Abstract
Background: Concerns about influenza vaccine effectiveness in older adults and the role of influenza strains encountered earlier in life led to this study.
Methods: Antibody responses against antigens in the 2011–2012 influenza vaccine at 21 days post vaccination were analyzed in 264 individuals aged 50–80 years. At Days 0 and 21, sera were tested for hemagglutination-inhibition titers against these vaccine strains and at Day 0 against a panel of 15 historical seasonal strains.
Results: The proportions of participants with seroprotective titers ≥1:40 to the vaccine strains at Days 0 and 21, respectively, were 37% and 66% for A(H1N1) and 28% and 63% for A(H3N2). An increasing number of responses ≥1:40 against historical strains was associated with seroprotective responses after vaccination among participants with a titer <1:40 at Day 0 for A(H1N1) and A(H3N2) vaccine strains (P < 0.01). In multivariable regression analyses among those with Day 0 titer <1:40, after controlling for age, sex, race, site and diabetes, Day 21 titers ≥ 1:40 for the vaccine A strains were significantly more likely as the number of seroprotective responses against historical strains increased (A(H1N1) odds ratio [OR] = 1.41, 95% confidence interval [CI] = 1.09–1.82 and A(H3N2) OR = 1.32, 95% CI = 1.07–1.62). The likelihood of seroconversion was significantly higher with an increasing number of responses to historical strains for A(H3N2) only (OR = 1.24, 95% CI = 1.01–1.52). Seroconversion was significantly less likely as Day 0 vaccine strain titers increased.
Conclusions: Seroprotective titers after influenza vaccination increased as the number of responses to historical strains increased.
Introduction
Influenza virus infections cause severe morbidity and mortality worldwide including as many as 200 000 influenza-associated hospitalizations in the US annually,Citation1 occurring mostly among the very young (<1 y) and the elderly (≥65 y).Citation2 The elderly account for nearly 90% of influenza deaths.Citation3 The severity of the influenza season varies from year to year and by strain of the infecting virus.
The severity of influenza infection for an individual may partially depend upon his or her ability to mount an effective immune response to viral infection. Primary infections elicit both CD8+ cytotoxic T cells (CTL) and antibodies. CTL are thought to play the main role in clearance of primary infections by eliminating virus-infected cells, while neutralizing antibodies, typically detected only after virus clearance, can effectively prevent future infections with antigenically related viruses.Citation4 Accordingly, neutralizing antibodies elicited to hemagglutinin after administration of inactivated vaccines represent a major source of vaccine-mediated immunological protection. Protection induced by influenza vaccination is diminished in the elderly compared with younger, healthy adults.Citation5-Citation7
Currently, most assessments of influenza vaccine immune response focus on hemagglutination inhibition (HI) antibody titers. However, antibody responses to current vaccine strains are only one measure of vaccine-induced protection.Citation8 Nascent immune responses may be affected by immunologic memory from past exposure to influenza virus infections or influenza vaccines. For example, the concept of antigenic seniority suggests that the most robust immune response is made after one’s first influenza infection. Therefore, fewer hemagglutinin-specific antibodies are produced after antigen exposures occurring later in life compared with exposures earlier in life.Citation9 This study investigated the association of pre-existing HI titers to historical influenza strains with antibody levels before and after receipt of the 2011–2012 trivalent influenza vaccine (TIV) among adults aged 50–80 y. We hypothesized that persons with broader antibody repertoires (i.e., antibody responses against a greater number of historical influenza strains) would produce enhanced immune responses after vaccination compared with persons with narrower repertoires, and that antibody repertoires would differ by age.
Results
Study population description
Demographic characteristics of the 264 participants are shown in . Average age was 62 (SD = 7.8 y) with 59% (157/264) aged 50–64 y. Fifty-eight percent of the cohort was female; 79.6% of participants self-identified their race as white, and 42% had diabetes (DM) with a significantly higher percentage in the older age group (P = 0.03). Fifty-six percent of the participants were enrolled at Marshfield.
Table 1. Demographic characteristics and underlying high-risk diseases, overall and stratified by age
Hemagglutination inhibition (HI) response to 2011–2012 TIV and variation by age
shows the percent of the study population who were seroprotected (titer ≥ 1:40) at Day 0 and HI responses to 2011–2012 vaccine strains. The proportions of participants whose HI titers were seroprotective at Day 0 and 21, respectively, were 37% and 66% for A(H1N1) and 28% and 63% for A(H3N2), with no differences in response by age group. Seroconversion percentages were lower, ranging from 34% to 38% for both A strains, with no differences by age group. GMT ratios with 95% CIs were 2.1 (1.9–2.5) for A(H1N1) and 2.3 (2.0–2.6) for A(H3N2), and did not differ by age group. Adjusted GMT ratios were similar to unadjusted GMT ratios. Together these data show that vaccination with 2011–2012 TIV elicited detectable virus-specific antibody responses to influenza A viruses in the majority of participants, though vaccination did not appear to dramatically increase HI.
Table 2. Hemagglutination-inhibition responses to 2011–2012 trivalent inactivated influenza vaccine antigens, overall and stratified by age group
At Day 0, GMTs and 95% CI for DM and for no DM were 21.8 (18.2–26.2) and 25.2 (21.5–29.6), respectively, for A(H1N1) and 19.5 (16.4–23.2) and 20.3 (17.5–23.5) for A(H3N2), respectively, and were not different by DM status. At Day 21, GMTs and 95% CI for DM and for no DM were 47.9 (39.2–58.5) and 53.3 (44.4–64), respectively, for A(H1N1) and 41.5 (33.5–51.5) and 49.6 (41.7–58.8) for A(H3N2), respectively, and were not different by DM status.
Geometric mean titers for vaccine and historical strains by age
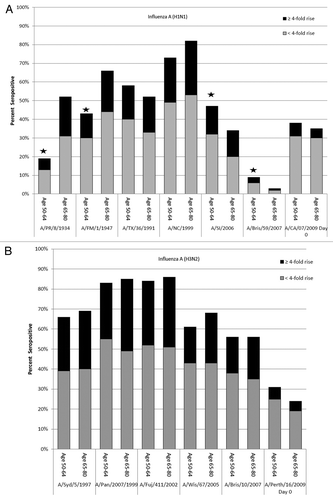
The historical and vaccine strain GMTs by age are examined in . For A(H1N1), the mean GMT against the current vaccine strain was significantly lower for the older group at Day 0. The GMTs against the oldest influenza strains (A/Puerto Rico/8/1934 and A/Fort Monmouth/1/1947) were significantly higher in the older group than the younger group (P < 0.001), consistent with the fact that these 2 virus strains circulated before the individuals in the younger group were born. In contrast, antibody titers to more recent strains, such as A/Solomon Islands/3/2006 and A/Brisbane/59/2007, were significantly lower in the older group than in the younger group (P < 0.05). No differences in GMTs between age groups were found for A(H3N2) for either the current vaccine strain or historical strains.
Table 3. Geometric mean titers for 2011–2012 influenza A vaccine and selected historical strains, overall and stratified by age
We compared log2 mean titers for A/Puerto Rico/8/1934 for those with and without a 4-fold response to A/California/7/2009 and found the comparison not significant (4.95 vs. 5.11, P = 0.59).
Level of historical seroprotective titers ≥ 1:40
shows the percentage of participants with a seroprotective HI titer against each historical strain by age group. Differences by age group are evident only for A(H1N1). Fifty percent of older participants had seroprotective titers (i.e., titers ≥ 1:40) against the A/Puerto Rico/8/1934 strain; compared with 20% of younger participants (P < 0.001)—65% of older participants had seroprotective titers against the A/Fort Monmouth/1/1947 strain compared with 43% of younger participants (P < 0.001). Conversely, for A/Brisbane/59/2007, seroprotection was 9.6% for younger participants compared with 2.8% for older participants (P = 0.033), and for A/Solomon Islands/3/2006, seroprotection was 34% for older participants and 47% for younger participants (P = 0.029). Immunological responses to historical A(H3N2) viruses did not differ by age group.
Figure 1. Percent of participants with seroprotective (titer ≥ 1:40) at Day 0 to A(H1N1) and A(H3N2) influenza virus strains. The overall height of the bars indicates the percent of participants with seroprotective titers (HI ≥ 1:40) to a given historical strain at Day 0. The upper part of each bar indicates the percent of those individuals who seroconverted (≥4-fold rise in titer) against the specified current influenza vaccine A virus strain. *Difference in percent seroprotected between age groups (P < 0.05). A/PR/8/1934 = A/Puerto Rico/8/1934; A/FM/1/1947 = A/Fort Monmouth/1/1947; A/TX/36/1991 = A/Texas/36/1991; A/NC/1999 = A/New Caledonia/20/1999; A/SI/2006 = A/Solomon Islands/3/2006; A/Bris/59/2007 = A/Brisbane/59/2007; A/CA/07/2009 = A/California/07/2009; A/Syd/5/1997 = A/Sydney/5/1997; A/Pan/2007/99 = A/Panama/2007/1999; A/Fuj/411/2002 = A/Fujian/411/2002; A/Wis/67/2005 = A/Wisconsin/67/2005; A/Bris/10/2007 = A/Brisbane/10/2007.
Association between historical counts of seroprotective titers to historical strains with HI response to the 2011–2012 influenza vaccine
The number of historical viruses of that type for which each specimen had a titer ≥ 1:40 was called the “historical count”—for example, if a given serum specimen were tested against 6 historical H1N1 viruses and had a seroprotective titer for 4 of them, the historical count was 4 for A(H1N1). Seroprotective titers at Day 21 increased as the number of historical strains with a seroprotection titer increased (tests for trend P < 0.001 for A(H1N1) and A(H3N2); data not shown). When this analysis was restricted to those seronegative at Day 0 (), seroprotection at Day 21 also increased as the number of historic A strains with a seroprotection titer increased (tests for trend P < 0.01 each). Multivariable logistic regression analyses that controlled for demographic variables was conducted, using Day 0 seroprotection, Day 21 seroprotection, Day 21 seroprotection among those seronegative at Day 0, and seroconversion as outcome variables, against both of the A strains in the 2011–2012 TIV ().
Figure 2. Proportion of participants with seroprotected (titers ≥ 1:40) at Day 21 by type and by the count of seropositive historical influenza strains among those seronegative at Day 0. The number of historical viruses for which each specimen had a titer ≥ 1:40 was called the “historical count”—for example, if a given serum specimen were tested against 6 historical viruses and had a titer ≥ 1:40 for 4 of them, the historical count was 4.
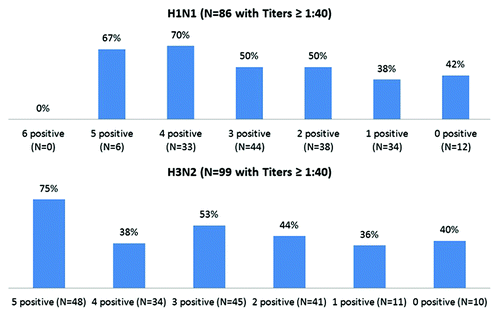
Table 4. Association of 2011–2012 influenza vaccine A strain seroprotection (HI titer ≥ 1:40) and seroconversion with count* of historical strains that have seroprotective titers (HI titer ≥ 1:40) at Day 0 from logistic regression: adjusted results
The likelihood of an individual having titers ≥ 1:40 before vaccination increased as the number (count) of seroprotective antibody titers against historical strains increased; this was found for both influenza A vaccine strains (). These data suggest that participants with “broader” antibody repertoires (i.e., responses to multiple distinct influenza viruses) prior to vaccination were more likely to have antibodies capable of cross-reacting with the 2011–2012 vaccine viruses.
Because seroprotective titers at Day 0 would be expected to persist through Day 21, seroprotective titer at Day 0 was included in the model examining seroprotection at Day 21 for each vaccine strain. This relationship was confirmed (, row 2). In addition, seroprotection at Day 21 was significantly more likely as the number (count) of seroprotective responses against historical strains increased (, row 3). After excluding individuals with seroprotective titers at baseline, seroprotection at Day 21 was significantly more likely as the number of seroprotective antibody titers against historical strains increased for both A(H1N1) and A(H3N2) (, row 4). Seroconversion to influenza A(H3N2) was significantly more likely as the historical count increased, but this relationship did not hold for A(H1N1). Higher Day 0 antibody titers were associated with a lower likelihood of seroconversion for both vaccine A strains, (, row 6). In summary, seroprotective Day 21 titers were associated with both Day 0 seroprotection to the vaccine strain and with the number of seroprotective responses to historical strains at Day 0 for influenza A—whereas, after controlling for Day 0 seroprotective titers, the number of historical strains recognized by participants’ antibody repertoires was associated with seroconversion only for A(H3N2). While all models adjusted for age, sex, race, site, and diabetes, none of these variables showed significant results.
Discussion
There is increasing awareness that the immune response to antigen exposure is shaped by an individual’s history of exposures to related antigens. Virtually all adults, and even many older children, show some baseline level of antibody immunity to influenza.Citation10 Accordingly, the kinetics of antibody responses to influenza vaccination in adults are typically rapid, consistent with the expansion of pre-existing memory responses.Citation11 Therefore, influenza immunization presents a unique challenge as its goal is to elicit a robust response to vaccine antigens in individuals with unknown and variable backgrounds of pre-existing immunity. This study investigated how pre-existing antibodies to historical influenza viruses influenced HI antibody titers against TIV vaccine strains before and after administration of the 2011–2012 TIV in adults aged 50–64 or 65–80 y.
Consistent with previous results, we found seroprotective serum antibody titers against both A strains in the 2011–2012 vaccine prior to vaccination in a substantial proportion of participants (overall, 28–37%). The fact that such a high proportion of participants had seroprotective titers on Day 0 is perhaps not surprising, because the viruses comprising the 2011–2012 vaccine were identical to those in the previous season’s vaccine. Nonetheless, our results further illustrate the concept that influenza vaccine is administered against a background of pre-existing immunity, which is often not explicitly measured.
Emerging evidence suggests that influenza vaccination generally stimulates expansion of memory B cells that produce antibodies recognizing epitopes that are shared between the vaccine virus and antigenically related viruses to which the individual has been exposed in the past.Citation12,Citation13 We postulated that individuals with a larger “antibody repertoire,” that is, detectable pre-existing antibody responses to multiple historical strains, would make stronger responses to TIV than individuals with more restricted repertoires, as these individuals would be more likely to have pre-existing antibodies capable of cross-reacting with contemporary viral antigens. Indeed, we found that participants with broader antibody repertoires at Day 0 had stronger antibody responses to TIV at Day 21, whether or not they had seroprotective titers to the 2011–2012 vaccine viruses at Day 0. These results were statistically significant for both A(H1N1) and A(H3N2). Although this study cannot directly assess the protective efficacy of specific antibody responses, it is interesting to note a recent study showing that pre-existing antibodies elicited by infection with antigenically related historical viruses protected ferrets from challenge with a 2009 H1N1 pandemic isolate. The robustness of protection was proportional to the antigenic relatedness between the historical virus and the 2009 isolate.Citation14 Notably, a relationship was also found between the number of historical influenza A strains recognized at Day 0 and the likelihood that individuals had pre-existing seroprotective titers against 2011–2012 A(H1N1) or A(H3N2) viruses. Because low pre-vaccination titers have been associated with a more rapid decline in titers following vaccination,Citation15 we speculate that individuals in our study with broader antibody repertoires may have experienced slower declines in antibody titer following vaccination than those with more limited repertoires. Several factors are likely to affect responsiveness to influenza vaccination. Individuals who have been exposed to a greater number of influenza strains, through infection and/or vaccination, may be more likely to harbor pre-existing memory responses that cross-react with vaccine strains. Host genetic factors may also predispose some individuals to respond more effectively to vaccination than others. Accordingly, recent studies have shown that increased antibody titer after influenza vaccination is associated with upregulation of multiple gene networks, particularly those involved in the IL-6-associated inflammatory response and in antigen processingCitation16,Citation17; polymorphisms in these genes or their regulatory elements may therefore influence overall vaccine “responsiveness.” The rapid upregulation of immune response genes in vaccine “responders” may also reflect the rapid expansion of pre-existing memory responses, rather than underlying genetic polymorphism. Neither these studies nor our data can separate the relative contributions of host genetics and immunological memory in vaccine responsiveness.
The 2009 A(H1N1) pandemic represented a departure from the expected linearity of influenza virus antigenic change. The hemagglutinin (HA) segment of the 2009 pandemic virus was derived from a swine influenza virus, and it was genetically and antigenically more closely related to HA segments from A(H1N1) viruses circulating between 1918 and ~1950 than it was to the HAs of recent seasonal A(H1N1) viruses.Citation18,Citation19 Accordingly, in previous studies, individuals ≥65 y old in 2009, who had been exposed to A(H1N1) viruses circulating before 1950, were the only participants found to have cross-reactive antibody responses to the pandemic virus.Citation20 Participants in our study aged y 65–80 had significantly stronger responses against A(H1N1) strains isolated in the 1930s and 1940s than participants aged 50–64 y, as would be expected, because individuals in the younger cohort were born after these older viruses circulated. Given the close antigenic relationship between A(H1N1) isolates from the 1930s and 1940s and the 2009 virus, we were surprised to find no difference in seroprotective titers against A/California/07/2009 by age group on Day 0 or Day 21. Indeed, the younger group showed slightly, but significantly, higher titers against the 2009 pandemic virus at Day 0. We speculate that these results reflect the fact that the pandemic virus had circulated for ~2 y before our study, and as a result many of our study participants had likely already been exposed to it through infection and/or vaccination. Younger subjects might therefore have made more robust responses than older subjects, particularly if the younger group contains a larger proportion of individuals who were infected with the pandemic virus.
The goal of vaccination is to increase the titer of influenza-specific antibodies regardless of whether participants have pre-existing antibodies—hence, the impact of pre-existing immunity on seroconversion was also examined. Relatively low rates of seroconversion in both age groups were found: Adjusted GMT ratios were between 1.5 and 2.3 for all vaccine antigens and age groups, and seroconversion was observed in 22.1–40.2% of participants, depending on antigen and age group. A significant association between the number of historical strains recognized with titer ≥ 1:40 and seroconversion after vaccination was observed only for A(H3N2) viruses. Seroprotective titers at Day 0 were significantly associated with seroprotective titers at Day 21, in accord with previous observations.Citation21,Citation22
Strengths and limitations
To our knowledge, this is the first study to assess how seroprotection to historical influenza viruses affected HI responses to the A strains in the 2011–2012 vaccine. However, our study included a limited number of participants, who resided in only 2 areas of the United States. Our results should not be generalized to the entire country due to this geographic restriction and because we intentionally enrolled a high percentage of individuals with diabetes and of middle- and older ages whose immune responses may differ from the general population. We did not gather prior vaccination history; it would not be possible to differeniate infection from vaccine response with the tests used in this study because antibody titers from recent strains cannot differentiate associations due to infection from associations due to vaccination. Finally, the limited number of A(H3N2) strains were from 1997 or later, based on what was readily available at the University of Pittsburgh lab.
Conclusions
We found that the breadth of the pre-existing antibody repertoire against influenza A viruses affects responsiveness to TIV vaccination. Individuals who recognize multiple historical influenza strains produce stronger antibody responses to A(H1N1) and A(H3N2) viruses, and are more likely to seroconvert to A(H3N2) viruses, following TIV administration. We also observed relatively low rates of seroconversion after TIV administration, and low ratios of post- to pre-vaccination GMTs. These findings are consistent with an emerging picture in which vaccination likely preferentially stimulates a pool of pre-existing memory B cells that produce antibodies recognizing epitopes from antigenically similar strains. More immunogenic influenza vaccine formulations are needed, both to boost pre-existing cross-reactive responses and to more effectively elicit de-novo responses.
Materials and Methods
Subjects
Study sites included outpatient medical facilities in Marshfield, Wisconsin and Pittsburgh, Pennsylvania. Individuals with diabetes mellitus (DM) were targeted so that they would comprise approximately one-half of the participants, due to the prevalence of DM and the possible impact on vaccine response. Eligible patients ages 50–80 y, who had not yet received the 2011–2012 TIV were enrolled from 8/31/11 to 11/11/11—influenza virus did not circulate widely in the community during that time period and the participants were not monitored for influenza during that time period. Participants were recruited from outpatient settings. Exclusion criteria included documented contraindications to TIV,Citation23 Guillain-Barré syndrome, dementia or Alzheimer disease, estimated life expectancy <2 y, medical treatment causing or diagnosis of an immunocompromising condition, or concurrent participation in another influenza vaccine research study. Consented participants had blood drawn before receiving the 2011–12 standard-dose intramuscular TIV (Day 0) and at approximately Day 21 (range, 17–28 d post vaccination) for serum. Serum samples were aliquoted and frozen at –80 °C until assayed. Study procedures, informed consent and data collection documents were reviewed and approved by Institutional Review Boards of Marshfield Clinic (IRB approval #COL10611), the University of Pittsburgh (IRB approval #PRO11060173), and the Centers for Disease Control and Prevention (IRB approvals #6215 and #6221).
Propagation of influenza viruses
Each serum sample was tested in HI assays against the A strains in the 2011–2012 TIV (A/California/7/2009 [H1N1]; A/Perth/16/2009 [H3N2]), as well as a panel of historical, seasonal isolates selected because they represented antigenically distinct viruses and were readily available to the Ross lab; they included 6 A(H1N1) and 5 A(H3N2). Reference sera from individuals vaccinated with either 2011–2012 TIV seasonal Fluzone vaccine (Sanofi-Pasteur) or 2009 pandemic H1N1 FluMist (MedImmune) vaccines were used as positive controls. B strains were not included due to funding constraints.
Hemagglutination inhibition assay
The HI assay protocol was adapted from the CDC laboratory-based influenza surveillance manual as previously describedCitation24 and assessed functional antibodies to the hemagglutinin able to inhibit agglutination of turkey erythrocytes. To inactivate non-specific inhibitors, sera were treated with receptor destroying enzyme prior to being tested.Citation25-Citation29 The HI titer was determined in assays by the reciprocal dilution of the last well that contained non-agglutinated red blood cells and was conducted in duplicate. Positive and negative serum controls were included for each plate.
Statistical methods
Key research questions included (1) Did vaccinees respond serologically to 2011–2012 TIV? (2) Did this response vary by age? (3) Were HI titers of ≥1:40 (seroprotection) against 2011–2012 TIV associated with detection of antibodies to an increasing number of historical strains? (4) Did response to historical strains vary by age? (5) Was seroconversion (≥4-fold rise in HI titers from Day 0 to Day 21, if Day 0 titers were ≥1:10 or a Day 21 titer of 1:40 if Day 0 titers were <1:10) to 2011–2012 TIV antigens associated with detection of antibodies to an increasing number of historical strains?Citation30 Three primary dichotomous outcomes were used—Day 0 titer ≥ 1:40, Day 21 titer ≥ 1:40, and seroconversion. Seronegative status was a titer < 1:40. Geometric mean titers (GMTs) and GMT ratios were calculated by (1) converting raw HI titers into log2-HI titers, (2) computing the means and 95% CIs, and (3) calculating the anti-log of those values. GMT ratios were calculated as the ratio of GMT at Day 21 to GMT at Day 0 (RGMT). This can be expressed as GMTD21/GMTD0 = antilog2 (mean[log2-HID21] – mean[log2-HID0]).
The sample size to test for non-inferiority between 2 groups is calculated from n = (1 + 1/u) (Zα + Zβ)2 σ2/(log[RGMT] − δ0), where RGMT is the ratio of GMT between groups. For a power of 0.8 and directional α of 0.025, a conservative estimate for RGMT of 0.8, and a δ0 of –0.69, 90 participants are needed in each group, for a total of 180.
To assess the response to the 2011–2012 vaccine, the mean pre- and post-vaccination log2-HI titers were compared by analysis of variance (ANOVA). The adjusted mean change from the pre- to the post-vaccination log2-HI titers, controlling for race, sex, site, diabetes status, and Day 0 baseline titers was obtained by analysis of covariance (ANCOVA). To compare age-based responses for percent seroprotection and percent seroconversion, participants were divided into 2 age groups—those aged 50–64 y (birth years 1947–1961) and those aged 65–80 y (birth years 1931–1946) and chi-square tests were used. In addition, the log2-transformed titers in the different age groups were compared by one-way ANOVA.
Cochran-Armitage trend tests were conducted to investigate the relationship between increasing number of titers ≥ 1:40 for historical strains of that type and post-vaccination titers ≥ 1:40.Citation31-Citation33 Using logistic regression and controlling for age, race, sex, site, diabetic status, and Day 0 baseline titers, associations between each of 2 outcome variables and number of seroprotective historical responses of that type were examined for each of the 2011–2012 TIV A strains. Variance inflation factors were used for detecting collinearity among independent variables. Statistical significance was set at P < 0.05.
Disclosure of Potential Conflicts of Interest
R.K.Z. and C.J.L. have research funding from Sanofi Pasteur, Inc. and Merck and Co, Inc. M.P.N. has research funding from Merck and Co., Inc. M.P.N. and C.J.L. consult for MedImmune, LLC. M.E.S. receives grant support from MedImmune, LLC.
Acknowledgments
The authors would like to thank Jedediah D Seltzer and Donald M Carter for technical assistance.
Funding
This investigation was supported by cooperative agreement U01 IP000467 from the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of those authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This research was also supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC.
References
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333 - 40; http://dx.doi.org/10.1001/jama.292.11.1333; PMID: 15367555
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54:1427 - 36; http://dx.doi.org/10.1093/cid/cis211; PMID: 22495079
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057 - 62; PMID: 20798667
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol 1997; 159:5197 - 200; PMID: 9548456
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 69; http://dx.doi.org/10.1016/j.vaccine.2005.08.105; PMID: 16213065
- Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis 1993; 167:584 - 92; http://dx.doi.org/10.1093/infdis/167.3.584; PMID: 8440930
- Centers for Disease Control and Prevention (CDC). Interim adjusted estimates of seasonal influenza vaccine effectiveness - United States, February 2013. MMWR Morb Mortal Wkly Rep 2013; 62:119 - 23; PMID: 23425960
- Webster RG. Immunity to influenza in the elderly. Vaccine 2000; 18:1686 - 9; http://dx.doi.org/10.1016/S0264-410X(99)00507-1; PMID: 10689149
- Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJ, Guan Y, Jiang CQ, Cummings DA. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog 2012; 8:e1002802; http://dx.doi.org/10.1371/journal.ppat.1002802; PMID: 22829765
- Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol 2007; 81:215 - 28; http://dx.doi.org/10.1128/JVI.01957-06; PMID: 17050593
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667 - 71; http://dx.doi.org/10.1038/nature06890; PMID: 18449194
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047 - 52; http://dx.doi.org/10.1073/pnas.1118979109; PMID: 22615367
- Ellebedy AH, Ahmed R. Re-engaging cross-reactive memory B cells: the influenza puzzle. Front Immunol 2012; 3:53; http://dx.doi.org/10.3389/fimmu.2012.00053; PMID: 22566934
- O’Donnell CD, Wright A, Vogel LN, Wei CJ, Nabel GJ, Subbarao K. Effect of priming with H1N1 influenza viruses of variable antigenic distances on challenge with 2009 pandemic H1N1 virus. J Virol 2012; 86:8625 - 33; http://dx.doi.org/10.1128/JVI.00147-12; PMID: 22674976
- Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine 2010; 28:3929 - 35; http://dx.doi.org/10.1016/j.vaccine.2010.03.067; PMID: 20394719
- Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Niño D, Arden N, Quarles JM, Couch RB, Belmont JW. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J Infect Dis 2011; 203:921 - 9; http://dx.doi.org/10.1093/infdis/jiq156; PMID: 21357945
- Franco LM, Bucasas KL, Wells JM, Niño D, Wang X, Zapata GE, Arden N, Renwick A, Yu P, Quarles JM, et al. Integrative genomic analysis of the human immune response to influenza vaccination. eLife 2013; 2:e00299; http://dx.doi.org/10.7554/eLife.00299; PMID: 23878721
- Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009; 459:931 - 9; http://dx.doi.org/10.1038/nature08157; PMID: 19525932
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197 - 201; http://dx.doi.org/10.1126/science.1176225; PMID: 19465683
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, DeVos JR, Gargiullo PM, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945 - 52; http://dx.doi.org/10.1056/NEJMoa0906453; PMID: 19745214
- Beyer WE, Palache AM, Sprenger MJ, Hendriksen E, Tukker JJ, Darioli R, van der Water GL, Masurel N, Osterhaus AD. Effects of repeated annual influenza vaccination on vaccine sero-response in young and elderly adults. Vaccine 1996; 14:1331 - 9; http://dx.doi.org/10.1016/S0264-410X(96)00058-8; PMID: 9004442
- Seidman JC, Richard SA, Viboud C, Miller MA. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses 2012; 6:52 - 62; http://dx.doi.org/10.1111/j.1750-2659.2011.00268.x; PMID: 21668661
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128 - 32; PMID: 21866086
- Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev 2006; 19:614 - 36; http://dx.doi.org/10.1128/CMR.00005-06; PMID: 17041137
- Bright RA, Medina MJ, Xu X, Perez-Oronoz G, Wallis TR, Davis XM, Povinelli L, Cox NJ, Klimov AI. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005; 366:1175 - 81; http://dx.doi.org/10.1016/S0140-6736(05)67338-2; PMID: 16198766
- Bright RA, Ross TM, Subbarao K, Robinson HL, Katz JM. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA vaccine. Virology 2003; 308:270 - 8; http://dx.doi.org/10.1016/S0042-6822(03)00008-4; PMID: 12706077
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 2006; 295:891 - 4; http://dx.doi.org/10.1001/jama.295.8.joc60020; PMID: 16456087
- Mitchell JA, Green TD, Bright RA, Ross TM. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine 2003; 21:902 - 14; http://dx.doi.org/10.1016/S0264-410X(02)00539-X; PMID: 12547601
- Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol 2000; 1:127 - 31; http://dx.doi.org/10.1038/77802; PMID: 11248804
- U.S. Department of Health and Human Services. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Silver Spring, MD, 2007.
- Cochran WG. Some Methods for Strengthening the Common χ2 Tests. Biometrics 1954; 10:417 - 51; http://dx.doi.org/10.2307/3001616
- Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics 1955; 11:375 - 86; http://dx.doi.org/10.2307/3001775
- Liu H. Cochran-Armitage Trend Test for Using SAS. SAS Institute Inc, 2004.
