Abstract
Salmonella enterica serovar Paratyphi A (S. Paratyphi A) is a human restricted pathogen that can cause systemic infection (paratyphoid fever) with recently increased incidence particularly in developing countries. Currently there is no licensed vaccine for prevention of infection from S. Paratyphi A. In this study the O-specific polysaccharide (OSP) of S. Paratyphi A was conjugated to diphtheria toxoid (DT) with and without adipic acid dihydrazide (ADH) as a linker. Binding of the OSP to a carrier protein was intended to convert a T-cell independent OSP response to a T-cell dependent response inducing higher levels of anti-OSP antibodies and immunological memory. These conjugates (OSP-AH-DT and OSP-DT) were evaluated for their immunogenicity in mice. The S. Paratyphi A OSP-DT conjugate induced a poor anti-OSP response less than that observed with LPS while the OSP-AH-DT conjugate induced a significantly higher antibody titer compared with LPS alone. The study also demonstrated diphtheria toxoid as a potential carrier protein for conjugate vaccine candidates using S. Paratyphi A OSP.
Introduction
Paratyphoid fever caused by Salmonella enterica serovar Paratyphi A (S. Paratyphi A) is a human enteric infection which can lead to bacteremia and enteric fever. Generally S. Typhi is believed to be the major cause of most episodes of the enteric fever, but several recent reports have shown an increasing prevalence of S. Paratyphi A infections resulting in a disease of major public health concern particularly in Asia.Citation1 Vaccination against S. Typhi has resulted in sharp decline of enteric fever in many areas of the world exposing an increase in paratyphoid episodes.Citation2 In spite of the increase in the number of enteric fever episodes caused by S. Paratyphi A, the paratyphoid vaccine development is lagging behindCitation3 and currently no vaccine against S. Paratyphi A is available.Citation4
Surface polysaccharides of many bacteria whether they are capsular polysaccharides or associated with the LPS in the case of gram-negative enteric bacteria are usually protective antigens.Citation5 The OSP purified from the LPS is poorly immunogenic and behaves as a T-cell independent antigen.Citation6 T-cell independent responses are characterized by low levels of anti-OSP antibodies and a lack of induction of memory cells and a failure to boost on subsequent doses.Citation6
Conjugation of a polysaccharide antigen to a carrier protein results in a T-cell dependent immune response characterized by higher titers of anti-polysaccharide antibodies having bactericidal activity and induction of memory cells.Citation7 This conjugation can be achieved by direct linkage of OSP with carrier protein or using an intermediate linker molecule such as adipic acid dihydrazide (ADH). S. Paratyphi A OSP-protein conjugates were previously shown to be safe and to elicit anti-OSP IgG antibodies in all age groups.Citation8 The currently licensed polysaccharide-protein conjugate vaccines include Hemophilus influenzae type b, Neisseria meningitidis, and Streptococcus pneumoniae while various conjugate vaccines candidates against E. coli, Shigella, Salmonella, Pseudomonas, and many other bacteria are at different stages of clinical trials.Citation9,Citation10
In this study we used direct and a linker molecule to conjugate OSP of S. Paratyphi A to diphtheria toxoid (DT) followed by an evaluation of the immunogenicity of the conjugates in mice. The use of DT as carrier protein in developing conjugate vaccine candidates against S. Typhi has been reported earlierCitation11 but to the best of our knowledge this is the first report of conjugating DT with S. Paratyphi A OSP.
Results
S. Paratyphi A OSP antigen preparation
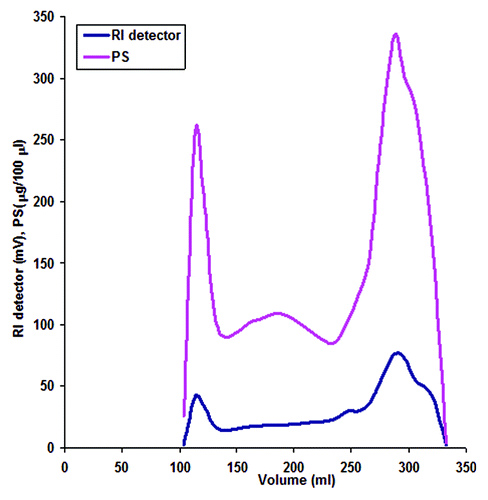
Growth of S. Paratyphi A and LPS extraction resulted in LPS yield as 29 mg per liter of culture. Results of silver staining of S. Paratyphi A LPS are shown in . After acid hydrolysis, Sephadex G-75 column fractions of the crude S. Paratyphi A OSP resulted in high (101 ml to 257 ml) and low (258 to 350 ml) molecular weight fractions based on Anthrone assay and refractive index (RI) detector (). Immunodiffusion assay of fractions showed that the high molecular weight portion of S. Paratyphi A OSP reacted with the anti LPS serum and was selected for preparation of conjugates. Nucleic acid and protein contamination in purified LPS and OSP were found to be less than 4% and 1% respectively. The LPS had >3000 endotoxin units (EU) per mg and the OSP less than 3 EU per mg which is acceptable according to WHO standards.Citation12 Derivatization of the OSP resulted in 0.87% ADH content in S. Paratyphi A OSP-AH. The O-acetyl content as measured by Hestrin assay was found to be 70 nmol/ml in the OSP and 16 nmol/ml in the OSP-AH.
Figure 1. Silver staining of S. Paratyphi A LPS on SDS PAGE. Lane 1: BanchMark prestained ladder (Invitrogen Cat# 10748–010). Lanes 2 and 3: S. Paratyphi A OSP 20 μg and 10 μg respectively. Lanes 4–7: Different amounts of S. Paratyphi A LPS in μg 0.5, 1, 2.5, and 5 respectively.

Figure 2. Sephadex G-75 profiles of S. Paratyphi A OSP. Elution volume was plotted against refractive index (RI) detector readings and polysaccharides (PS) concentrations as measured by Anthrone assay. The fractions from 101 ml to 257 ml were pooled together as higher molecular weight antigenic part of S. Paratyphi A OSP.
S. Paratyphi A OSP-AH-DT conjugate
The OSP and DT yields in the final OSP-AH-DT conjugate (compared with the starting OSP and DT added to the conjugation reaction) were 43.8% and 27.3% respectively and the OSP/DT ratio was found to be 1.6.
S. Paratyphi A OSP-DT conjugate
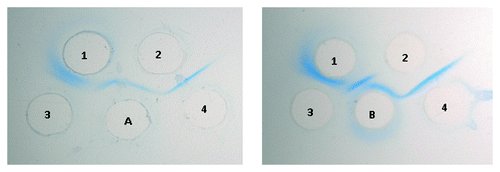
The OSP conjugated directly to DT without linker yielded higher recoveries of both OSP and DT compared with the conjugate generated with the linker. The OSP and DT yields in the final OSP-DT conjugate were 80.2% and 41.4% for polysaccharide and protein respectively and the OSP/DT ratio was 1.94. The results of immuno-diffusion assays for both conjugates are shown in .
Figure 3. Double Immunodiffusion of conjugates against anti-OSP and anti-DT sera. Wells A and B: S. Paratyphi A OSP-AH-DT conjugate and S. Paratyphi A OSP-DT conjugate (100 μg each) respectively. Wells 1 and 2: Anti-OSP and anti-DT (15 μl each) respectively. Wells 3 and 4: S. Paratyphi A OSP and DT (100 μg each) respectively.
Immunogenicity evaluation of the conjugates
The immuno-diffusion result confirmed the presence of OSP antigen in the conjugate prepared without a linker, however, the anti-OSP response was poor and less than that induced by LPS alone (). In contrast the conjugate with the ADH linker induced a significantly higher (P = 0.0446) anti-OSP response compared with the response to LPS alone.
Table 1. Immunogenicity evaluation of the prepared S. Paratyphi A conjugates with DT
Discussion
Bacteria of the genus Salmonella cause a variety of diseases in humans as well as animals commonly referred as salmonellosis. In humans, severe disease and bacteremia are associated primarily with S. Typhi and S. Paratyphi A serotypes. Symptoms of paratyphoid fever in most cases cannot be distinguished from those of typhoid fever (caused by S. Typhi), but it seems to follow a distinct route of transmission: whereas typhoid fever is spread predominantly within the household, paratyphoid fever is mainly transmitted outside the patient’s home.Citation13 The emerging incidence of S. Paratyphi A infections has gained great concern, particularly because of the fact that in the areas where paratyphoid incidence is high there is a risk that even a highly effective S. Typhi vaccine may appear as poorly efficacious.Citation4 The old whole-cell bacterial vaccine (TAB) against S. Typhi and S. Paratyphi A and B was de-licensed because of multiple factors including high incidence of reactogenicity, gradual hydrolysis of essential O-acetyl group of LPSCitation7 and inability of parenterally injected inactivated gram-negative bacteria to induce high levels of IgG LPS antibodies.Citation14,Citation15 Since no vaccine against paratyphoid is available, a combination vaccine covering both serotypes seems needed, particularly in Asia.Citation16
Salmonella LPS consists of the 3-deoxy-D-manno-octulosonic acid (KDO) terminus of a conserved core region linked to lipid A on one side and to a variable OSP chain on the other side. The serovar specific OSP of S. Paratyphi A is the immunodominant portion of the molecule that extends as a repeating polymerCitation17 and consists of a trisaccharide backbone (rhamnose, mannose, and galactose), with a branch of paratose from the C-3 mannose (which confers serogroup specificity: factor 2) and glucose from the C-6 of galactose.Citation7,Citation8 The OSP-DT conjugation most likely occurred via activated hydroxyl groups on the sugar repeat units of the OSP, in contrast, in the binding in the OSP-AH-DT conjugate the ADH most likely was bound to carboxyl groups on the KDO groups on the core polysaccharide region and subsequently the DT was bound to the ADH. The differences in the position of the conjugation on the OSP may explain the differences observed in the anti-OSP response, or it may be due to the distance between the OSP and the DT permitting better access to the antigen sites on the OSP in the conjugate with the spacer. Another possible reason could be a higher free polysaccharide content in the conjugate without the linker (OSP-DT) compared with the one with the linker (OSP-AH-DT). Although free polysaccharide was not directly measured the conjugate OSP-AH-DT was a pool of fractions 59–83 ml from the CL-6B column, free OSP eluted at 85–145 ml, and OSP-DT was a collection of fractions 53–101 ml. The OSP-AH-DT was likely to contain very little free OSP whereas OSP-DT likely contained some free OSP.
The OSP-protein linkage in conjugate vaccines converts T-cell independent polysaccharide antigen into a T-cell dependent antigen, eliciting better immune response with immunological memory. There is experimental evidence that serum antibodies to the OSP of group B and D salmonellae, whether actively induced or passively administered, confer protection to mice.Citation18-Citation20
Various conjugation strategies have been reported including recent conjugation chemistry to link the S. Paratyphi A O:2 and the carrier protein CRM197, using the terminus 3-deoxy-D-manno-octulosonic acid (KDO), thus leaving the O:2 chain unmodified. These new conjugates were found to have bactericidal activity against S. Paratyphi A.Citation4 S. Paratyphi A OSP was also bound to tetanus toxoid (TT) with ADH linker (SPA-TT1) and without ADH (SPA-TT2), the conjugate with the linker was more immunogenic (not statistically significant) than the conjugate without the linker. Only SPA-TT2 was injected into younger children and it did not elicit a booster response in the 2 to 4 y-old group.Citation8 This is in contrast to the booster response elicited in this age group by pneumococcus type 6B-TT, Vi-rEPA, and Shigellaflexneri 2a-rEPA conjugates.Citation21,Citation22
Conjugation of S. Paratyphi A OSP with diphtheria toxoid (DT) has not been previously reported. We have conjugated OSP of S. Paratyphi A with DT and evaluated immunogenicity in mice. Two conjugates of S. Paratyphi A OSP were prepared with DT: one using an ADH linker molecule and the other directly linking OSP to DT. The conjugate with the linker showed a significantly higher anti-OSP response compared with LPS alone whereas the conjugate prepared by directly linking OSP to DT was poorly immunogenic. The results presented here demonstrated that OSP purified from S. Paratyphi A can be conjugated to DT carrier protein. The enhanced immune response seen with the OSP-AH-DT conjugate is indicative of a successful conjugation and warrants further development as a vaccine candidate.
Materials and Methods
Purification of polysaccharide antigens
An isolate of S. Paratyphi A from Guangxi Province, China was used for fermentation and was obtained from the collection held at the International Vaccine Institute (IVI). The seed inoculum was added as 6% (volume/volume) to each of the two 10 L fermentors containing 8 L of TSB (tryptic soya broth Merck, Cat. # 105459). The fermentation was controlled at a temperature of 32 °C with 30% dissolved oxygen and pH 7. The fermentation process was of 10 h duration. Optical density of the culture was measured every hour at 600 nm. The bacterial cells were killed by addition of formalin to a final concentration of 1% and kept stirring at 200 rpm overnight. Bacteria were harvested by centrifugation at 7000 g at 4 °C for one hour. The wet cell-pallet was processed further for extraction and purification of LPS by hot-phenol methodCitation23 and freeze-dried. Purified S. Paratyphi A LPS were electrophoresed on SDS-PAGE and checked by silver staining.Citation24 Purification of S. Paratyphi A OSP was done by acid hydrolysis as previously described.Citation23 Briefly, the LPS of S. Paratyphi A at 10 mg/ml was made up to 1% glacial acetic acid and incubated in boiling water for 90 min. The pH was bought up to 7.0 using 1 N sodium hydroxide and the mixture was then ultracentrifuged at 96 000 g for 5 h at 4 °C after addition of a drop of 1 M calcium chloride. The supernatant containing the OSP was dialyzed against purified water and freeze-dried. The OSP was then made up to 10 mg/ml in purified water and applied to chromatography column (2.6 × 70 cm) using Sephadex G-75 media against purified water. Fractions were collected and checked for the presence of polysaccharides (by Anthrone assayCitation25) and antigenicity (by immuno-diffusion assayCitation26). The high molecular weight fractions of OSP were pooled and freeze-dried for later preparation of S. Paratyphi A OSP conjugates.Citation7 The AKTA Basic FPLC chromatographic system (GE Healthcare) was used during all chromatography experiments with automated fractionation and attached UV and refractive index (RI) detection systems. Nucleic acid contamination was measured spectrophotometrically at A260. Protein estimation was done by Coomassie assay reagent (Pierce, Cat. # 23200) as instructed by the manufacturer. Limulus Ameobocyte Lysate (LAL) test,Citation27 was used to determine endotoxin levels in S. Paratyphi A LPS and OSP.
Derivatization of S. Paratyphi A OSP
The OSP was dissolved in MES buffer as 10 mg/ml. The ADH and EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydro-chloride) were added as powder to the OSP solution to make final concentrations of 500 mM and 100 mM respectively. After 30 min reaction time, an additional amount of EDC was added to make a final concentration of 200 mM. Reaction was allowed to continue at room temperature for two hours with pH maintained at 5.0 to 6.0. The reaction mixture was then dialyzed against saline overnight. The dialysate was applied to the chromatography column Sephardex G-25 (1.6 × 50 cm) for desalting and the void volume fractions were harvested and freeze-dried and designated OSP-AH. The trinitrobenzene sulfonic acid (TNBS) assayCitation28 was performed to measure the hydrazide group and Hestrin assayCitation29 was performed on OSP and OSP-AH to determine the O-acetyl concentration (C-3 of rhamnose in OSP is partially O-acetylatedCitation30) as it is essential for the immunogenicity of S. Paratyphi A OSP.Citation7
Conjugation of S. Paratyphi A OSP-AH to DT
Derivatized OSP was conjugated to DT as previously reported.Citation7 Briefly the OSP-AH was dissolved in saline at 20 mg/ml. The solution was put in an ice bath and EDC powder added slowly to a final concentration of 15mM. The DT solution was added into the reaction mixture slowly to make the final ratio OSP-AH /DT = 1/1 (wt/wt). The reaction mixture was stirred on ice for 4 h with pH maintained between 5.0 and 5.5, followed by then dialysis against saline overnight at 4 °C using 6~8 Kd cut off membrane. The dialyzed conjugate mix was run through a chromatography column containing Sepharose CL-6B (1.6 × 84 cm) and eluted with saline. Anthrone assay, Coomassie protein assay, and immuno-diffusion assay were performed on collected fractions. The void volume fractions, which showed precipitation lines with anti-LPS and anti-DT serum were pooled and designated conjugate OSP-AH-DT.
Conjugation of S. Paratyphi A OSP to DT without linker
The OSP was conjugated directly to DT using 1-cyano-4-dimethylaminopyridiniom tetrafluoroborate (CDAP, Sigma, Cat. # C2776) activation.Citation7 The S. Paratyphi A OSP was dissolved in saline at 20 mg/ml and the pH adjusted between 5.0 to 6.0. The CDAP was added slowly to make the final ratio OSP/ CDAP = 1/1 (wt/wt). After addition the pH was adjusted to 7.5–8.5 using 0.2 M solution of TEA (Tri-ethylamine). After two minutes of reaction, DT was slowly added to make the final ratio OSP/DT = 1/1 (wt/wt). The reaction mixture was stirred at room temperature and the pH maintained at 8.0–8.5 for two hours followed by dialysis against saline overnight at 4 °C. The dialyzed conjugate mixture was then loaded onto the chromatography column Sepharose CL-6B (1.6 × 84 cm) and eluted with saline. Anthrone protein and immuno-diffusion assays were performed on collected fractions. The void volume fractions, which showed precipitation lines with anti-LPS and anti-DT serum, were pooled and designated OSP-DT conjugate.
Preparation of standard hyper immune sera
Formalin inactivated S. Paratyphi A cells were diluted in saline to a final OD600 of 1.0. These cells were then used to inject 8 wk old female ICR miceCitation11; the sera were analyzed for the presence of antibodies against S. Paratyphi A LPS using double immuno-diffusion assay.Citation26
Mice immunization
Six-week-old female ICR mice (Groups of 10) were injected subcutaneously on day-1, day-28 and day-56 as follows: Group 1 was injected with saline only, group 2 with LPS only, group 3 and 4 with OSP-AH-DT conjugate and OSP-DT conjugate respectively. The dose of polysaccharide in all groups was 2.5 μg per injection and heart bleeding was done after 2 wk of the last injection.
Immunoassay and data analysis
The anti-OSP IgG levels in serum were determined by enzyme-linked immunosorbent assay (ELISA) using Nunc Maxisorp plates (coated with purified LPS of S. Paratyphi A as described previously.Citation31,Citation32 For calculations of antibody titers, ELISA data were processed with Program ELISA for Windows, Centers for Disease Control and Prevention.Citation33 Titers of the test sera were determined by interpolation of OD values.Citation11
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The research facilities and funds for this work were provided by National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan, International Vaccine Institute (IVI), Seoul, and funded by the Government of the Republic of Korea and Higher Education Commission (HEC) of Pakistan.
References
- Liang W, Zhao Y, Chen C, Cui X, Yu J, Xiao J, Kan B. Pan-genomic analysis provides insights into the genomic variation and evolution of Salmonella Paratyphi A. PLoS One 2012; 7:e45346; http://dx.doi.org/10.1371/journal.pone.0045346; PMID: 23028950
- Dong B-Q, Yang J, Wang X-Y, Gong J, von Seidlein L, Wang ML, Lin M, Liao HZ, Ochiai RL, Xu ZY, et al. Trends and disease burden of enteric fever in Guangxi province, China, 1994-2004. Bull World Health Organ 2010; 88:689 - 96; http://dx.doi.org/10.2471/BLT.09.069310; PMID: 20865074
- Sahastrabuddhe S, Carbis R, Wierzba TF, Ochiai RL. Increasing rates of Salmonella Paratyphi A and the current status of its vaccine development. Expert Rev Vaccines 2013; 12:1021 - 31; http://dx.doi.org/10.1586/14760584.2013.825450; PMID: 24053396
- Micoli F, Rondini S, Gavini M, Lanzilao L, Medaglini D, Saul A, Martin LBO. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS One 2012; 7:e47039; http://dx.doi.org/10.1371/journal.pone.0047039; PMID: 23144798
- Robbins JB, Schneerson R, Szu SC, Pozsgay V. Bacterial polysaccharide-protein conjugate vaccines. Pure Appl Chem 1999; 71:745 - 54; http://dx.doi.org/10.1351/pac199971050745
- Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc 2005; 77:293 - 324; http://dx.doi.org/10.1590/S0001-37652005000200009; PMID: 15895165
- Konadu E, Shiloach J, Bryla DA, Robbins JB, Szu SC. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect Immun 1996; 64:2709 - 15; PMID: 8698499
- Konadu EY, Lin FY, Hó VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas AB, Li J, et al. Phase 1 and phase 2 studies of Salmonella enterica serovar paratyphi A O-specific polysaccharide-tetanus toxoid conjugates in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun 2000; 68:1529 - 34; http://dx.doi.org/10.1128/IAI.68.3.1529-1534.2000; PMID: 10678970
- Finn A. Bacterial polysaccharide-protein conjugate vaccines. Br Med Bull 2004; 70:1 - 14; http://dx.doi.org/10.1093/bmb/ldh021; PMID: 15339854
- Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis 2010; 50:241 - 6; http://dx.doi.org/10.1086/649541; PMID: 20014951
- Ali A, An SJ, Cui C, Haque A, Carbis R. Preparation and evaluation of immunogenic conjugates of Salmonella enterica serovar Typhi O-specific polysaccharides with diphtheria toxoid. Hum Vaccin Immunother 2012; 8:189 - 93; http://dx.doi.org/10.4161/hv.18350; PMID: 22426380
- WHO technical report series, no. 840. Requirements for Vi polysaccharide typhoid vaccine. WHO, Geneva, Switzerland. 1994.
- Vollaard AM, Ali S, van Asten HA, Widjaja S, Visser LG, Surjadi C, van Dissel JT. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA 2004; 291:2607 - 15; http://dx.doi.org/10.1001/jama.291.21.2607; PMID: 15173152
- Baumgartner JD, Heumann D, Calandra T, Glauser MP. Antibodies to lipopolysaccharides after immunization of humans with the rough mutant Escherichia coli J5. J Infect Dis 1991; 163:769 - 72; http://dx.doi.org/10.1093/infdis/163.4.769; PMID: 2010630
- McCabe WR, DeMaria A Jr., Berberich H, Johns MA. Immunization with rough mutants of Salmonella minnesota: protective activity of IgM and IgG antibody to the R595 (Re chemotype) mutant. J Infect Dis 1988; 158:291 - 300; http://dx.doi.org/10.1093/infdis/158.2.291; PMID: 3042871
- Wilde H. Enteric fever due to Salmonella typhi and paratyphi A a neglected and emerging problem. Vaccine 2007; 25:5246 - 7; http://dx.doi.org/10.1016/j.vaccine.2007.04.053; PMID: 17560692
- Whitfield C, Kaniuk N, Frirdich E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J Endotoxin Res 2003; 9:244 - 9; http://dx.doi.org/10.1177/09680519030090040501; PMID: 12935355
- Colwell DE, Michalek SM, Briles DE, Jirillo E, McGhee JR. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol 1984; 133:950 - 7; PMID: 6203984
- Reeves MW, Evins GM, Heiba AA, Plikaytis BD, Farmer JJ 3rd. Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J Clin Microbiol 1989; 27:313 - 20; PMID: 2915026
- Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis 1992; 15:346 - 61; http://dx.doi.org/10.1093/clinids/15.2.346; PMID: 1381621
- Ashkenazi S, Passwell JH, Harlev E, Miron D, Dagan R, Farzan N, Ramon R, Majadly F, Bryla DA, Karpas AB, et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J Infect Dis 1999; 179:1565 - 8; http://dx.doi.org/10.1086/314759; PMID: 10228084
- Sarnaik S, Kaplan J, Schiffman G, Bryla D, Robbins JB, Schneerson R. Studies on Pneumococcus vaccine alone or mixed with DTP and on Pneumococcus type 6B and Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugates in two- to five-year-old children with sickle cell anemia. Pediatr Infect Dis J 1990; 9:181 - 6; http://dx.doi.org/10.1097/00006454-199003000-00007; PMID: 2336298
- Chu CY, Liu BK, Watson D, Szu SS, Bryla D, Shiloach J, Schneerson R, Robbins JB. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga’s bacillus) bound to tetanus toxoid. Infect Immun 1991; 59:4450 - 8; PMID: 1937803
- Tsai CM. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand 1986; 14:25 - 33; http://dx.doi.org/10.1016/S0092-1157(86)80006-3; PMID: 2420803
- Scott TA, Melvin EH. Determination of dextran with anthrone. Anal Chem 1953; 25:1656 - 61; http://dx.doi.org/10.1021/ac60083a023
- Ouchterlony O, Nilson LA. in: D. M. Weir, L. A. Herzenberg, C. Blackwell, L. A. Herzenberg (Eds.) Immunodiffusion and Immunoelectrophoresis in Handbook of Experimental Immunology. Oxford: Blackwell, 1986, pp. 32.1-32.50.
- Levin J, Bang FB. The role of endotoxin in the extracellular coagulation in the Limulus.. Bull Johns Hopkins Hosp 1964; 115:265 - 74; PMID: 14209047
- Chu C, Schneerson R, Robbins JB, Rastogi SC. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun 1983; 40:245 - 56; PMID: 6601061
- Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem 1949; 180:249 - 61; PMID: 18133390
- Hellerqvist CG, Lindberg B, Samuelsson K, Lindberg AA. Structural studies on the O-specific side-chains of the cell-wall lipopolysaccharide from Salmonella parathyphi A var. durazzo. Acta Chem Scand 1971; 25:955 - 61; http://dx.doi.org/10.3891/acta.chem.scand.25-0955; PMID: 5117491
- Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla DA, Robbins JB. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun 1993; 61:1023 - 32; PMID: 8432585
- Szu SC, Li XR, Schneerson R, Vickers JH, Bryla D, Robbins JB. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun 1989; 57:3823 - 7; PMID: 2807549
- Plikaytis BD, Holder PF, Carlone GM. Program ELISA for Windows user's manual, version 1.00. Centers for Diease Control and Prevention, Atlanta, GA. 1996.
