Abstract
This commentary reviews recent changes in Centers for Disease Control (CDC) vaccine storage guidelines that were developed in response to an investigative report by the Office of the Inspector General. The use of temperature data loggers with probes residing in glycol vials is advised along with storing vaccines in pharmaceutical refrigerators. These refrigerators provide good thermal distribution but can warm to 8 °C in less than one hour after the power is discontinued. Consequently, electric grid instability influences appropriate refrigerator selection and the need for power back-up. System Average Interruption Duration Index (SAIDI) values quantify this instability and can be used to formulate region-specific guidelines. A novel aftermarket refrigerator regulator with a battery back-up power supply and microprocessor control system is also described.
On June 5, 2012, The Office of the Inspector General (OIG) released a report criticizing Centers for Disease Control (CDC) management of the Vaccines for Children (VFC) program. The OIG sampled VFC providers nation-wide. Twenty-eight percent failed to rotate stock and stored expired vaccines with non-expired vaccines. Seventy-six percent exposed vaccines to improper temperatures for greater than 5 cumulative hours over a 2 wk study period.Citation1 The extent of cold chain failure was greater than levels documented in a prior data-logger study of county clinics in Houston.Citation2
In response to the OIG report, the CDC issued new guidelines for vaccine storage. Current recommendations include 24-h digital data logging of temperature using a glycol-encased probe. Pharmaceutical refrigerators are preferred over the household variety. All units should be separate stand-alone refrigerators and freezers. The use of combination refrigerator/freezer units is discouraged due to venting of cold freezer air into the refrigerated section. If the combination units are to be used at all, vaccines are not to be placed near the vents in the refrigerator compartment and a separate dedicated freezer should be used to store frozen vaccines.Citation3
The new CDC guidelines are based on a National Institute of Standards and Technology Interagency Report (NISTIR) comparing household refrigerator/freezers to pharmaceutical grade refrigerators. Pharmaceutical units demonstrated superior thermal distribution and stability. However, their performance during power outages was surprisingly poor. Pharmaceutical refrigerators, especially those with glass doors, rapidly reached an internal temperature of 8 °C in 45 to 140 min while household units were able to maintain temperatures for 60 to 240 min. The NISTIR stated that vaccines were likely to be rendered unusable in just an hour or two after a power outage.Citation4
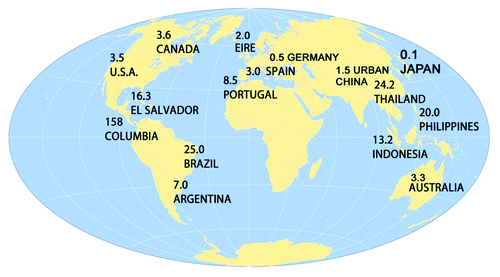
The System Average Interruption Duration Index (SAIDI) is an electrical grid reliability measure equal to the average annual outage duration in hours for each customer served. SAIDI values for selected countries are listed in .Citation5,Citation6
The US has an average of 1.5 power outages per year each lasting 3.5 h—a very high value for an industrialized nation. This power outage duration greatly exceeds the time needed for a pharmaceutical refrigerator to heat up and spoil vaccines.
Australia, a country with an electrical grid slightly better than the US, is promoting the adoption of pharmaceutical grade refrigerators.Citation7 Clinics that have replaced household refrigerators with these units are experiencing difficulties. A study by D’Onise measured the impact of this policy. Pharmaceutical refrigerators increased the risk of cold chain failure due to heating by a factor of three and were 15 times more likely than household units to fail during a power outage. Since pharmaceutical refrigerators hold a greater volume of vaccine stock, their average financial cost of loss was approximately double that of household refrigerators.Citation8 If the US implements a similar policy, we should anticipate similar poor outcomes.
The latest version of the CDC Vaccine Tool Kit mentions that automated backup electrical generators should be considered in the event of anticipated power outages.Citation9 The cost of purchasing and installing a modestly priced electrical generator powered by natural gas is approximately $7000.Citation10 Consequently, mandating the use of pharmaceutical grade refrigerators will increase total refrigeration costs since a back–up power generator will be required to compensate for electrical grid insufficiencies.
In an attempt to find a less expensive solution to these vaccine refrigerator problems, I met with Dr Marie Odin, a Professor in Biomedical Engineering at Rice University. She secured funding for laboratory space from ConocoPhillips and introduced me to Andres Martin-de-Nicolas, an electrical engineering/computer science major.
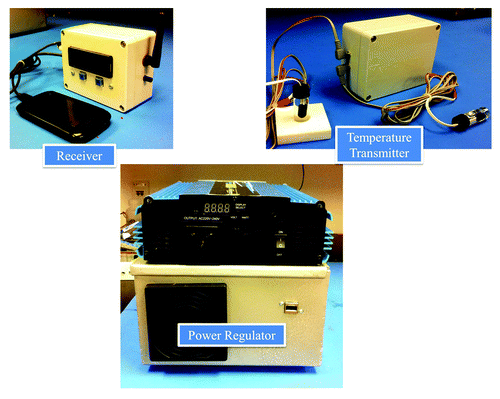
We have developed a low cost after-market regulator that integrates an inverter, battery charger and transfer switch into one system to provide back-up battery power in the event of an electrical outage. Installation and configuration require no technical skills. The device plugs into the refrigerator, a deep-cycle battery and an existing 220/120 volt electrical outlet. The total component cost of the prototype in is under $500.
This device utilizes a set of NIST-traceable thermistor probes encased in vials of glycol to model the thermal mass of individual vaccine vials. These sensors are distributed within the refrigerator to account for compartmental temperature variance. Sensor data are wirelessly transmitted and logged to a USB flash drive. Power outages or improper storage temperatures activate an alarm buzzer and release a programmed SMS text message alert to the vaccine manager.
This apparatus also has the ability to over-ride and replace the native thermostat of a wide variety of stand-alone and combinational fridge/freezer units. During an initial calibration period, profiling software pairs the refrigeration equipment with the device. A thermostat program then takes control of maintaining the 2–8 °C environment. In the event that the profiler detects a particular refrigerator is incapable of safely storing vaccines, the user is advised that the unit is not recommended for vaccine storage.
Laboratory test results with a 160 A-hour deep cycle battery (C and D Technologies UPS 12–615 MRXF) resulted in the following back-up power durations: 18 h for a 28 cu. ft. refrigerator/freezer, 30 h for an 18 cu. ft. refrigerator/freezer and greater than 60 h for a 7 cu. ft. chest freezer utilized as a refrigerator. We hope to partner with a manufacturer and conduct field-testing during 2014.
In summary, electrical grid stability must be considered when formulating vaccine refrigeration policies. Pharmaceutical grade refrigerators should only be used in areas with SAIDI values of less than 1 h or in concert with back-up power. In areas with SAIDI values less than 2 h, a well-insulated stand-alone household refrigerator demonstrating uniform thermal distribution may be a good choice. If the SAIDI is greater than 2 h, a battery back- up or a permanently installed generator should be considered. For remote regions with SAIDI values greater than 60, solar powered refrigerators would be a sensible choice.
Disclosure of Potential Conflicts of Interest
The authors invented a vaccine refrigeration device described in the article that is licensed by Baylor College of Medicine.
References
- Office of the Inspector General. Department of Health and Human Services. Vaccines for Children Program: Vulnerabilities in Vaccine Management. June 2012. Available from: http://oig.hhs.gov/oei/reports/oei-04-10-00430.pdf
- McColloster P, Vallbona C. Graphic-output temperature data loggers for monitoring vaccine refrigeration: implications for pertussis. Am J Public Health 2011; 101:46 - 7; http://dx.doi.org/10.2105/AJPH.2009.179853; PMID: 21088272
- Centers for Disease Control.Vaccine storage and handling interim guidance. October 2012. Available from: http://www.cdc.gov/vaccines/recs/storage/interim-storage-handling.pdf
- Chojnacky M, Miller W, Strouse G. Thermal analysis of refrigeration systems used for vaccine storage. National Institute of Standards and Technology Report NISTIR 7753. September 2010. Available from: http://www.nist.gov/pml/div685/grp01/upload/NISTIR7753-Thermal-Analysis-of-a-Dual-zone-Refrigerator-and-Pharmaceutical-Refrigerator-for-Vaccine-Storage.pdf
- Microgrids and distributed energy resource management. Saviva research review. April, 2013. Available from: http://www.savivaresearch.com/wp-content/uploads/2013/05/April-2013-DERMS.pdf
- Moreira C. Mind the physical infrastructure, says Uptime Institute. Enterprise IT News. December 5, 2012. Available from: http://www.enterpriseitnews.com.my/coverage/item/1752-mind-the-physical-infrastructure-says-uptime-institute.html
- Australian Government Department of Health and Ageing. Strive for five: national vaccine storage guidelines, 2nd edition. 2013.
- D’Onise K, Almond S, MacDonald B, Watson M, Scrimgeour S. Have purpose-built vaccine refrigerators reduced the cost of vaccine losses in South Australia?. Aust N Z J Public Health 2012; 36:572 - 6; http://dx.doi.org/10.1111/j.1753-6405.2012.00932.x; PMID: 23216500
- Centers for Disease Control. Vaccine Storage &Handling TOOLKIT. 2012. Available from http://www.cdc.gov/vaccines/recs/storage/toolkit/storage-handling-toolkit.pdf
- Perratore E. Generator Buying guide. Consumer Rep 2013. Available from: http://www.consumerreports.org/cro/generators/buying-guide.htm