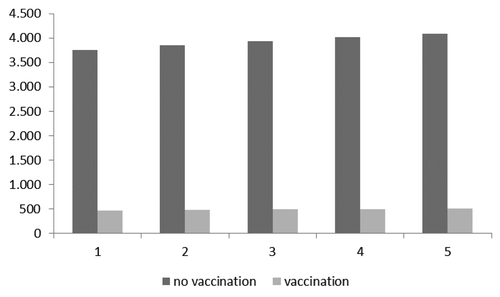
shows the pneumococcal CAP cases expected in the first group (HR subjects aged 50–79) with and without vaccination.
Figure 1. Expected cases with and without vaccination in the first targeted group (HR subjects aged 50–79).
Costs needed for the vaccination of the first targeted cohort in 5 y of follow-up are reported in . The total costs per year with or without immunization of the first cohort are reported in . During the first year, the implementation of the vaccination program requires more than 2-fold the resources needed for the treatment of pneumococcal CAP cases expected without vaccination. However, vaccination costs notably decrease even from the second year.
Table 1. Costs of vaccination program for the first targeted cohort
Table 2. Costs expected with and without vaccination of the first targeted group
The BIA for the base case scenario confirms the great initial expense and the following savings, up to €29 005 660 after 5 y ().
Table 3. Budget impact analysis (BIA) for the first targeted group
considers savings and avoided cases achievable in 5 y with vaccination. For the first targeted group, total pneumococcal CAP cases expected with a vaccination program were assumed to be 509, while those expected without vaccination were estimated to be 4083 (with a reduction of 3574 cases). Therefore, the final savings per pneumococcal CAP case is equal to €8116, what relationship between avoided costs and avoided cases.
Table 4. Avoided costs and cases for the first targeted group
The sensitivity analysis was performed by varying the effectiveness of the vaccine (+/−10%): estimated savings after 5 y could be €34 662 134 in the first case, and €23 402 745 in the worst scenario (data not shown).
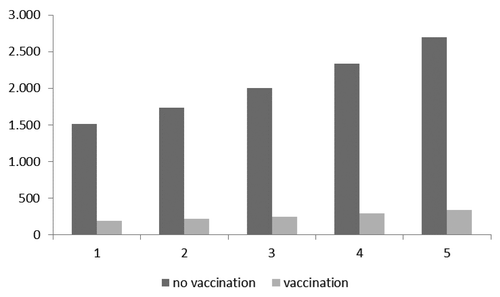
As for the second targeted group (HR subjects aged 50–64 plus 65 y-old subjects), shows the pneumococcal CAP cases expected with and without vaccination.
Figure 2. Expected cases with and without vaccination in the second targeted group (HR individuals aged 50–64 + those aged 65 y).
shows vaccination costs in 5 y. The costs per year with or without immunization of the second cohort are reported in . In this case also, at the first year the costs with vaccination seem to be higher than those for the treatment of pneumococcal CAP cases in the second scenario; however, even from the second year it’s possible to observe notable savings.
Table 5. Costs of vaccination program for the second targeted group
Table 6. Costs expected with and without vaccination for the second targeted group
BIA showed at the end of the follow-up achievable savings equal to €10 006 017 ().
Table 7. Budget impact analysis (BIA) for the second targeted group
reports savings resulting from the difference between the 2 scenarios. The number of expected cases (2694) among the second targeted cohort without vaccination could be reduced by vaccination to 337. With this strategy, the final savings per CAP case is equal to €4245.
Table 8. Avoided costs and cases for the second targeted group
Sensitivity analysis showed savings equal to €10 879 772 when considering a 10% increase in the effectiveness of the vaccine and equal to €9 132 263 with a 10% decrease (data not shown).
Discussion and Conclusions
The new paradigm of evidence based medicine for decision-making has widely grown in recent years, making available to public health care policies—and to professionals and managers as well—tools to assess the clinical and welfare rationality of public choices.
The evidence-based health care approach currently support most of the control activities and decision-makers’ choices, thus becoming a real asset in all health care organization levels: national first, and then regional.
This study represents an example of how these indicators could be useful to manage and control diseases with a high burden.
Although pneumococcal diseases are assumed to be not always correctly identified and/or notified, they represent one of the main cause of morbidity and mortality.Citation23 In order to reduce their burden, and related direct or indirect costs, adequate vaccination policies are needed.
The great number of risk factors for pneumococcal diseases and their interactions highlight the importance of immunoprophylaxis.
Therefore, contrarily to other analyses,Citation24,Citation25 focused on age-based vaccination strategies, this study considered some risk groups as targeted cohorts for adult vaccination. The inclusion of these groups followed indications of the Italian Ministry of Health, which suggests the consideration of risk conditions for pneumococcal immunization in adults.
Multimorbidity patterns were difficult to apprise, due to the absence of appropriate data collection systems; this would produce artificial estimates and information far from adhering to reality.
The economic evaluation of pneumococcal vaccine for adult groups represents an essential instrument to support health policies. In fact, due to the cost restriction, stakeholders should know the value for money of a new immunization strategy, but also its budget impact as in the short as in the long period. As described above, this impact comes from the difference between cost of vaccination program and savings achievable through the vaccine-related reduction of cases.
The pharmacoeconomics evaluation performed in this study showed that both hypothesized immunization strategies could produce savings. Results showed that the vaccination of HR subjects of 50–79 y could allow about 28 million euro of savings. However, this strategy requires a high investment in a short period. On the contrary, the second hypothesis implies less initial costs, but generates lower savings.
It has to be noted that the present analysis did not consider the serotype coverage, which could be considered a limitation of the procedure.
However, the analysis considered only direct costs using the perspective of the National Health Service. Therefore, the conservative nature of this evaluation disregards further implications that could be advantageous for the local health system.
Moreover, the analysis takes into account only the pneumococcal pneumonia cases and does not include the other vaccine-preventable pneumococcal diseases, and it referred only to hospitalized cases—however, the impact of vaccination may be wider, both clinically and economically, if non-hospitalized pneumococcal pneumonia cases are included. Furthermore, the infection in high-risk subjects is likely more severe than considered and, consequently, the economic impact of vaccination is more extensive. Lastly, vaccination advantages do not interest only immunized subjects but also the community, due to the herd immunity effects. Also regarding this aspect, the present analysis is conservative, because further savings deriving from the increase of targeted cohorts were not considered.
In the health care system, policy-makers’ choices basically depend on 2 elements which are concatenated together. On the one hand, there are the financial resources available; on the other, the epidemiological context of reference that will decree the priorities on the allocation of resources.
Findings of this study support the vaccination for HR adults, as recommended by EMA. This strategy could represent a sustainable and savings-producer health policy. Our findings could help to choice between the 2 proposed hypotheses, in order to better address economical resources.
Materials and Methods
This economic evaluation was developed to analyze for a 5-y period the impact of an adult pneumococcal vaccination program in the Campania region. The model considered 2 vaccination strategies: at first, the group of HR subjects aged 50–79 (about 1 million of individuals in the region), and later HR individuals aged 50–64, together with all those aged 65 y (about 400 000 subjects). The impact of PCV vaccination programs was compared with a no-vaccination scenario. HR subjects were considered those with chronic diseases such as diabetes, hypertension, nephropathies, COPD and cardiovascular diseases.
The analyses were performed on the resident population in Campania on January 1, 2009, as reported by the National Institute for Statistics (Istituto Nazionale di Statistica, ISTAT), without considering sex and origin.Citation25 On the base of the average national coverage for the last influenza immunization program in Italy in the elderly, vaccination coverage of the targeted cohorts was supposed to be 60%.Citation24
CAP incidence was considered equal to 3.34%.Citation23
The clinical outcome of the analysis were hospitalized pneumococcal CAP cases in adult population (50–79 y). Data were obtained from the hospital discharge forms (Scheda di Dimissione Ospedaliera, SDO) of respiratory departments which participate to the training regional network. During the years 2010–2011, 18 965 CAP cases were reported on these SDOs.Citation26 Assuming that the overall rate of CAP due to Spn is about 40%, the number of pneumococcal CAP per year among reported cases was estimated to be 3793.Citation27
On the basis of previous studies, the vaccine efficacy against pneumococcal pneumonia was assumed to be 87.5%, then this value was used to calculate the number of avoided cases for each vaccination strategy.Citation22,Citation24,Citation28
Expected cases were corrected for the global mortality rate, as obtained from 2010 ISTAT data.Citation29
The economic model was based on the difference between the costs sustained with (vaccine plus treatment for expected cases) and without (only expected cases) a vaccination program.
As regard the first vaccination strategy, all the subjects at risk aged 50–79 y were considered for immunization at first year; since the second year only 50 y old were included in the vaccination program. As for the second strategy, individuals at risk aged 50–64 y and those aged 65 y were considered for immunization at first year, while 50 y old and 65 y old were included for subsequent years.
The cost of the vaccine was 42.5 Euro per dose; the cost of a CAP case due to Spn was assumed to be the average of costs for complicated and non-complicated pneumonia cases, equal to €3809.Citation30 Costs were updated to a rate of 3%. To test the strength of results, a sensitivity analysis was applied by considering a +/− 10% variation in the vaccine efficacy.
| Abbreviations: | ||
| Spn | = | Streptococcus pneumoniae |
| CAP | = | community-acquired pneumonia |
| HR | = | high risk |
| COPD | = | chronic obstructive pulmonary disease |
| PCV13 | = | 13-valent pneumococcal conjugated vaccine |
| PPV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| FDA | = | Food and Drugs Administration |
| EMA | = | European Medicine Agency |
| BIA | = | budget impact analysis |
| ISTAT | = | National Institute for Statistics (Istituto Nazionale di Statistica) |
| SDO | = | Scheda di Dimissione Ospedaliera |
Disclosure of Potential Conflicts of Interest
One of the authors (P.B.) received a grant from Pfizer Italia to support a part-time researcher position.
References
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67:71 - 9; http://dx.doi.org/10.1136/thx.2009.129502; PMID: 20729232
- Health Protection Agency. Centre for Infections, London, UK. Invasive Pneumococcal Disease (IPD) in England & Wales after 7-valent conjugate vaccine (PCV7); potential impact of 10 and 13-valent vaccines. Available at http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1245581527892.
- Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010; 28:4955 - 60; http://dx.doi.org/10.1016/j.vaccine.2010.05.030; PMID: 20576535
- Cadeddu C, De Waure C, Gualano MR, Di Nardo F, Ricciardi W. 23-valent pneumococcal polysaccharide vaccine (PPV23) for the prevention of invasive pneumococcal diseases (IPDs) in the elderly: is it really effective?. J Prev Med Hyg 2012; 53:101 - 3; PMID: 23240169
- Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000; 30:100 - 21; http://dx.doi.org/10.1086/313608; PMID: 10619740
- Giorgi-Rossi P, Merito M, Borgia P. Cost-effectiveness of introducing the conjugated pneumococcal vaccine to routine free immunizations for infants in Lazio, Italy. Health Policy 2009; 89:225 - 38; http://dx.doi.org/10.1016/j.healthpol.2008.05.016; PMID: 18639952
- French N. Use of pneumococcal polysaccharide vaccines: no simple answers. J Infect 2003; 46:78 - 86; http://dx.doi.org/10.1053/jinf.2002.1113; PMID: 12634068
- Obert J, Burgel PR. Pneumococcal infections: association with asthma and COPD. Med Mal Infect 2012; 42:188 - 92; PMID: 22444165
- Smith SA, Poland GA. Use of influenza and pneumococcal vaccines in people with diabetes. Diabetes Care 2000; 23:95 - 108; http://dx.doi.org/10.2337/diacare.23.1.95; PMID: 10857977
- Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, Zavitkovsky A, Jackson LA. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008; 26:4947 - 54; http://dx.doi.org/10.1016/j.vaccine.2008.07.016; PMID: 18662735
- Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29:3398 - 412; http://dx.doi.org/10.1016/j.vaccine.2011.02.088; PMID: 21397721
- Icardi G, Sticchi L, Bagnasco A, Iudici R, Durando P. Pneumococcal vaccination in adults: rationale, state of the art and perspectives. J Prev Med Hyg 2012; 53:78 - 84; PMID: 23240164
- della Salute M. Piano Nazionale Prevenzione Vaccinale 2012-14. Gazzetta Ufficiale n. 47 (12 March 2012). Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf
- Ministero della Salute. Circolare n. 11 del 19 Novembre 2001. Vaccinazione antipneumococcica in età pediatrica. Available at: http://www.salute.gov.it/imgs/C_17_normativa_78_allegato.pdf
- Ministero della Salute. Circolare del 29 Luglio 2010. Circolare Prevenzione e controllo dell’influenza: raccomandazioni per la stagione 2010-2012. Available at: http://www.trovanorme.salute.gov.it/renderNormsanPdf?anno=0&codLeg=34861&parte=1%20&serie=
- Vila-Corcoles A, Ochoa-Gondar O, Guzmán JA, Rodriguez-Blanco T, Salsench E, Fuentes CM, EPIVAC Study Group. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis 2010; 10:73; http://dx.doi.org/10.1186/1471-2334-10-73; PMID: 20298596
- Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, Llor C, EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43:860 - 8; http://dx.doi.org/10.1086/507340; PMID: 16941367
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; CD000422; PMID: 18253977
- Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC, Starkovich PT, Dunstan M, Carste B, Shay DK, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007; 25:4029 - 37; http://dx.doi.org/10.1016/j.vaccine.2007.02.062; PMID: 17391816
- The Committee for Medicinal Products for Human Use. European Medicines Agency. Prevenar 13. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/001104/WC500112838.pdf.
- de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015 - 23; http://dx.doi.org/10.1086/529142; PMID: 18444818
- Schwarz TF, Flamaing J, Rümke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29:5195 - 202; http://dx.doi.org/10.1016/j.vaccine.2011.05.031; PMID: 21619909
- Viegi G, Maio S, Pistelli F, Baldacci S, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease: health effects of air pollution. Respirology 2006; 11:523 - 32; http://dx.doi.org/10.1111/j.1440-1843.2006.00886.x; PMID: 16916323
- Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother 2013; 9; http://dx.doi.org/10.4161/hv.23268; PMID: 23295824
- Popolazione residente al 1 Gennaio 2009 per età, sesso e stato civile. Demo.istat. Demografia in cifre. Istituto Nazionale di Statistica. Available at: http://demo.istat.it
- Agenzia Regionale Sanitaria. Dati relativi alle polmoniti in Campania anni 2010-2011v, codici 481-486. Available at: http://www.arsan.campania.it/
- Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012; 67:540 - 5; http://dx.doi.org/10.1136/thoraxjnl-2011-201092; PMID: 22374921
- Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21:810 - 5; http://dx.doi.org/10.1097/00006454-200209000-00005; PMID: 12352800
- Decessi e mortalità in Campania. Istituto Nazionale di Statistica. Available at: http://demo.istat.it
- Agenzia Nazionale per i Servizi Sanitari Regionali. (Age.na.s.). Ricoveri ospedalieri, i sistemi tariffari regionali vigenti nell’anno 2009. Regione Campania. Available at: http://www.agenas.it/index.htm
