Abstract
Despite substantial efforts in recent years toward the development of new vaccines and drugs against tuberculosis (TB), success has remained elusive. Immunotherapy of TB with mycobacterial Hsp65 as a DNA vaccine (DNA-hsp65) results in a reduction of systemic bacterial loads and lung tissue damage, but the high homology of Hsp65 with the mammalian protein raises concern that pathological autoimmune responses may also be triggered. We searched for autoimmune responses elicited by DNA-hsp65 immunotherapy in mice chronically infected with TB by evaluating the humoral immune response and comprehensive histopathology using stereology. Cross-reactive antibodies between mycobacterial and mammalian Hsp60/65 were detected; however, no signs of pathological autoimmunity were found up to 60 days after the end of the therapy.
Tuberculosis (TB) remains a leading cause of death with more than 8 million cases every year and 1.4 million deaths worldwide, representing one of the three most impacting infectious diseases for human health.Citation1 One of the reasons for the limited success in the combat of TB is the lack of new drugs to shorten disease treatment, which typically lasts at least 6 mo. As a result, immunotherapy for TB has gained new attention because it may also provide an adjuvant effect for conventional chemotherapy by improving the immune response of the patient against the bacillus. In this regard, three candidates for TB immunotherapy have been discussed specifically in the literature: (1) M. vaccae, (2) a post-infection vaccine called RUTI made from detoxified, fragmented M. tuberculosis cells, and (3) a DNA vaccine encoding the heat-shock protein 65 of M. leprae (DNA-hsp65).Citation2-Citation4 M. vaccae is the only immunotherapy that has been tested in TB patients and, despite high individual variability, it showed some improvement in treatment outcome.Citation5
Considering the well-known pathological reaction triggered in individuals sensitized or infected with M. tuberculosis, known as the Koch phenomenon,Citation6 it is important to evaluate the variety of autoimmune responses triggered by immunotherapeutic candidates based on mycobacterial products, in M. tuberculosis infected hosts. This is particularly important for Hsp65-based immunobiological agents, such as DNA-hsp65 vaccine, because of its high homology with the human Hsp60 and because of the reported association of this protein as a target antigen in some human autoimmune diseases, including arthritis, atherosclerosis, and diabetes.Citation7-Citation9 For this reason, we have evaluated, in vitro and in vivo, autoimmune responses in mice that have been challenged with a high dose virulent M. tuberculosis strain and immunized with DNA-hsp65 for therapeutic purposes.
The experimental protocol (Ethics Committee approval 094/2009) was performed as recently described,Citation10 where 8-wk-old BALB/c mice were challenged with 1 × 105 H37Rv M. tuberculosis cells via intra-tracheal route. Mice started to receive immunotherapy with DNA-hsp65 at day 30 post-infection. A total of four doses of 100 μg of DNA each were administered at 10 d intervals (50 μg in each quadriceps). Control mice received saline or empty vector pVAX1 (Invitrogen). At days 10 and 60 after the last dose of DNA, equivalent to 70 and 120 d after infection, respectively, the mice were euthanized for sample collection. These time points are referred to as short and long follow-up, respectively. In this model we typically observe a significant decrease in M. tuberculosis colony forming units (CFU) counts in lungs, spleen, and liver of DNA-hsp65-treated mice, in parallel with a decrease in lung inflammation, indicating a beneficial effect of the therapy.Citation10,Citation11 These immunotherapeutic effects of DNA-hsp65 are mainly associated with the induction of anti-Hsp65 specific T cells producing IFN-γ, as previously demonstrated.Citation12 Moreover, as shown for other HSP family members, it is possible that Hsp65 also has an adjuvant activity by assisting antigen processing/presentation because it was reported that Hsp65 possesses a proteolytic activity for several types of peptides, including peptides derived from itself.Citation13 It was also shown that the abolition of this property, by site direct mutagenesis, interferes with the disease outcome of NZB/NZW F1 lupus-prone mice that were immunized with Hsp65, and as a consequence, the disease was accelerated.Citation14
To evaluate the humoral immune response following DNA-hsp65 therapy of TB, we first determined the production of anti-Hsp65 antibodies by ELISA. The levels of IgG1 antibodies specific against the M. leprae Hsp65 recombinant protein (rHsp65) were detected in all of the experimental groups at short and long time points () but were significantly higher after DNA-hsp65 immunization 10 d after the end the therapy, indicating the success of the immunization capacity of the DNA-hsp65 vaccine to induce specific antibodies. Although the levels of these antibodies were maintained in DNA-hsp65-immunized mice, the saline and vector groups presented an increase of these cross-reactive antibodies, indicating the progression of the infection (). Contrary to the findings for the IgG1 isotype, IgG2a anti-rHsp65 antibodies were highly produced in all of the infected groups (i.e., saline, vector, and DNA-hsp65) at both time points (). This indicates that the M. tuberculosis infection itself is able to elicit the production of IgG2a antibodies that cross-react with the M. leprae rHsp65. In addition, the levels of IgG2a were considerable higher than those of IgG1; these observations are consistent with the previously reported predominant Th1 immune response to mycobacterial Hsp65.Citation11,Citation15,Citation16
Figure 1. Production of specific and cross-reacting antibodies against mycobacterial Hsp65, human Hsp60 and DNA after M. tuberculosis infection in mice untreated or treated with DNA-hsp65 immunotherapy. (A) anti-IgG1 and (B) IgG2a antibodies reactive to recombinant M. leprae Hsp65 protein. (C) Detection of antibodies that cross-react with human Hsp60. (D) anti-DNA antibodies. The mean value obtained in healthy non-infected animals in each measurement was subtracted from all the individuals in each group prior to performing statistical analysis. *P < 0.05 compared with all other groups at the same time point by one-way ANOVA and Tukey’s multiple comparisons post-test. F1, female F1 of NZB/NZW mice used as positive controls.
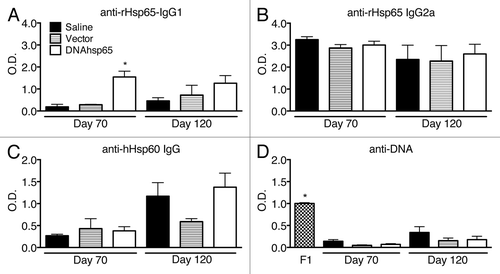
Because some degree of cross-reactivity was observed in infected mice recognizing the vaccine-encoded protein in all of the experimental groups, we next evaluated whether produced antibodies could also cross-react with the homolog human protein (Hsp60). In view of the high homology between the human and the murine Hsp60s (97%), this may be considered a valid way to evaluate induced autoreactivity in this context. Indeed, the infection alone also triggered the production of antibodies that recognize the human Hsp60 (), indicating infection-induced autoreactivity. It is interesting to note that the presence of these antibodies was clearly detected in the long follow-up sample only, indicating that this was a very late phase feature of the infection itself and was not particularly increased by the vaccine because the antibody levels were not different among DNA-hsp65-treated and untreated infected animals. Nonetheless, immunization of healthy mice with the DNA-hsp65 (not challenged with M. tuberculosis) can induce the production of antibodies that cross react with the human Hsp60, as demonstrated before.Citation17 However, this antibody production is not associated to tissue damage, supporting our interpretation that the DNA-hsp65 vaccine does not seem to be involved in triggering pathological autoimmune reactions. It is important to highlight that in this previous work, we observed the induction of anti-Hsp60 cross-reactive antibodies that last up to 210 d after the beginning of the immunization with DNA-hsp65, and in our present study, the evaluations went until 90 d from the first injection of DNA-hsp65. Considering these data, it is tempting to speculate that anti-Hsp-60 cross-reactive antibodies could last at least a similar time in animals infected with M. tuberculosis.
We also evaluated whether the immunization with plasmid DNA as a vector or in the form of DNA-hsp65 vaccine triggered the production of anti-DNA antibodies, which display significant relevance in pathological autoimmunity. A strong production of anti-DNA antibodies was observed in the positive control sample from NZB/NZW F1 lupus-prone mice but not in the experimental groups (). Taken together, the evaluation of the humoral immune response showed that the M. tuberculosis infection in mice elicits antibodies that cross-react with both the M. leprae Hsp65 and the human Hsp60 but does not trigger anti-DNA antibodies. Moreover, the immunotherapy with DNA-hsp65 of animals infected with M. tuberculosis does not boost the production of anti-Hsp60 cross-reactive antibodies. Nevertheless, the characterization of the anti-Hsp60 antibodies regarding IgG1 or IgG2a subtypes could also provide a better idea regarding if these cross-reactive antibodies increase mainly because of the presence of M. tuberculosis or because of DNA-hsp65 immunization in the infected animals, a shortcoming of the study that needs further evaluation. However, the evaluation of the humoral immune response, per se, does not discriminate the potentially pathological autoimmunity, and the immunized groups—with either the plasmid alone or with the DNA-hsp65 vaccine—could eventually develop pathological autoimmunity and tissue aggression where Hsp60 is upregulated during inflammation. Thus, we performed an exhaustive search for signs of pathological autoimmunity following the immunization of infected mice with either the vector or the DNA-hsp65 vaccine. Two different laboratories of pathology performed a single-blinded evaluation of samples obtained from 18 different organs (listed below). Six to ten animals per experimental group were included in the analysis. In addition, to obtain a reliable evaluation that takes into account the entire volume of each organ, the preparation of the samples for the histopathological analysis involved the application of stereological principles that allow for the evaluation of the whole organ compared with random sections commonly obtained to perform histopathological analysis.Citation18,Citation19 Therefore, the organs were sliced in two different manners, depending on whether they were isotropic or anisotropic, before the inclusion in paraffin. For isotropic organs, defined as those in which it is not possible to define the orientation of the section at the histological level (i.e., adrenal gland, articulation and bones, eyes, liver, lungs, lymph nodes, pancreas, skin, spleen, ovary, thymus, thyroid), ten sections from each organ were obtained at different levels at equal intervals, covering the entire organ, and stained for hematoxylin and eosin (HE). For anisotropic organs (i.e., brain, heart, kidneys, large intestine, small intestine, and skeletal muscle), the orientator methodology was applied to convert the organ to isotropic (), and then all of the resultant pieces of the organ were immersed together in paraffin, sliced, and stained, as described before. The alterations found in the histopathological evaluation were classified as minor or major, depending on the frequency and the size of the lesions observed. Minor alterations were found in essentially all organs, which included foci of congestion, hemorrhage, and foci of lymphoplasmacytic inflammatory infiltration (). Considering that these alterations were of minor intensity and were observed in all of the experimental groups, including in healthy control animals, they were not considered to be associated either with the infection or with the immunization with plasmid or DNA-hsp65 vaccine.
Figure 2. Preparation of isotropic and anisotropic organs for paraffin embedding and histological sectioning. Isotropic organs, such as the spleen (A), do not require special procedures and were fixed and embedded in paraffin after their extraction. Anisotropic organs, such as the kidney (B), were pre-sectioned following the orientator method.Citation18 For this method, (1) the organ was cut at random; (2) resultant fragments were cut again with a perpendicular section to the first plane, and (3) step 2 was performed again with the fragments obtained in step 2. After step 3, fragments are considered uniformly isotropic. Then, all of the fragments were immersed in paraffin and sectioned as in A. Grid unit on graph paper equals to 0.5 cm2.
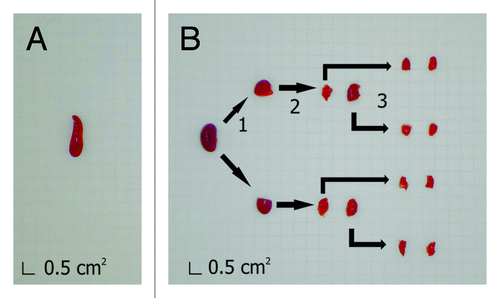
Table 1. Frequency of minor lesions observed in the histopathology evaluation*
In contrast, major alterations were observed in the lungs, lymph nodes, thymus, spleen, and liver of infected mice but not in healthy controls, indicating that the alterations were due to active TB disease (). With the exception of the lungs, the pathological findings consisted of slight lymphoid hyperplasia with mononuclear cell infiltration, with similar intensity among the experimental groups, which is consistent with commonly reported findings due to M. tuberculosis infection.Citation20,Citation21 The alterations in the lungs presented the characteristics of TB pneumonia, consisting of a multifocal process of granulomatous aspect with parenchymatous, subpleural and peribronchovascular distribution, with the typical presence of foamy macrophages surrounded by lymphocytes and some neutrophils. The DNA-hsp65-treated group displayed a more focalized process than the more spread aspect observed in the saline and vector-injected animals, as described recently.Citation10
Figure 3. Histopathological analysis of mice infected with M. tuberculosis and treated or not with DNA-hsp65 immunotherapy. Sections of lung, spleen, lymph node, thymus, and liver were obtained from not infected, saline, vector, and DNA-hsp65 groups. The lung samples presented an active inflammatory process with granuloma-like structures across the tissue in samples from saline and vector injected groups. These lesions were less prominent and smaller in DNA-hsp65-treated animals. In contrast, samples from all of the infected groups presented a slight lymphoid hyperplasia in spleen, lymph node, thymus, and liver. The figure shows the results at 120 d after infection. Similar findings were observed at day 70. Tissue was fixed with 10% buffered formalin, and 5 μm sections were stained with hematoxylin and eosin as described in the text. Magnification panels, 100 ×.
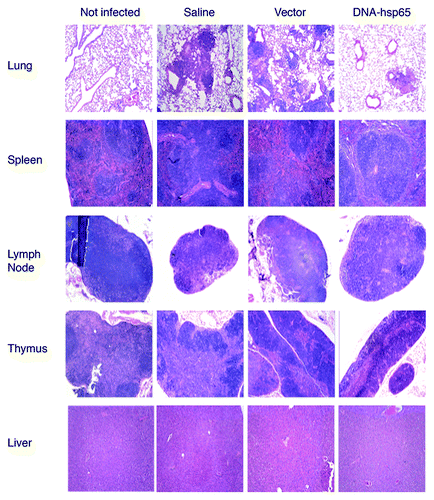
Considering that Hsp60 is a widely expressed molecule across the tissues and increases its expression in stress conditions,Citation22 we could expect that DNA-hsp65 treated animals show signs of autoimmune attack in organs where tuberculosis infection is not common and/or worsen the lesions in the organs where M. tuberculosis is present, as is observed during the Koch phenomenon. In this manner, the stereological analysis, commonly used for meticulous search of lesions or transgene expression,Citation23-Citation25 although not able by itself to discriminate between the lesions caused by the infection from those that could be triggered by the breakdown of tolerance to Hsp60/65, indicates that DNA-hsp65 treatment resulted in diminution of lung damage and did not trigger pathological autoimmunity.
Taken together, our present data on the humoral immune response in addition to the results from the stereologic histopathological evaluation of treated and untreated M. tuberculosis-infected mice indicate that, although cross-reactive antibodies to Hsp60 are elicited in these animals, no histological alteration compatible with autoimmune inflammation could be attributed to the vaccine or plasmid up to 60 d after the end of the therapy. These findings are in accordance with previous data from our group in a similar post-vaccination long-term follow-up evaluation (up to 210 d) in normal BALB/c mice, as mentioned before.Citation17
Our data contrast with the observations of Taylor and colleagues,Citation26 who reported lung damage and no effect in bacterial counts in mice immunized with Hsp65. Other similar deleterious results were reported in the same study with a vaccine encoding Ag85, another well-studied antigen by different laboratories for a new anti-TB vaccine, some of which were evaluated in clinical trials.Citation27,Citation28 Considering that several laboratories that have tested the efficacy of Hsp65 and Ag85-based vaccines in prophylactic and therapeutic schemes did observe a significant reduction in both lung inflammation and bacterial counts, it has been discussed that the harmful effects observed by Taylor et al.Citation26 could be due to endotoxin contamination of the vaccines used.Citation29 In addition, the downregulation of Th2 related genes and the reduction in the overall Th17 response in mice with active TB that received DNA-hsp65 therapy were also observed by our group, together with a higher lung tissue preservation.Citation10,Citation30 These studies suggest that DNA-hsp65 immunization does not only act as a pro-Th1 molecule and a Th2/Th17 inhibitor but also triggers an immunomodulatory response, fine-tuning inflammation. It seems that DNA-hps65 immunization elicits a complex immune response that can be affected not only by the host’s genetic background but also by other ongoing immune responses, such as in infections and in pathological autoimmunity. Accordingly, systemic lupus erythematosus and autoimmune uveitis can be worsened by Hsp65 immunization,Citation14,Citation31 but the immunization with vector DNA or DNA-hsp65 results in the reduction of joint inflammation and pancreatic islet infiltration in models of arthritis and diabetes,Citation32,Citation33 indicating immunoregulatory properties of this immunobiological agent. Interestingly, regulatory T (Treg) cells seem to be part of this immunoregulatory effect because mice vaccinated with DNA-hsp65 prior to M. tuberculosis challenge presented a higher number of CD4+Foxp3+ T cells than mice immunized with plasmid alone or than those infected but not immunized.Citation34 Considering that the uncontrolled inflammation may culminate in important deleterious effects in an ongoing infection,Citation6,Citation35,Citation36 we favor the hypothesis that Treg cells may also have an important participation in the beneficial immune response induced by the DNA-hsp65 by controlling excessive inflammation. The role of Treg cells in the immunoregulatory effect of DNA-hsp65 immunization needs to be investigated further.
In conclusion, our results show that mice chronically infected with M. tuberculosis and treated with DNA-hsp65, which reduces bacterial loads systemically and inflammation in lungs, present in parallel the production of cross-reactive antibodies against Hsp60/65 but do not present pathological autoimmunity up to 60 d after the end of the therapy. Evaluations in animals treated with a combination of chemotherapy and immunotherapy at longer follow-up as well as in animals genetically predisposed to develop autoimmune diseases could be valuable to further investigate the potentially harmful effects of DNA-hsp65 immunotherapy of TB.
| Abbreviations: | ||
| TB | = | tuberculosis |
| Hsp | = | heat-shock protein |
| DNA-hsp65 | = | DNA vaccine encoding Hsp65 |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research received support from Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (Grant number 2009/06793-7). We thank Dr Carlos A Mandarim-de-Lacerda for excellent discussion prior initiation of the study.
References
- Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med 2013; 368:745 - 55; http://dx.doi.org/10.1056/NEJMra1200894; PMID: 23425167
- Stanford J, Stanford C, Grange J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front Biosci 2004; 9:1701 - 19; http://dx.doi.org/10.2741/1292; PMID: 14977580
- Prabowo SA, Gröschel MI, Schmidt ED, Skrahina A, Mihaescu T, Hastürk S, Mitrofanov R, Pimkina E, Visontai I, de Jong B, et al. Targeting multidrug-resistant tuberculosis (MDR-TB) by therapeutic vaccines. Med Microbiol Immunol 2013; 202:95 - 104; http://dx.doi.org/10.1007/s00430-012-0278-6; PMID: 23143437
- Guo S, Zhao J. Immunotherapy for tuberculosis: what’s the better choice?. [Landmark Ed] Front Biosci (Landmark Ed) 2012; 17:2684 - 90; http://dx.doi.org/10.2741/4079; PMID: 22652806
- Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS One 2011; 6:e23826; http://dx.doi.org/10.1371/journal.pone.0023826; PMID: 21909406
- Rook GA, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol 1996; 50:259 - 84; http://dx.doi.org/10.1146/annurev.micro.50.1.259; PMID: 8905081
- Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet 1993; 341:255 - 9; http://dx.doi.org/10.1016/0140-6736(93)92613-X; PMID: 8093914
- Huang MN, Yu H, Moudgil KD. The involvement of heat-shock proteins in the pathogenesis of autoimmune arthritis: a critical appraisal. Semin Arthritis Rheum 2010; 40:164 - 75; http://dx.doi.org/10.1016/j.semarthrit.2009.10.002; PMID: 19969325
- Cohen IR. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol 1991; 9:567 - 89; http://dx.doi.org/10.1146/annurev.iy.09.040191.003031; PMID: 1680347
- Zárate-Bladés CR, Rodrigues RF, Souza PR, Rios WM, Soares LS, Rosada RS, Brandão IT, Masson AP, Floriano EM, Ramos SG, et al. Evaluation of the overall IFN-γ and IL-17 pro-inflammatory responses after DNA therapy of tuberculosis. Hum Vaccin Immunother 2013; 9:1093 - 103; http://dx.doi.org/10.4161/hv.23417; PMID: 23324590
- Bonato VL, Gonçalves ED, Soares EG, Santos Júnior RR, Sartori A, Coelho-Castelo AA, Silva CL. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology 2004; 113:130 - 8; http://dx.doi.org/10.1111/j.1365-2567.2004.01931.x; PMID: 15312144
- Silva CL, Lowrie DB. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect Immun 2000; 68:3269 - 74; http://dx.doi.org/10.1128/IAI.68.6.3269-3274.2000; PMID: 10816472
- Portaro FC, Hayashi MA, De Arauz LJ, Palma MS, Assakura MT, Silva CL, de Camargo AC. The Mycobacterium leprae hsp65 displays proteolytic activity. Mutagenesis studies indicate that the M. leprae hsp65 proteolytic activity is catalytically related to the HslVU protease. Biochemistry 2002; 41:7400 - 6; http://dx.doi.org/10.1021/bi011999l; PMID: 12044173
- Marengo EB, de Moraes LV, Faria M, Fernandes BL, Carvalho LV, Tambourgi DV, Rizzo LV, Portaro FC, Camargo AC, Sant’anna OA. Administration of M. leprae Hsp65 interferes with the murine lupus progression. PLoS One 2008; 3:e3025; http://dx.doi.org/10.1371/journal.pone.0003025; PMID: 18716655
- Souza PR, Zárate-Bladés CR, Hori JI, Ramos SG, Lima DS, Schneider T, Rosada RS, Torre LG, Santana MH, Brandão IT, et al. Protective efficacy of different strategies employing Mycobacterium leprae heat-shock protein 65 against tuberculosis. Expert Opin Biol Ther 2008; 8:1255 - 64; http://dx.doi.org/10.1517/14712598.8.9.1255; PMID: 18694348
- Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun 1998; 66:169 - 75; PMID: 9423854
- Lima DS, Zarate-Blades CR, Souza PRM, Trombone AP, Santos RR, Brandao IT, et al. No Evidence of Pathological Autoimmunity Following Mycobacterium Leprae Heat-Shock Protein 65-DNA Vaccination in Mice. European Journal of Inflammation 2009; 7:77 - 85
- Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc 2003; 75:469 - 86; http://dx.doi.org/10.1590/S0001-37652003000400006; PMID: 14605681
- Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol (1985) 2007; 102:459 - 67; http://dx.doi.org/10.1152/japplphysiol.00808.2006; PMID: 16973815
- Nobrega C, Nunes-Alves C, Cerqueira-Rodrigues B, Roque S, Barreira-Silva P, Behar SM, Correia-Neves M. T cells home to the thymus and control infection. J Immunol 2013; 190:1646 - 58; http://dx.doi.org/10.4049/jimmunol.1202412; PMID: 23315077
- Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb) 2011; 91:497 - 509; http://dx.doi.org/10.1016/j.tube.2011.03.007; PMID: 21733755
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol 2013; 14:630 - 42; http://dx.doi.org/10.1038/nrm3658; PMID: 24026055
- Alladi PA, Mahadevan A, Vijayalakshmi K, Muthane U, Shankar SK, Raju TR. Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochem Int 2010; 57:530 - 9; http://dx.doi.org/10.1016/j.neuint.2010.06.018; PMID: 20615443
- Dedeoglu A, Ferrante RJ, Andreassen OA, Dillmann WH, Beal MF. Mice overexpressing 70-kDa heat shock protein show increased resistance to malonate and 3-nitropropionic acid. Exp Neurol 2002; 176:262 - 5; http://dx.doi.org/10.1006/exnr.2002.7933; PMID: 12093104
- Hecker JG, Hall LL, Irion VR. Nonviral gene delivery to the lateral ventricles in rat brain: initial evidence for widespread distribution and expression in the central nervous system. Mol Ther 2001; 3:375 - 84; http://dx.doi.org/10.1006/mthe.2001.0272; PMID: 11273780
- Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun 2003; 71:2192 - 8; http://dx.doi.org/10.1128/IAI.71.4.2192-2198.2003; PMID: 12654841
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al, MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381:1021 - 8; http://dx.doi.org/10.1016/S0140-6736(13)60177-4; PMID: 23391465
- Hokey DA, Ginsberg A. The current state of tuberculosis vaccines. Hum Vaccin Immunother 2013; 9:2142 - 6; http://dx.doi.org/10.4161/hv.25427; PMID: 23792698
- Lowrie DB. DNA vaccines for therapy of tuberculosis: where are we now?. Vaccine 2006; 24:1983 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.11.010; PMID: 16316711
- Zárate-Bladés CR, Bonato VL, da Silveira EL, Oliveira e Paula M, Junta CM, Sandrin-Garcia P, Fachin AL, Mello SS, Cardoso RS, Galetti FC, et al. Comprehensive gene expression profiling in lungs of mice infected with Mycobacterium tuberculosis following DNAhsp65 immunotherapy. J Gene Med 2009; 11:66 - 78; http://dx.doi.org/10.1002/jgm.1269; PMID: 19035575
- Commodaro AG, Marengo EB, Peron JP, Brandao W, Arslanian C, Melo RL, Baldon EJ, Belfort R Jr., Sant’anna OA, Rizzo LV. The Role of M. leprae Hsp65 Protein and Peptides in the Pathogenesis of Uveitis. Autoimmune Dis 2012; 2012:197648; http://dx.doi.org/10.1155/2012/197648; PMID: 22966424
- Santos-Junior RR, Sartori A, De Franco M, Filho OG, Coelho-Castelo AA, Bonato VL, Cabrera WH, Ibañez OM, Silva CL. Immunomodulation and protection induced by DNA-hsp65 vaccination in an animal model of arthritis. Hum Gene Ther 2005; 16:1338 - 45; http://dx.doi.org/10.1089/hum.2005.16.1338; PMID: 16259568
- Santos Júnior RR, Sartori A, Bonato VL, Coelho Castelo AA, Vilella CA, Zollner RL, Silva CL. Immune modulation induced by tuberculosis DNA vaccine protects non-obese diabetic mice from diabetes progression. Clin Exp Immunol 2007; 149:570 - 8; http://dx.doi.org/10.1111/j.1365-2249.2007.03433.x; PMID: 17590177
- Fedatto PF, Sérgio CA, Paula MO, Gembre AF, Franco LH, Wowk PF, Ramos SG, Horn C, Marchal G, Turato WM, et al. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells. Immunology 2012; 137:239 - 48; http://dx.doi.org/10.1111/imm.12006; PMID: 22891805
- Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol 2012; 34:753 - 70; http://dx.doi.org/10.1007/s00281-012-0351-7; PMID: 23076807
- Ottenhoff TH. The knowns and unknowns of the immunopathogenesis of tuberculosis. Int J Tuberc Lung Dis 2012; 16:1424 - 32; http://dx.doi.org/10.5588/ijtld.12.0479; PMID: 23044443
