Abstract
To address influenza B lineage mismatch and co-circulation, several quadrivalent inactivated influenza vaccines (IIV4s) containing two type A strains and both type B lineages have recently been approved in the United States. Currently available trivalent inactivated vaccines (IIV3s) or trivalent live attenuated influenza vaccines (LAIV3s) comprise two influenza A strains and one of the two influenza B lineages that have co-circulated in the United States since 2001. The objective of this analysis was to evaluate the cost-effectiveness of a policy of universal vaccination with IIV4 vs. IIV3/LAIV3 during 1 year in the United States. On average per influenza season, IIV4 was predicted to result in 30 251 fewer influenza cases, 3512 fewer hospitalizations, 722 fewer deaths, 4812 fewer life-years lost, and 3596 fewer quality-adjusted life-years (QALYs) lost vs. IIV3/LAIV3. Using the Fluarix QuadrivalentTM (GlaxoSmithKline) prices and the weighted average IIV3/LAIV3 prices, the model predicts that the vaccination program costs would increase by $452.2 million, while direct medical and indirect costs would decrease by $111.6 million and $218.7 million, respectively, with IIV4. The incremental cost-effectiveness ratio (ICER) comparing IIV4 to IIV3/LAIV3 is predicted to be $90 301/QALY gained. Deterministic sensitivity analyses found that influenza B vaccine-matched and mismatched efficacies among adults aged ≥65 years had the greatest impact on the ICER. Probabilistic sensitivity analysis showed that the cost per QALY remained below $100 000 for 61% of iterations. In conclusion, vaccination with IIV4 in the US is predicted to reduce morbidity and mortality. This strategy is also predicted to be cost-effective vs. IIV3/LAIV3 at conventional willingness-to-pay thresholds.
Introduction
Influenza, a contagious viral infection, results in substantial morbidity and mortality. Approximately 5–20% of the United States population are infected with seasonal influenza each year, resulting in between 3000 and 49 000 deaths annually, depending on the influenza attack rate and circulation each year.Citation1 The costs associated with influenza are also substantial, with annual direct medical costs estimated to be >$10 billion and indirect costs >$16 billion ($ 2003).Citation2
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual vaccination for all US individuals aged ≥ 6 mo.Citation3 Currently available influenza vaccines are either trivalent inactivated vaccines (IIV3s) or trivalent live attenuated influenza vaccines (LAIV3s). These contain two influenza A strains (H1N1 and H3N2) and one influenza B lineage (either Victoria or Yamagata).Citation4 During the 10 influenza seasons from 2001/2002 to 2010/2011, type B virus accounted for between 1% and 44% of specimens tested (mean 21%); and between 2% and 100% (mean 50%) of type B specimens tested matched the lineage in the vaccine for that year.Citation5 Predicting which type B lineage will predominate is difficult;Citation6 therefore, quadrivalent inactivated vaccines (IIV4s), which contain both influenza B lineages, are being developed.
The objective of this analysis was to evaluate the cost-effectiveness of universal influenza vaccination with IIV4 compared with trivalent vaccines (IIV3/LAIV3).
Results
Health outcomes
Based on published coverage rates, it was predicted that 116 878 708 individuals would receive influenza vaccination during a single season. IIV4 vaccination was predicted to result in fewer influenza cases, hospitalizations, deaths, and both life years (LYs) and quality adjusted life years (QALYs) lost ().
Table 1. Base-case health outcomes
Costs
The model predicts that the vaccination program costs (vaccine acquisition and administration) would increase by $452.2 million, with a $330.3 million reduction in influenza-related costs for IIV4 compared with IIV3/LAIV3 ($111.6 million direct medical and $218.7 million indirect) (). Excluding vaccination program costs, all cost categories are predicted to be reduced with IIV4 apart from caregiver work absenteeism, due to lower estimated efficacies of IIV4 and IIV3 vs. LAIV3 among children.Citation7,Citation8
Table 2. Base-case cost outcomes
Incremental cost effectiveness ratio (ICER)
Using the wholesale acquisition cost (WAC) of Fluarix QuadrivalentTM prices and the weighted average IIV3/LAIV3 prices, the predicted base-case ICER comparing IIV3/LAIV3 to IIV4 was $90 301/QALY gained. The estimates of total cost used for the calculation of cost per QALY gained do not include costs associated with productivity lost due to mortality. This is consistent with US Panel on Cost-Effectiveness recommendations,Citation9 as it is assumed that productivity losses are accounted for in the QALYs lost. Using similar logic, productivity losses due to acute disease were included in the costs, as no QALY losses were accrued for acute illness.
Alternative scenario analyses
IIV4 was predicted to be highly cost-effective compared with IIV3/LAIV3 for scenarios of low IIV3 vaccine match of circulating B lineages but similar average B lineage circulation to the base case (as observed in 2005/2006; $37 045/QALY gained), while in years of high mismatch and high circulation of influenza B (such as 2008/2009), IIV4 was predicted to dominate IIV3/LAIV3 (i.e., less costly and more effective) (). However, the IIV4 strategy was predicted to be less cost-effective compared with IIV3/LAIV3 than in the base case in scenarios assuming low IIV3 vaccine mismatch of circulating B lineages but similar average B lineage circulation overall (year 2006–2007; ~$257 000/QALY gained). In the year that had the lowest circulation of influenza B among those >65 y of age (2010–11, 4%), the model predicted an ICER of $340 772, while in the year with the highest circulation of influenza B among >65 y (2007–2008, 51%) the predicted ICER was $8632.Citation10
Table 3. Alternative scenario cost-effectiveness analyses
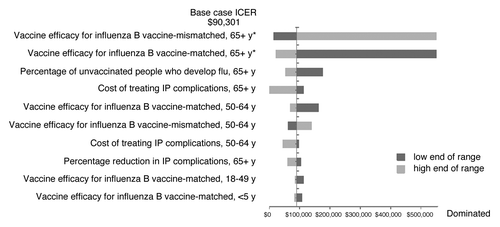
Deterministic sensitivity analyses (DSAs)
When key parameters were varied through their 95% confidence interval of the base-case values, influenza B matched and mismatched vaccine efficacy in adults aged ≥65 y had the greatest impact on the ICER ().
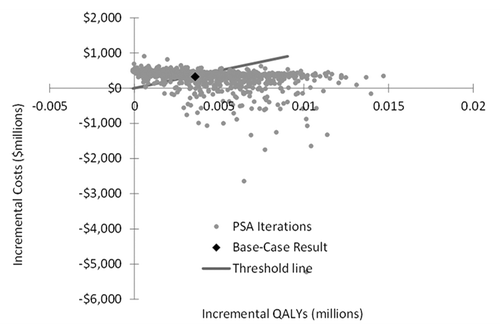
Probabilistic sensitivity analysis (PSA)
Most (61%) of the 1000 iterations fell below the $100 000 per QALY gained threshold (). The iterations that fell above the $100 000 per QALY gained threshold tended to be those for which a low influenza B matched vaccine efficacy estimate in adults aged ≥65 y was drawn.
Discussion
Our analysis predicts that, under the base-case model assumptions of vaccine coverage, efficacy, and cost; vaccination with IIV4 is expected to be more effective than IIV3/LAIV3, preventing >30 000 cases of influenza, >3500 hospitalizations, and >700 deaths on average each influenza season.
These outcomes are broadly comparable with those from a recent evaluation conducted by the Centers for Disease Control and Prevention (CDC),Citation6 which calculated net differences in rates of influenza, hospitalizations, and deaths with IIV4 vs. IIV3 vaccine in the United States. They predicted reductions of 0–1.33 million influenza cases (mean 274 158), 0–12 472 hospitalizations (mean 2144), and 0–663 deaths (mean 137) annually between 1999–2000 and 2008–2009, depending on each year’s match between the circulating B lineage and the lineage included in IIV3 (100% match in 1999–2010 to 2% match in 2007–2008). Our estimates of influenza cases and hospitalizations prevented were within these ranges, but our model predicted more deaths averted. This is due, at least in part, to the fact that the CDC study restricted deaths to patients with International Classification of Diseases Ninth Revision (ICD-9) codes indicating influenza or pneumonia, while our model included all deaths occurring in patients with underlying respiratory and circulatory disease. However, our approach has been suggested to be more likely to provide an appropriate estimate of mortality, whereas considering only pneumonia and influenza mortality might provide an underestimation.Citation11
Another key difference between the CDC studyCitation6 and the current model is that the CDC study calculated differences based on data from each individual influenza season from 1999–2000 to 2008–2009, whereas our model predicted outcomes by applying recent population and vaccination coverage data (2010–2011 season) to the average influenza circulation data from the past 11 seasons (2001–2002 to 2011–2012). Additionaly, we stratified the population by age, whereas the CDC analysis did not, and therefore may not have captured the variability in influenza-associated hospitalizations and mortality that exists by age.Citation2 Furthermore, vaccine coverage estimates in the CDC modelCitation6 were substantially lower than those used in the current model, because the CDC study was based on vaccination practices prior to the universal influenza vaccination recommendation in 2010.Citation3 Lastly, influenza incidence in the CDC modelCitation6 was estimated from influenza-associated pneumonia and influenza mortality,Citation12-Citation15 and was lower than the incidence data used in the current model, which was estimated from surveillance data.Citation2,Citation14
An updated economic evaluation based on the CDC dataCitation6 has recently been published.Citation16,Citation17 Mean yearly additional costs to third party payers for using IIV4 instead of IIV3 were estimated to be $152 million and $334 million for incremental costs of IIV4 over IIV3 of $2.50 and $5, respectively;Citation17 somewhat lower than our estimated vaccination cost of $452 million. Mean yearly total costs to society were estimated to be reduced by $133 million (for the $2.50 incremental cost) or increased by $49 million (for the $5 incremental cost);Citation17 compared with our estimate of an additional cost of $122 million.
In our base-case analysis, IIV4 was estimated to increase the overall strategy costs by almost $122 million. This reflects an increase in the vaccination program cost, driven by the increment in the IIV4 price over the IIV3 price. This increase is partially offset by the reduction in yearly disease- and treatment-associated costs of approximately $330.3 million, which includes $111.6 million in medical costs and $218.7 million in indirect costs (such as loss in productivity due to death). The use of IIV4 is predicted to reduce the number of cases of influenza, physician visits, and associated hospitalizations, as well as influenza-related deaths, thereby reducing the QALY losses due to death vs. current standard of care. When the differences in QALYs and overall costs associated with each alternative strategy were compared, the ICER was $90 301/QALY gained compared with IIV3/LAIV3. However, the current model did not include every element associated with the costs and burden of influenza (such as emergency room visits and rehabilitations costs post hospitalization for severe episodes), and is therefore expected to be conservative and underestimate the true impact of IIV4.
In two alternative scenarios, we analyzed the impact of high- and low-matched B lineage for IIV3/LAIV3. As expected, during high-match influena B lineage seasons, IIV4 was projected to be less cost-effective than in the base case, but for the low-match influena B lineage seasons, IIV4 was projected to be highly cost-effective compared with IIV3/LAIV3. These alternative analyses demonstrate that results are sensitive to changes in the match between circulating strains/lineages and those in IIV3.
As with any model, the current model is a simplified representation of the vaccination, disease, and treatment processes, and hence it cannot reflect all outcomes or population heterogeneity within the age groups. Due to limited age- and strain-/lineage-specific data and yearly variations in influenza epidemiology, there is a degree of uncertainty in estimating the model inputs. Moreover, information such as vaccination and co-morbidity status of hospitalized and fatal cases were not available; these probabilities could therefore not be reliably adjusted and the model outcomes are conservative with respect to potential benefit of vaccination. The percentage of children who are vaccine-naïve is not readily estimable. It is likely that the probability of getting vaccinated is higher among children vaccinated the previous year, so the assumption used in the model, that the percentage vaccine naïve, and therefore requiring two doses, was equal to the percentage of children not vaccinated during the previous season, could have led to an overestimation of vaccine-naïve children among those vaccinated, and therefore an overestimation of associated vaccination costs. We attempted to address the uncertainty in model parameters by performing multiple sensitivity analyses using the most conservative estimates within the range of plausibility. Improvements in data and research, such as the availability of surveillance data by strain and lineage at a more granular, age-stratified level, would allow for better parameterization of models with respect to the impact of strains/lineages on different populations.
There are currently no efficacy studies that directly investigate the efficacy for matched and mismatched IIV3, therefore Tricco et al.Citation18 was used to estimate the impact of matched vs. mismatched vaccine efficacy due to influenza B. An additional review by DiazGranados et al.Citation19 published at the same time revealed similar efficacy of influenza by vaccine, and by influenza strain and match.
Estimates of the incremental efficacy of IIV4 were based on estimates of strain- and lineage-specific efficacy of IIV3 vaccine combined with the distribution of circulating A and B influenza virus in the United States, and not estimated directly from clinical trials. Moreover, age-specific B lineage efficacy data were not available. Risk of infection and subsequent hospitalization with influenza B may be higher among children and those aged ≥ 65 y.Citation20 Therefore, while influenza B may disproportionally affect children, the highest burden of influenza in general, and thus the highest benefit of IIV4 vaccination, remains among the elderly under the current assumptions. However, we had insufficient data to estimate age- and strain/lineage-specific disease incidence or hospitalization rates. Canadian age-and strain-specific data suggest that there is wide year-to-year variation in the strain distribution by age, including some years in which B disease is higher among adults aged 65 y and older than among younger individuals.Citation10 Because of this uncertainty, we assumed in the base case that the relative prevalence of B strains does not differ by age, however, we performed alternative analyses under several different assumptions based on the Canadian data. There are several studies comparing the clinical spectrum and disease severity of influenza A and influenza B in the United States that suggest that influenza A and B are clinically similar.Citation21-Citation24 A recent large case-series studyCitation21 of individuals 6 mo of age and older compared the clinical presentation, risk of radiographic pneumonia, and hospital admission among patients with medically attended influenza A and influenza B infections over four seasons. When data from all 4 seasons (2004/05–2007/08) were combined, no individual symptom or group of symptoms distinguished influenza A and B infections in children or adults. Therefore, in our model the proportion of influenza A or B responsible for disease was weighted by average circulation, and prognosis of infection was assumed to be similar between influenza A and B.
Additional data on the clinical outcomes of patients with influenza A or B could also better inform models and improve our understanding of how influenza frequency and related diseases differ by type. In a multi-cohort transmission model, there could be instances where vaccinating children could be cost-effective, while vaccinating those aged ≥65 y may not be, even if influenza outcomes in the elderly were more severe, if direct and indirect protection of both age groups would be acchieved by vaccinating children. This protection would apply to all influenza vaccines (both IIV3s and IIV4s), but would be expected to increase the incremental effect of the IIV4 vaccine.
We opted to use a static model and as such, the impact of herd effect was not addressed. It is important, however, to keep in mind that mathematical models have predicted that vaccinating 20% of children against influenza could almost halve the number of influenza cases in people of all ages in the United States; while 80% coverage among children could prevent >90% of cases overall.Citation25 However, a recent observational study found no difference in the rate of influenza-related hospitalizations among the elderly even when school-aged vaccination rates were >40%.Citation26 Published studies have shown a protective effect of childhood influenza vaccination in the elderly, but they had high vaccine coverage rates (50–85%,Citation27 60–70%,Citation28 and >85%).Citation23 We used national coverage rates in the model (21–67%, depending on age),Citation29-Citation31 which are well below the coverage rates in these published studies. Therefore, it is unknown whether, at current coverage levels, the assumption of herd effect in US influenza vaccine cost-effectiveness analyses is warranted. However, it would be useful to be able to predict what the potential benefits would be should influenza vaccination coverage in children improve. As herd effect against influenza is largely mediated by vaccination of children, it should be recognized that inclusion of a herd effect could improve the economic profile of IIV4 assuming it primarily replaces IIV3. IIV4 replaces both IIV3 and LAIV, as in our base case analysis, the impact of herd effect may be less predictable, as IIV4 could has a lower efficacy than the overall current practice of IIV3/LAIV among children. Given this uncertainty, we chose to consider only the direct effects of vaccination in our analysis. A transmission model accounting for different populations and the relationships between the different circulating influenza strains and lineages would further inform cost-effectiveness analyses. This is especially true with respect to influenza infection dynamics, as well as co-circulation and mismatch challenges.
We further note that our model assumes that the availability of IIV4 is not restricted by any limitation in manufacturing capacity. Reduced vaccine availability could result in more annual influenza cases relative to current levels;Citation6 however, the relative cost-effectiveness would not be affected. Over-the-counter medicine costs and emergency room visits were also not included in the model, both of which could be potentially significant contributors to the overall cost implications of influenza disease management. Despite these limitations, we conclude that under a wide range of plausible assumptions, IIV4 vaccination should result in increased program costs due to a vaccine price increment, but lower treatment-related costs, as well as reduced productivity losses and improved clinical outcomes such as reduced hospitalizations and death compared with the current practice.
Conclusions
The current model predicts that replacing IIV3/LAIV3 with IIV4 in the United States could prevent >30 000 cases of influenza, >3500 hospitalizations, and >700 deaths on average each year compared with IIV3/LAIV3. Using the GlaxoSmithKline Fluarix QuadrivalentTM prices, vaccination program costs would be predicted to increase by $452.2 million, while direct medical and indirect costs would decrease by approximately by $111.6 million and $218.7 million, respectively. The predicted ICER comparing IIV4 to IIV3/LAIV3 was $90 301/QALY gained, therefore, IIV4 is predicted to be cost-effective at conventional willingness-to-pay thresholds (ICER <$100 000 per QALY gained).
Influenza vaccine coverage level data from 2010–2011 indicate that 2009–2010 (pandemic year) coverage levels have been maintained.Citation30 If coverage increases over the next few years as a result of the February 2010 ACIP recommendation of universal influenza vaccination in the United States,Citation3 benefits realized by replacing IIV3/LAIV3 with IIV4 will likely be higher than estimated in this analysis.
Methods
Perspective
Results are presented from the societal perspective, which considers medical costs (physician visits, inpatient and outpatient care, and antiviral medications) and non-medical costs (patients’ and caregivers’ productivity losses owing to time lost from work due to acute illness and lifetime productivity loss due to fatal cases).
Intervention strategies
The model predicts and compares outcomes for: (1) the current recommended practice of vaccination of all US residents with IIV3 or LAIV3 and (2) replacement of IIV3/LAIV3 with IIV4.
Time frame and analytic horizon
The time horizon for intervention was 1 y, as influenza can occur on an annual basis. However, some costs, and certainly QALY losses due to mortality, occurring during the intervention period extend beyond the intervention period. Therefore, in order to ensure that all costs and outcomes were fully assessed within the analyses, costs and outcomes beyond the one year of vaccination were accrued over individuals’ lifetimes. .
The economic model
Overview
We adapted a previously published decision-tree model of seasonal influenza vaccination to examine costs and outcomes associated with recommended universal vaccination in the United States with IIV4 compared with IIV3 or LAIV3.Citation32 The population was stratified into five age groups: <5, 5–17, 18–49, 50–64, and ≥65 y. Total population and age distribution in the United States were obtained from the US Census Bureau based on 2011 data.Citation33 The model estimated cases and outcomes of laboratory-confirmed influenza within these age groups.
Model structure
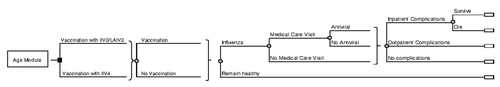
The decision of interest was whether to implement a strategy of universal vaccination in the US population with IIV4 vs. IIV3/LAIV3 (). Under each vaccination strategy, individuals may or may not receive the specified vaccine based on coverage assumptions, and may or may not contract influenza based on published estimates of the risk of influenza and vaccine efficacy.
Over the course of the model, a proportion of patients who contract influenza seek medical care and a proportion of these may receive an antiviral. Some influenza patients may develop a complication, which requires either outpatient or inpatient care, and a proportion of those with inpatient complications die (). Influenza-associated hospitalizations and deaths were defined based on underlying respiratory and circulatory ICD-9 codes rather than pneumonia and influenza ICD-9 codes, as most complications of influenza occur after influenza has resolved, and consequently may not have an ICD-9 code for influenza.Citation14
Model outcomes
The model assessed the clinical outcomes and costs associated with each case of influenza. Medical costs (e.g., physician visits, diagnosis, drugs, and hospitalization; $ 2011) were assigned to each case in the model. Over-the-counter medicine costs and emergency room visits were not included. Non-medical costs included workdays lost by symptomatic individuals or their caregivers. The model also estimated QALYs and LYs lost due to fatal cases of influenza. All costs incurred after the base year and lifetime QALYs lost due to death were discounted at 3% per year.
Base-case model inputs
Model parameters were estimated for each age group from published and publicly-available sources.
Vaccine coverage ()
Table 4. Vaccine coverage and probability of influenza estimates
Estimated age-specific influenza vaccine coverage was based on CDC data from the 2010/2011 influenza season.Citation29-Citation31 The proportions of IIV3 and LAIV3 use were estimated from Behavioral Risk Factor Surveillance System data,Citation34 National Immunization Survey data (CDC, personal communication), and CDC data.Citation30 Estimates of vaccine coverage were combined with US population dataCitation33 to derive the total number of individuals vaccinated overall and by age.
Influenza incidence among unvaccinated individuals ()
The incidence of influenza among unvaccinated persons was estimated from a published study of the burden of influenza in the United States.Citation2 Age-specific differences in the proportions of people infected with influenza A vs. B were not taken into account due to lack of data. Healthcare utilization prognosis of an episode and mortality were assumed to be similar for influenza A and influenza B, based on evidence from US community studies.Citation21-Citation24
Vaccine efficacy ()
Table 5. Vaccine efficacy estimates
Estimates of age-specific efficacies for IIV3 and LAIV3 were obtained primarily from meta-analyses.Citation7,Citation8,Citation18,Citation35 For children and the elderly, estimates were available for overall IIV3 efficacy, but not for matched or mismatched influenza B efficacy. Since IIV3 efficacy is lower in children and the elderly,Citation7,Citation35,Citation36 efficacy assumptions for matched and mismatched efficacy were based on adult efficacy estimates, taking into account the lower expected efficacy. Combining strain-specific efficacy estimates and assumptions for influenza A and B with CDC 11-y average circulating influenza A level (79%)Citation5 and 11-y average influenza B vaccine matching (50%)Citation5 yielded overall IIV3 efficacy estimates. IIV4 efficacy for influenza B was assumed to be equivalent to the efficacy of a matched IIV3, and overall estimated IIV4 efficacies were calculated by applying this efficacy to the proportion of circulating B not covered by IIV3/LAIV3.
Influenza treatment and complications (Table S1)
The probability of seeking an outpatient visit to treat influenza, receiving an antiviral (olsetamivir or zanamivir), and having a complication requiring outpatient treatment were estimated from the published literature.Citation2,Citation3,Citation37-Citation41 Among children, an outpatient complication was defined as acute otitis media, pneumonia, or sinusitis not leading to hospitalization among patients with laboratory-confirmed influenza. In adults, an outpatient complication was defined as any outpatient event requiring administration of an antibiotic among persons with laboratory-confirmed influenza.
Estimates of inpatient complications (influenza-attributable respiratory and circulatory hospitalizations) were derived from 1993–2008 data, using ICD-9 codes for respiratory and circulatory disorders, to identify excess admissions with these conditions during an influenza season.Citation15 Influenza-associated hospitalizations were not restricted to those associated with laboratory-confirmed influenza because admitted patients are not routinely swabbed to confirm influenza, and are often hospitalized for influenza-related complications after resolution of influenza infection.
Antiviral effectiveness against outpatient and inpatient complications of laboratory-confirmed influenza were estimated from systematic reviews of influenza treatment.Citation39,Citation42 Mortality risks among those hospitalized for influenza-related complications were derived from published estimates.Citation11,Citation14,Citation15 As with hospitalizations, influenza-related deaths were defined based on codes for underlying respiratory and circulatory disorders, using data from 1993–2008 to correspond with the time period used to calculate hospitalizations.
Medical costs (Table S2)
IIV3 and LAIV3 costs were estimated from 2011 influenza vaccine prices.Citation43 Based on ACIP recommendations, children <9 y old who have not been vaccinated previously should receive two doses of vaccine.Citation3 Therefore, the proportion of vaccine-naïve children was assumed to be equal to the proportion of children not vaccinated in the previous influenza season (2009–2010).Citation3 It was assumed that 55% and 43% of vaccines used in children aged <5 y and 5–18 y, respectively, were obtained at the public cost and the remainder at private cost.Citation44
Costs associated with vaccine administration and the cost of a physician visit for the treatment of influenza were estimated from the 2011 Physicians’ Fee and Coding Guide and 2010 State Medicaid paid vaccine administration rate (2011 rates were not available at time of analysis).Citation45,Citation46 It was assumed that 35% of children <18 y, 9–11% of adults 18–64 y, and all adults ≥65 y were covered by Medicaid or Medicare, and the remainder by private insurance or self-pay.Citation47 Adverse effects of vaccination (e.g., local injection site reactions)Citation3 were not considered in the model. As with vaccine acquisition costs, a proportion of children received the cost of administration of two doses.Citation3
The costs of antiviral drugs (oseltamivir and zanamivir) were estimated from national average wholesale prices published in the 2011 Red BookCitation48 weighted by the proportion of patients receiving each drug, derived from a physician survey of prescribing practices.Citation39 Medical costs for patients with complications requiring outpatient and inpatient care were estimated from published claims analyses of costs associated with influenza complications.Citation2,Citation3,Citation11,Citation37,Citation38,Citation49
Non-medical costs (Table S3)
Lost workdays due to acute diseaseCitation2,Citation37,Citation38 were assigned to all influenza cases; it was assumed that adults not participating in the workforce work inside the home, and that workdays are lost by adult caregivers in caring for influenza cases among children. Average daily wages were obtained from the US Bureau of Labor Statistics.Citation50 Estimates of lifetime lost productivity of fatal cases were obtained from published literature.Citation2
LY and QALY estimates (Table S4)
Utility values, preference-based measures of quality of life ranging from 0 to 1 used to calculate QALYs, were obtained from published literature.Citation51 Temporary reductions in quality of life due to acute disease were not considered in the model as the effect is transitory and would therefore have a negligible impact on overall QALYs. Moreover, as productivity losses due to acute illness are included in the analyses, inclusion of quality of life decrements for acute illness would overestimate influenza-associated morbidity. Age-specific utility estimates were applied to population estimates and projected life expectancy of fatal cases to calculate total QALYs lost due to death.Citation33,Citation51,Citation52 It was assumed that the average age at death among cases aged 5–64 y was the same as the average age of all-cause death in each age group in the US population. However, because the ages at death for fatal influenza cases among children <5 y and adults ≥65 y are not equal to the average ages at death in these age groups in the US population, their quality adjusted life expectancies were estimated based on average ages of influenza death (1.3 y and 84 y for those aged <5 y and ≥65 y, respectively).Citation11
Outcomes
Health and cost
The model estimates patient outcomes (cases of influenza; influenza-attributable respiratory and circulatory hospitalizations and deaths; and LYs and QALYs lost due to influenza- for clinical outcomes by age, see Table S5) and costs under each strategy. An ICER < $100 000 per QALY gained was considered to be cost-effective. This is a conservative assumption when comparted to the World Health Organization recommendation that a cost-effective intervention is less than three times the gross domestic product (GDP) per capita.Citation53 The GDP per capita was approximately $47 000 in the United States in 2011,Citation54 which fixes the US threshold at approximately $141 000.
Incremental cost-effectiveness threshold
In the calculations of the incremental cost per QALY gained, the price of IIV4 was set as the weighted average wholesale acquisition cost (WAC), public/private price of Fluarix QuadrivalentTM. The cost estimates used for ICER calculations did not include costs associated with productivity lost due to mortality. This is consistent with the US Panel on Cost-Effectiveness recommendations,Citation9 as we assumed that productivity losses were accounted for in the QALYs lost. Using similar logic, productivity losses due to acute disease were included in the costs, as no QALY losses were accrued for acute illness.
Alternative scenario analyses
Where year-to-year variations in the estimate were noted, alternative scenario analyses were conducted to test the impact of plausible alternative assumptions on the ICERs. If a strategy was both more costly and resulted in more QALYs lost, that strategy was considered “dominated.”
One scenario involved varying the percentage of circulating vaccine-matched B influenza; years were selected where the annual circulation of influenza B overall was similar to the mean of 20.7%. The percentages of circulating vaccine-matched B influenza from 2006–2007 (76.5%) and 2005–2006 (18.7%) were chosen to represent high-match and low-match seasons, with similar overall influenza B circulation (20.8% and 20.3% respectively).Citation5
A second scenario analysis was conducted in which circulation of influenza B was higher than the mean and accompanied with low-match, 2008–2009 influenza B circulation was 34.3% and vaccine-matched influenza B was 16.6%.
Additional scenario analyses were performed to investigate the possible variation in the distribution of influenza A and B disease by age (<65 y and ≥65 y). As these data were not available for the United States, data from the Canadian Public Health Agency were used as a surrogate.Citation10 We evaluated two scenarios, based on years in which percentage of disease due to B strains was lower among adults ≥65 y than among adults <65 y (2010–11: 4% vs. 12%), and where the percentage of disease due to B strains among adults ≥65 y was higher than among adults <65 y (2007–8: 51% vs. 41%).
DSA
A DSA was conducted by varying parameters one at a time through the 95% confidence intervals of the base-case values and calculating the cost-effectiveness at each extreme value. Age-specific estimates of vaccination coverage, vaccine efficacy, probability of visiting a physician, receipt of an antiviral, developing complications and mortality, and cost of influenza treatment and complications were evaluated.
PSA (Table S6)
A PSA was performed to assess the impact of uncertainty in the precision of parameter estimates on model results. Parameters were expressed as probability distributions around their estimated means and a Monte Carlo simulationCitation55 was performed, in which values were drawn at random with replacement in 1000 iterations, yielding a distribution of results. Parameters handled in this fashion included: vaccination coverage, probabilities of influenza among unvaccinated individuals, vaccine efficacy, probabilities of physician visit for treatment of influenza, probabilities of receiving antiviral, probabilities of developing complications and mortality, antiviral effectiveness, productivity loss due to illness, quality adjusted life expectancy, and value of lifetime lost productivity. Distributions were derived from the published literature and expert judgment. For each of these parameters, it was assumed that age-specific estimates were independent of the estimates in the other age groups. The percentage of iterations in which the IIV4 strategy was cost-effective compared with current practice is reported.
Genevieve Meier is an employee of, and owns stock in, the GlaxoSmithKline group of companies. Derek Misurski is a former employee of the GlaxoSmithKline group of companies and had restricted shares at the time of his employment. OptumInsight received funding from the GlaxoSmithKline group of companies to complete the work disclosed in this manuscript and consultancy fees. Karen Clements, Lisa McGarry, and Narin Pruttivarasin are employees or former employees of OptumInsight.
| Abbreviations: | ||
| ACIP | = | Advisory Committee on Immunization Practices |
| CDC | = | Centers for Disease Control and Prevention |
| DSA | = | deterministic sensitivity analysis |
| ICD-9 | = | International Classification of Diseases Ninth Revision |
| ICER | = | incremental cost-effectiveness ratio |
| IIV3 | = | trivalent inactivated vaccine |
| IIV4 | = | quadrivalent inactivated vaccine |
| LAIV3 | = | trivalent live attenuated influenza vaccine |
| LY | = | life year |
| MD | = | medical doctor |
| PSA | = | probabilistic sensitivity analysis |
| QALY | = | quality adjusted life year |
| WAC | = | wholesale acquisition cost |
Additional material
Download Zip (177 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and agreed with the submission of the publication.
We thank Gabriel Gomez-Ray who provided assistance in model programming on behalf of OptumInsight, Dr Jenny Lloyd and Prachee Panda who provided medical writing services on behalf of GlaxoSmithKline, and Heather Santiago (publication manager, GlaxoSmithKline) for editorial assistance and manuscript coordination.
Trademark Statement
Fluarix QuadrivalentTM is a registered trade mark of the GlaxoSmithKline group of companies.
References
- Centers for Disease Control and Prevention (CDC). Seasonal Influenza (Flu): Questions & Answers. Available from: http://www.cdc.gov/flu/about/qa/preventingflu.htm
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086 - 96; http://dx.doi.org/10.1016/j.vaccine.2007.03.046; PMID: 17544181
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:RR-8 1 - 62; PMID: 20689501
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep 2012; 61:613 - 8; PMID: 22895385
- Centers for Disease Control and Prevention (CDC). Flu Activity & Surveillance Reports.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.12.098; PMID: 22226861
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; CD004879; PMID: 18425905
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36 - 44; http://dx.doi.org/10.1016/S1473-3099(11)70295-X; PMID: 22032844
- Gold MR, Siegel JE, Russel LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press, 1996.
- NACI Members. Canada Communicable Disease Report. Canada: Public Health Agency, Canada. Fluwatch: www.publichealth.gc.ca. Accessed; 21 Jan 2014.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Chowell G, Miller MA, Viboud C. Seasonal influenza in the United States, France, and Australia: transmission and prospects for control. Epidemiol Infect 2008; 136:852 - 64; http://dx.doi.org/10.1017/S0950268807009144; PMID: 17634159
- Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 17:Suppl 1 S3 - 10; http://dx.doi.org/10.1016/S0264-410X(99)00099-7; PMID: 10471173
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057 - 62; PMID: 20798667
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54:1427 - 36; http://dx.doi.org/10.1093/cid/cis211; PMID: 22495079
- Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012; 30:7443 - 6; http://dx.doi.org/10.1016/j.vaccine.2012.10.025; PMID: 23084849
- Lee BY, Bartsch SM, Willig AM. Corrigendum to “The economic value of a quadrivalent versus trivalentinfluenza vaccine”. [Vaccine 30 (2012) 7443–7446] Vaccine 2013; 31:2477 - 9; http://dx.doi.org/10.1016/j.vaccine.2013.04.013
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; http://dx.doi.org/10.1186/1741-7015-11-153; PMID: 23800265
- DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine 2012; 31:49 - 57; http://dx.doi.org/10.1016/j.vaccine.2012.10.084; PMID: 23142300
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28:Suppl 4 D45 - 53; http://dx.doi.org/10.1016/j.vaccine.2010.08.028; PMID: 20713260
- Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, Belongia EA. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respir Viruses 2012; 6:37 - 43; http://dx.doi.org/10.1111/j.1750-2659.2011.00263.x; PMID: 21668663
- Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med 2007; 4:e247; http://dx.doi.org/10.1371/journal.pmed.0040247; PMID: 17683196
- Monto AS, Davenport FM, Napier JA, Francis T Jr.. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis 1970; 122:16 - 25; http://dx.doi.org/10.1093/infdis/122.1-2.16; PMID: 5433709
- Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. Int J Infect Dis 2007; 11:40 - 7; http://dx.doi.org/10.1016/j.ijid.2005.10.008; PMID: 16678464
- Weycker D, Edelsberg J, Halloran ME, Longini IM Jr., Nizam A, Ciuryla V, Oster G. Population-wide benefits of routine vaccination of children against influenza. Vaccine 2005; 23:1284 - 93; http://dx.doi.org/10.1016/j.vaccine.2004.08.044; PMID: 15652671
- Hull HF, McBean M, O’Connor H. Assessment of herd immunity in the elderly following vaccination of school children with live, attenuated influenza vaccine. Infectious Disease Society of America 48th Annual Meeting, Vancouver, Canada 2010.
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889 - 96; http://dx.doi.org/10.1056/NEJM200103223441204; PMID: 11259722
- Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect 2006; 134:71 - 8; http://dx.doi.org/10.1017/S0950268805005650; PMID: 16316494
- Centers for Disease Control and Prevention (CDC). Final estimates for 2009-10 Seasonal Influenza and Influenza A (H1N1) 2009 Monovalent Vaccination Coverage - United States, August 2009 through May, 2010. Available from: http://www.cdc.gov/flu/fluvaxview/coverage_0910estimates.htm.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010-11 season - United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. Available from: http://www.cdc.gov/flu/fluvaxview/coverage_1011estimates.htm
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccination coverage among children aged 6 months-18 years --- eight immunization information system sentinel sites, United States, 2009-10 influenza season. MMWR Morb Mortal Wkly Rep 2010; 59:1266 - 9; PMID: 20930704
- Aballéa S, Chancellor J, Martin M, Wutzler P, Carrat F, Gasparini R, Toniolo-Neto J, Drummond M, Weinstein M. The cost-effectiveness of influenza vaccination for people aged 50 to 64 years: an international model. Value Health 2007; 10:98 - 116; http://dx.doi.org/10.1111/j.1524-4733.2006.00157.x; PMID: 17391419
- US Census Bureau. . 2011.
- Merrill RM, Beard JD. Influenza vaccination in the United States, 2005-2007. Med Sci Monit 2009; 15:PH92 - 100; PMID: 19564838
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; CD004876; PMID: 20166072
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010; CD001269; PMID: 20614424
- Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey. . Vital and health statistics Series 10 2009; 1 - 81
- Centers for Disease Control and Prevention (CDC). Influenza vaccination levels among persons aged > or =65 years and among persons aged 18-64 years with high-risk conditions--United States, 2003. MMWR Morb Mortal Wkly Rep 2005; 54:1045 - 9; PMID: 16237375
- Dharan NJ, Beekmann SE, Fiore A, Finelli L, Uyeki TM, Polgreen PM, Fry AM. Influenza antiviral prescribing practices during the 2007-08 and 2008-09 influenza seasons in the setting of increased resistance to oseltamivir among circulating influenza viruses. Antiviral Res 2010; 88:182 - 6; http://dx.doi.org/10.1016/j.antiviral.2010.08.010; PMID: 20739002
- Heikkinen T, Silvennoinen H, Peltola V, Ziegler T, Vainionpaa R, Vuorinen T, Kainulainen L, Puhakka T, Jartti T, Toikka P, et al. Burden of influenza in children in the community. J Infect Dis 2004; 190:1369 - 73; http://dx.doi.org/10.1086/424527; PMID: 15378427
- Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003; 163:1667 - 72; http://dx.doi.org/10.1001/archinte.163.14.1667; PMID: 12885681
- Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, Ades AE, Sutton A, Cooper N, Elliot AJ, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. [iii-iv.] Health Technol Assess 2009; 13:1 - 265, iii-iv; PMID: 19954682
- IMS Health, DDD(tm). Total Sales 2011. 2012.
- 2010 VFC Eligible Children. Data on file.
- Physicians Fee & Coding Guide 2011. Mag Mutual Healthcare Solutions.
- Centers for Medicare & Medicaid Services. VFC Comparison of Regional Maximum Rate for Vaccine Administration to Current State Rate.
- United States Census Bureau. Health Insurance Coverage Status and Type of Coverage - All Persons by Age and Sex: 1999 to 2012. Available from: http://www.census.gov/hhes/www/hlthins/data/historical/files/hihistt3B.xls.
- Book R. 2011 Pharmacy Fundamental Reference. Montvale, NJ: Thomson, 2011.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333 - 40; http://dx.doi.org/10.1001/jama.292.11.1333; PMID: 15367555
- Bureau of Labor Statistics. Employment, Hours, and Earnings from the Current Employment Statistics Survey (National) 2006-2012
- Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making 2006; 26:391 - 400; http://dx.doi.org/10.1177/0272989X06290497; PMID: 16855127
- Arias E. United States life tables, 2006. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System 2010; 58:1 - 40
- World Health Organization. Cost-effectiveness thresholds. Available from: http://www.who.int/choice/costs/CER_thresholds/en/
- Economics T. United states GDP per capita PPP. Available from: http://www.tradingeconomics.com/united-states/gdp-per-capita-ppp
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making 1985; 5:157 - 77; http://dx.doi.org/10.1177/0272989X8500500205; PMID: 3831638