 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Clinical trials of the human rotavirus vaccine Rotarix™ (RV1) have demonstrated significant reductions in severe rotavirus gastroenteritis (RVGE) in children worldwide. However, no correlate of vaccine efficacy (VE) has yet been established. This paper presents 2 analyses which aimed to investigate whether serum anti-RV IgA measured by ELISA 1 or 2 mo post-vaccination can serve as a correlate of efficacy against RVGE: (1) In a large Phase III efficacy trial (Rota-037), the Prentice criteria for surrogate endpoints was applied to anti-RV IgA seropositivity 1 mo post-vaccination. These criteria determine whether a significant vaccine group effect can be predicted from the surrogate, namely seropositivity (anti-RV IgA concentration >20 U/mL); (2) Among other GSK-sponsored RV1 VE studies, 8 studies which assessed immunogenicity at 1 or 2 mo post-vaccination in all or a sub-cohort of enrolled subjects and had at least 10 RVGE episodes were included in a meta-analysis to measure the regression between clinical VE and VE predicted from immunogenicity (VE1). In Rota-037, anti-RV IgA seropositivity post-vaccination was associated with a lower incidence of any or severe RVGE, however, the proportion of vaccine group effect explained by seropositivity was only 43.6% and 32.7% respectively. This low proportion was due to the vaccine group effect observed in seronegative subjects. In the meta-analysis, the slope of the regression between clinical VE and VE1 was statistically significant. These two independent analyses support the hypothesis that post-vaccination anti-RV IgA seropositivity (antibody concentration ≥20 U/mL) may serve as a useful correlate of efficacy in clinical trials of RV1 vaccines.
Introduction
Investigations into potential immune correlates of rotavirus (RV) protection have focused on those relating to local mucosal immune responses (secretory immunoglobulin A [IgA]) and systemic responses including total serum IgA, total serum IgG and neutralizing activity.Citation1-Citation5 Consequently, secretory IgA is currently considered to be the most acceptable laboratory parameter as a measure of immune responsiveness to RV vaccines, and is generally used as a measure of seroconversion in clinical trials.Citation1,Citation6-Citation11 Studies of natural RV infection and vaccine trials indicate an association between the detection of serum RV antibody and protection, however, no correlate of efficacy has been validated.Citation1,Citation6-Citation10
Rotarix™ (GlaxoSmithKline [GSK] Vaccines) is an attenuated human RV (RV1) vaccine, currently licensed in over 115 countries and offers protection against RV gastroenteritis (RVGE) hospitalisation and RVGE of any severity due to multiple circulating RV strains.Citation12,Citation13
This paper presents two analyses which aim to investigate the predictive value of post RV1 vaccination serum IgA concentrations from seropositive subjects (antibody concentration ≥20 U/mL) on vaccine efficacy (VE) against RV disease. In the course of the clinical development of RV1, the serum IgA assay has been modified, with limited impact on the range of concentrations below the assay cut-off. Therefore, the analyses presented in this paper were limited to data from seropositive subjects. The two analyses comprised: (1) applying the Prentice criteriaCitation14 for surrogate endpoints to anti-RV IgA antibody concentrations ≥20 U/mL one month post-vaccination in a large Phase III efficacy trial (Rota-037), (2) a meta-analysis involving 8 RV1 VE studies to measure the correlation between clinical VE and VE predicted from immunogenicity (VEI) (i.e., 1 minus the relative risk of the seronegativity rate).
Results
1. Prentice criteria for surrogate endpoints in study Rota-037
In study Rota-037, subjects were allocated to a 3 dose RV1 vaccine group, a 2 dose RV1 vaccine/1 dose placebo group and a 3 dose placebo group according to a 1:1:1 randomization ratio. The study enrolled 4939 subjects, of which 4417 subjects comprised the efficacy according-to-protocol (ATP) cohort. Immunogenicity data one month post-dose 3 were missing for 317 subjects, thus 4100 subjects were included in the Prentice criteria analysis.
Since a correlate is expected to be independent of the number of doses and since the primary study objective was to show efficacy of the RV1 vaccine pooled group as compared with placebo, the Prentice criteria analysis was applied regardless of the number of RV1 vaccine doses i.e., using a pooled RV1 vaccine group and the placebo group.
The distribution of anti-RV-IgA antibody concentration one month post-vaccination for the 4100 subjects is summarized by group in . The table presents also the total number and percentage of subjects reporting RVGE (any and severe) after the blood sample. The results suggest that:
Table 1. Rota-037; number and percentage of subjects with any RVGE/severe RVGE by rotavirus IgA antibody concentration
In the RV1 vaccine group, anti-RV-IgA antibody concentrations ≥20 U/mL appear to be associated with a reduced risk of both any and severe RVGE.
In the placebo group, anti-RV-IgA antibody concentrations above 50 U/mL appear to be associated with a reduced risk of both any and severe RVGE. However the number of subjects with an anti-RV-IgA antibody concentration between [20–50] U/mL was limited to 52 (4%) subjects.
Anti-RV-IgA concentrations above 50 U/mL were associated with the same level of protection in both the RV1 vaccine and placebo groups; a lower RVGE incidence of both any and severe episode(s) was observed in both the RV1 vaccine and placebo groups.
These observations are formalized using the Prentice criteria described in the method section and applied to the study as shown in . Model 1 shows the statistical significance of the vaccine group effect; model 2 shows the statistical significance of seropositivity effect. Although the vaccine group effect in model 1 and seropositivity effect in model 2 are both associated with a P value below 0.0001, the seropositivity rate is more significant as shown by the larger log-likelihood ratio test. Model 3 shows the vaccine group effect for subjects when adjusting for the seropositivity status, and the seropositivity effect when adjusting for the vaccine group effect. The seropositivity effect appears to be stronger than the vaccine group effect, but this does not capture the full vaccine group effect. The proportion of vaccine group effect explained by seropositivity accounts for 43.6% and 32.7% of the vaccine group effect on any RVGE and severe RVGE, respectively. This low proportion of explained vaccine group effect is mainly due to the lower proportion of subjects reporting RVGE in the vaccine group as compared with the placebo group among seronegative subjects (). Accordingly, in this analysis seropositivity alone does not fully capture the vaccine group effect.
Table 2. Prentice criteria applied to Rota-037 study
Meta-analysis to assess the correlation between immunogenicity and clinical efficacy
presents the scatter plots of the observed relative risk of reporting RVGE (any and severe) in the vaccine group as compared with the placebo group (i.e., 1−VE) vs. the observed relative risk of the seronegativity rate (i.e., 1−VEI). Each dot represents a study with a horizontal line showing the 95% confidence interval (CI) around 1−VEI and a vertical line showing the 95% CI around 1−VE. The estimated regression line is shown as a black line (———) with numerical expression annotated.
Figure 1. Meta-analysis: Scatter plots of the relative risk (RR) with 95% CIs for efficacy and immunogenicity by study. Scatter plots between the relative risk of reporting RVGE ( = 1−VE) and the relative risk of seronegative concentration one month post-vaccination ( = 1−VEI).
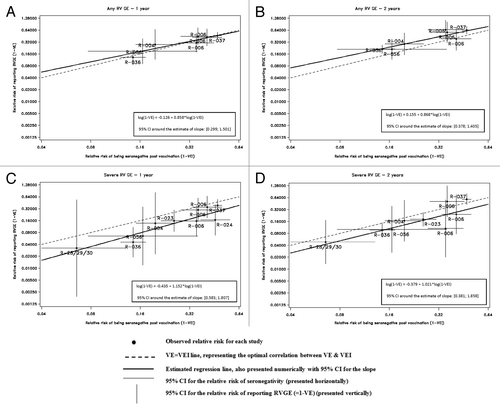
In the absence of a predictive value of VEI for VE one would expect a horizontal estimated line. However, over 1 y follow up of any RVGE, the estimated regression line presents a slope that is statistically significantly different from 0 indicating a relationship between VE predicted from the seropositivity rate and clinical VE (). The regression line is generally above the dash line (– – – – –) which represents an exact fit between VEI and VE (i.e., X = Y). This suggests that VEI may be an overestimation of the true efficacy against any RVGE over 1 y follow up. With respect to assessment of the predictive value of VEI for clinical VE against any RVGE reported within 2 y following vaccination, the separation of the regression line (VEI) above the dash line is greater than over 1 y indicating a larger over-estimation of the true VE against any RVGE (; Table S1).
The regression analyses associated with severe RVGE are presented in over a 1 y and 2 y follow-up, respectively. Again the estimated regression lines present a slope that is statistically significantly different from 0 indicating a relationship between VE predicted from the seropositivity rate and clinical VE. However, in this case the estimated regression line is below the dash line which represents an exact fit between VEI and VE. This suggests that VEI may be an under-estimation of the true efficacy against severe RVGE. The separation of the regression line (VEI) below the dash line is greater over 1 y than over 2 y indicating a larger under-estimation of the true VE against severe RVGE.
Sensitivity analysis excluding Rota-037 provided similar findings (data not shown).
A summary of results for VE and immunogenicity by study is provided in Table S1.
Discussion
Clinical trials powered according to clinical efficacy endpoints are generally resource intensive, requiring large, long-term studies. A validated laboratory marker of clinical protection would provide a robust basis for extrapolation of clinical efficacy data derived in specific settings to other regions or populations. Clinical efficacy trials of the RV1 vaccine conducted in diverse settings have shown that vaccination significantly reduces the occurrence of RVGE caused by multiple circulating RV strains.Citation15 However, no immunological parameter has thus far been validated as a surrogate for efficacy. Two complementary analyses were performed to assess whether post-vaccination anti-RV serum IgA seropositivity can serve as a surrogate for vaccine efficacy.
In the first analysis, the Prentice criteria for surrogate endpoints and the proportion of vaccine group effect explained when applied to a Phase III efficacy trial (Rota-037) showed statistical evidence that seropositivity was associated with greater efficacy compared with seronegativity (as per the observed statistical significance of γ in model 3). However, it accounted for only 43.6% and 32.7% of the vaccine group effect on any RVGE and on severe RVGE respectively. Subjects enrolled to the Rota-037 study conducted in Malawi and South Africa had a relatively low seropositivity rate post RV1 vaccination among the studies in the RV1 clinical development plan, representing 64% of vaccinated subjects compared with other studies (Table S1B). Therefore if the vaccine has a protective effect in seronegative subjects, the study represents a worst case scenario to assess the proportion of vaccine efficacy correlated with seropositivity rate. Indeed, a protective vaccine group effect in as much as 37% of vaccinated subjects could not be correlated with seropositivity due to the limitation of the assay to quantify immune responses below 20 U/mL. In a study presenting higher seropositivity rates post-vaccination, a higher proportion of vaccine group effect would be expected. Although such analyses could have been conducted in other studies, these would have been less powered compared with Rota-037, which involved the largest number of subjects with available seropositivity data post-vaccination.
The meta-analysis has the advantage to cover an extensive population in geographically diverse settings with different RV strain genotypes and length of follow-up.Citation16 To ensure the results were independent from the Rota-037 data used in the first analysis, a sensitivity analysis was performed excluding the study. This analysis provided further support to the analysis in Rota-037 whereby immunogenicity was shown to correlate with clinical efficacy. Similar results were obtained, as indicated by the slope of the estimated regression line being statistically significantly different from 0 indicating a relationship between VE predicted from the post-vaccination seropositivity rate and clinical VE. However, VEI likely overestimates the true efficacy against any RVGE, more so in the second compared with the first year of life, and underestimates the true efficacy against severe RVGE, more so in the first compared with the second year of life. An important assumption made in the meta-analysis was to consider that the immunogenicity sub-cohort used to assess VEI was representative of the efficacy cohort used to assess VE. In some studies, such as Rota-036, the immunogenicity sub-cohort represented less than 50% of the efficacy cohort. To ensure that the meta-analysis results were not biased by this assumption, clinical efficacy limited to the immunological sub-cohort was recomputed for these studies. Although the fact that there is no evidence of a difference is not proof of comparability, it was reassuring to observe no evidence of a difference in VE for subjects included in the immunogenicity sub-cohort and for subjects not included in the immunogenicity sub-cohort.
In clinical trials of candidate RV vaccines, correlations between RV antibody titers and protection have been inconsistent.Citation6,Citation8,Citation15 The results of the analyses presented here support the hypothesis that serum anti-RV IgA antibodies correlate with efficacy and are very likely an important factor in the host defense mechanism, although probably only one of several effectors of protection.Citation9 Similar findings have recently been reported from an extensive review of available RV1 peer-reviewed articles and reports.Citation10
In conclusion, two different analyses indicate that post-vaccination anti-RV IgA seropositivity (i.e., antibody concentration ≥20 U/mL) may serve as a useful correlate of efficacy in clinical trials of human RV vaccines.
Methods
Efficacy surveillance
All RV1 efficacy studies were randomized, double-blind, placebo controlled, Phase II/III, and compared 2 or 3 doses of RV1 vaccine to placebo with respect to the occurrence of RVGE (Table S2).Citation12,Citation17-Citation23
In each study, healthy infants aged 6 to 14 weeks at first vaccination were enrolled. Study methodologies were consistent in terms of data collection, active surveillance for GE episodes and the collection and processing of samples: RVGE surveillance started either from first dose of study vaccine or 2 weeks after the last study vaccine dose. Surveillance ended when the subject was approximately 1 or 2 y of age dependent on the study (Table S2). The definitions of a GE episode and the procedures for data and biological sample collection have been described.Citation12,Citation24-Citation27 Clinical severity was assessed according to the 20-point Vesikari scale (<7 = mild, 7–10 = moderate, ≥11 = severe).Citation28 In studies Rota-023, Rota-024, and Rota-028/029/030, only severe RVGE was assessed;Citation12 in all other studies all severities of GE were recorded.
Analyses of stool samples collected during GE episodes were performed using validated procedures in a GSK laboratory or GSK validated laboratory. The presence of RV antigen was assessed using ELISA, either Rotaclone™, or an assay developed at the Cincinnati Children’s Hospital previously used in RV vaccine trials.Citation29,Citation30
Humoral response
In each RV1 efficacy study, post-vaccination blood samples were collected 1–2 mo after the last vaccination in all subjects or a sub-cohort of subjects. In the latter case, the study randomization was stratified for the sub-cohort and enrolment into the sub-cohort was contingent on obtaining informed consent and targeted sample size. Blood samples were tested for anti-RV IgA antibody using an ELISA assay with a cut-off of 20 U/mL.Citation31 Seropositivity was defined as anti-RV-IgA concentration ≥ 20U/mL.
Statistical methods
Two statistical methods were used to investigate whether seropositivity measured one or two months post-vaccination can serve as a correlate of efficacy against RVGE:
Prentice criteria for surrogate endpoints in study Rota-037
The Prentice criteria were used in the Rota-037 study to determine whether anti-RV IgA seropositivity one month post-vaccination can serve as a surrogate (i.e., predictor) of the occurrence of RVGE.Citation14 The 4 Prentice criteria to establish a surrogate endpoint are:
The vaccine group effect must be significantly associated with the occurrence of RVGE. This objective was achieved in Rota-037 studyCitation17 and is presented below under model 1.
The vaccine group effect must be significantly associated with the surrogate i.e., anti-RV IgA seropositivity one month post-vaccination. This was achieved in the Rota-037 study.Citation17
The surrogate effect must be significantly associated with the occurrence of RVGE. This is presented below under model 2.
The surrogate effect on the occurrence of RVGE must capture the vaccine group effect. This is presented below under model 3.
Results from criteria (1), (3), and (4) are presented in this paper using a logistic model for p, the probability of reporting RVGE after the one month post-vaccination blood sample: (model 1) Logit(p) = α + β × G; (model 2) Logit(p) = α + γ × C; (model 3) Logit(p) = α + β × G + γ × C; with G = 1 for subjects receiving the vaccine and 0 for subjects receiving placebo, with C = 1 for seropositive subjects and 0 for seronegative subjects.
Note that the fourth criterion requires that the vaccine group effect significance disappears in model 3 when the surrogate is accounted. However, this criterion is acknowledged as being too stringent and is unlikely to be satisfied completely. In practice, it is expected that a surrogate endpoint may explain part but not all the treatment effect.Citation32 As a result the proportion of explained vaccine group effect by the surrogate as proposed by Lin et al.Citation32 is also computed as
These models were based on all subjects with anti-RV IgA results available one month post-vaccination and who belonged to the ATP cohort of efficacy, defined as all RV naive subjects with follow up beyond 2 weeks post final vaccination.
Meta-analysis to assess the correlation between immunogenicity and clinical efficacy
All GSK sponsored RV1 efficacy studies reported before 01 Dec 2011, for which immunogenicity at one or two months post-vaccination were available, were a priori eligible for this meta-analysis (see Table S2 for the list of eligible studies). However, out of these, Rota-007 was excluded due to a low number of RVGEs. Therefore 8 studies contributed to the meta-analysis. Note that 3 of these studies, Rota-023, Rota-024, and Rota-028/029/030 only recorded severe RVGE. Therefore these studies were not included in the meta-analysis of any RVGE.
If seropositivity at one/two months post-vaccination is indicative of protection, one would expect that VE inferred from immunogenicity (VEI) would be correlated with clinical VE. In each study clinical VE and VEI were estimated as:
where P1 and P0 are the proportion of subjects reporting RVGE in the vaccine group and the placebo group, respectively. This was based on the ATP cohort for efficacy as defined for Rota-037.
where S+1 and S+0 are the proportion of seropositive subjects in the vaccine group and the placebo group, respectively. This was based on the ATP cohort for immunogenicity defined as all subjects who complied with the vaccination and blood sampling schedule, for whom immunogenicity data were available, and who had no RV other than vaccine strain in stool samples.
The 95% CI for VE and VEI were based on exact Poisson rate ratio between groups.Citation33The predicted value of VEIi for the clinical VEi in study 1 was assessed using the following regression: log(1−VEi) = μ + ω × log(1−VEIi) + εI, with εi being a random error normally distributed. The log transformation was used to normalize the distribution.Citation34
To account for the known variability of VE and VEI from each study, the intercept (μ) and the slope (ω) of the regression were estimated with 95% CI using imputation techniques. Mathematical details are available in the Supplemental Material.
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| ELISA | = | enzyme-linked immunosorbent assay |
| GE | = | gastroenteritis |
| RV | = | rotavirus |
| RVGE | = | rotavirus gastroenteritis |
| VE | = | vaccine efficacy |
Additional material
Download Zip (282.4 KB)Disclosure of Potential Conflicts of Interest
B.C., N.K., H.H.H., and C.V. are employed by the GlaxoSmithKline group of companies. H.H.H. and C.V. also hold stock ownership from the sponsoring company; K.M.N. declares that institutional grants were obtained from GAVI for conducting studies on Rotavirus; A.D.S. has no conflict of interest to be declared; N.C. declares to have received grants from the GlaxoSmithKline group of companies to conduct clinical trial and is board member of GSK and SPMSD UK Rotavirus Vaccine advisory boards; S.M. declares to have received consulting fee or honorarium, support for travel to meetings for the study or other purposes and outside the scope of this submitted work S.M. has done consultancy and received payment for lectures including service on speakers bureaus for various pharmaceutical companies and his institution has received grants to conduct clinical trials.
Funding
GlaxoSmithKline Biologicals SA funded all costs associated with the development and the publishing of the present manuscript.
Acknowledgments
The authors thank Dr Richard L Ward (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) for critical review of this paper; Dr Sarah Benns (Independent Medical Writer, United Kingdom) for assistance in preparation of the manuscript and Manjula K for editorial assistance and manuscript coordination (GlaxoSmithKline Vaccines).
Trademarks
Rotarix is a registered trademark of the GlaxoSmithKline group of companies.
References
- Velázquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, Glass RI, Pickering LK, Ruiz-Palacios GM. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 2000; 182:1602 - 9; http://dx.doi.org/10.1086/317619; PMID: 11069230
- Chiba S, Yokoyama T, Nakata S, Morita Y, Urasawa T, Taniguchi K, Urasawa S, Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet 1986; 2:417 - 21; http://dx.doi.org/10.1016/S0140-6736(86)92133-1; PMID: 2874413
- Hjelt K, Grauballe PC, Paerregaard A, Nielsen OH, Krasilnikoff PA. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol 1987; 21:39 - 47; http://dx.doi.org/10.1002/jmv.1890210106; PMID: 3025356
- Matson DO. Protective immunity against group A rotavirus infection and illness in infants. Arch Virol Suppl 1996; 12:129 - 39; PMID: 9015110
- O’Ryan ML, Matson DO, Estes MK, Pickering LK. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis 1994; 169:504 - 11; http://dx.doi.org/10.1093/infdis/169.3.504; PMID: 8158022
- Wood D, WHO Informal Consultative Group. WHO informal consultation on quality, safety and efficacy specifications for live attenuated rotavirus vaccines Mexico City, Mexico, 8-9 February 2005. Vaccine 2005; 23:5478 - 87; http://dx.doi.org/10.1016/j.vaccine.2005.07.035; PMID: 16129525
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337 - 46; http://dx.doi.org/10.1056/NEJMoa1006261; PMID: 21793745
- Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol 2012; 2:419 - 25; http://dx.doi.org/10.1016/j.coviro.2012.05.003; PMID: 22677178
- Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis 2002; 34:1351 - 61; http://dx.doi.org/10.1086/340103; PMID: 11981731
- Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284 - 94; http://dx.doi.org/10.1093/infdis/jit166; PMID: 23596320
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine 2006; 24:2718 - 31; http://dx.doi.org/10.1016/j.vaccine.2005.12.048; PMID: 16446014
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, da Silva VB, Mascarenhas JD, Noronha VL, Clemens R, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. J Pediatr (Rio J) 2007; 83:217 - 24; http://dx.doi.org/10.2223/JPED.1600; PMID: 17380232
- Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431 - 40; http://dx.doi.org/10.1002/sim.4780080407; PMID: 2727467
- De Vos B, Han HH, Bouckenooghe A, Debrus S, Gillard P, Ward R, Cheuvart B. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J 2009; 28:261 - 6; http://dx.doi.org/10.1097/INF.0b013e3181907177; PMID: 19289978
- Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J 2001; 20:63 - 75; http://dx.doi.org/10.1097/00006454-200101000-00013; PMID: 11176570
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289 - 98; http://dx.doi.org/10.1056/NEJMoa0904797; PMID: 20107214
- Zeng SQ, Halkosalo A, Salminen M, Szakal ED, Karvonen A, Vesikari T. Norovirus gastroenteritis in young children receiving human rotavirus vaccine. Scand J Infect Dis 2010; 42:540 - 4; http://dx.doi.org/10.3109/00365541003652556; PMID: 20524782
- Linhares AC, Verstraeten T, Wolleswinkel-van den Bosch J, Clemens R, Breuer T. Rotavirus serotype G9 is associated with more-severe disease in Latin America. Clin Infect Dis 2006; 43:312 - 4; http://dx.doi.org/10.1086/505493; PMID: 16804845
- Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Da Silveira TR, Rivera L, Rivera Medina DM, Saez-Llorens X, Gonzalez Ayala SE, De León T, et al, Rota-024 Study Group. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 2011; 30:e103 - 8; PMID: 21378594
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, Garcia-Corbeira P, Damaso S, Han HH, Bouckenooghe A. Immunogenicity and safety of the human rotavirus vaccine Rotarix co-administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine 2010; 28:5272 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.05.057; PMID: 20538094
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, Muto H, Smolenov I, Suryakiran PV, Han HH. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.05.017; PMID: 21640780
- Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, De Vos B. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J 2004; 23:937 - 43; http://dx.doi.org/10.1097/01.inf.0000141722.10130.50; PMID: 15602194
- Salinas B, Pérez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzábal JP, Cervantes Y, Costa Clemens S, Damaso S, Hardt K, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24:807 - 16; http://dx.doi.org/10.1097/01.inf.0000178294.13954.a1; PMID: 16148848
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore 2006; 35:38 - 44; PMID: 16470273
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259 - 67; http://dx.doi.org/10.3109/00365549009027046; PMID: 2371542
- Gilchrist MJ, Bretl TS, Moultney K, Knowlton DR, Ward RL. Comparison of seven kits for detection of rotavirus in fecal specimens with a sensitive, specific enzyme immunoassay. Diagn Microbiol Infect Dis 1987; 8:221 - 8; http://dx.doi.org/10.1016/0732-8893(87)90053-8; PMID: 2835201
- Ward RL, Bernstein DI, Young EC, Sherwood JR, Knowlton DR, Schiff GM. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis 1986; 154:871 - 80; http://dx.doi.org/10.1093/infdis/154.5.871; PMID: 3021869
- Ward RL, Bernstein DI, Shukla R, Young EC, Sherwood JR, McNeal MM, Walker MC, Schiff GM. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis 1989; 159:79 - 88; http://dx.doi.org/10.1093/infdis/159.1.79; PMID: 2535868
- Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997; 16:1515 - 27; http://dx.doi.org/10.1002/(SICI)1097-0258(19970715)16:13<1515::AID-SIM572>3.0.CO;2-1; PMID: 9249922
- Tang ML, Ng HK. Comment on: confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med 2004; 23:685 - 90, author reply 891-2; http://dx.doi.org/10.1002/sim.1683; PMID: 14755397
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41:55 - 68; http://dx.doi.org/10.2307/2530643; PMID: 4005387
