Abstract
Background: Mumps, a communicable, acute and previously well-controlled disease, has had recent and occasional resurgences in some areas.
Methods: A randomized, double-blind, controlled and multistep phase I study of an F-genotype attenuated mumps vaccine produced in human diploid cells was conducted. A total of 300 subjects were enrolled and divided into 4 age groups: 16–60 years, 5–16 years, 2–5 years and 8–24 months. The groups were immunized with one injection per subject. Three different doses of the F-genotype attenuated mumps vaccine, A (3.5 ± 0.25 logCCID50), B (4.25 ± 0.25 logCCID50) and C (5.0 ± 0.25 logCCID50), as well as a placebo control and a positive control of a licensed A-genotype vaccine (S79 strain) were used. The safety and immunogenicity of this vaccine were compared with those of the controls.
Results: The safety evaluation suggested that mild adverse reactions were observed in all groups. No serious adverse event (SAE) was reported throughout the trial. The immunogenicity test showed a similar seroconversion rate of the neutralizing and ELISA antibody in the 2- to 5-year-old and 8- to 24-month-old groups compared with the seroconversion rate in the positive control. The GMT of the neutralizing anti-F-genotype virus antibodies in the vaccine groups was slightly higher than that in the positive control group.
Conclusions: The F-genotype attenuated mumps vaccine evaluated in this clinical trial was demonstrated to be safe and have effective immunogenicity vs. control.
Introduction
Mumps is a common acute viral disease that spreads easily and quickly via the respiratory route in children.Citation1 The virus primarily causes inflammation and swelling of single or double parotid glands and can subsequently lead to encephalitis, meningitis or orchitis.Citation2 Therefore, most countries target mumps as one of the leading communicable diseases in their childhood immunization programs.Citation3 Since the 1970s, when the attenuated mumps vaccine was licensed (typically prepared from the Jerry-Lynn [JL] strain),Citation4 the vaccine has been extensively administered around the world.Citation5 Immunization has contributed to the dramatic decline in mumps incidence in most countries and regions. For example, in the US, the incidence of mumps has declined from 100/100 000 in the 1960s to 0.1/100 000 in 2001, with a degree of reduction of 99.9%.Citation6 On mainland China, the incidence of mumps has declined from 100–1000/100 000 in the 1970s to 10/100 000 in 2001.Citation7 However, in the past decade, this well-controlled communicable disease has been reported to resurge occasionally in adolescents in countries such as the US, the UK and Canada, which are all countries with well-established vaccination programs.Citation8-Citation11 On mainland China, the administration of the S79 strain (originated from JL strain) live attenuated mumps vaccine prepared from five passages since master seed of JL strain was initiated in the 1990s in children.Citation12 This vaccine was initially administered as a monovalent vaccine and has been followed by the Measles, Mumps, Rubella Combined Live Vaccine (MMR) since 2005.Citation13 The incidence of mumps was greatly reduced after the use of the mumps vaccine but has remained at a level between 20/100 000 and 30/100 000 since 2005.Citation7 It has been speculated that the low immune protection rate of this vaccine is most likely related to the single dose-only immunization program. Some researchers reported that the GMT value of neutralizing antibodies of the S79 vaccine was approximately 10, while the immune protection rate was only 54.35%.Citation14 However, Dayan et al. suggested that it might be necessary to develop a new effective mumps vaccine or to alter the current mumps vaccine immunization strategy to achieve complete control of mumps epidemics.Citation15
As demonstrated by recent studies of the mumps virus, the variety in mumps virus genotypes is attributable to genetic variation directly induced by the long-term transmission of the mumps virus in a population. Currently, 13 distinctively confirmed genotypes (A-M) of the mumps virus have been identified,Citation16 and they are distributed in different geographical regions based on the nucleotide sequence of the small hydrophobic (SH) gene. This gene is the most variable gene in the mumps genome, and a new genotype is defined when the nucleotide differences of the SH gene are ≥6%.Citation17 Based on epidemiological studies on mainland China, the predominantly circulating viral strain is believed to be of the F genotype.Citation18 Although cross-neutralization assays showed that the viral strains of different genotypes are capable of exhibiting cross-antigenic characteristics to some extent,Citation19 the development of a new vaccine specifically targeted to a geographic pandemic viral strain with the same genotype trend is a reasonable goal.Citation20,Citation21
Based on the characterization of the predominantly circulating mumps virus on mainland China, a new attenuated mumps vaccine was developed using a genotype-F viral strain that was selectively isolated from a mumps outbreak and attenuated in human diploid cells. In this paper, the phase I clinical trial of this new mumps vaccine is described, and the results are summarized.
Results
In total, 435 volunteers participated in the interview and physical examination for this trial. Of these volunteers, 135 were excluded because they failed to meet the eligibility criteria, and 300 were enrolled in the trial (). The baseline level of these volunteers is described in . The dose escalation started on 15 October 2012 with the adult group (16–60 y) and was followed by school-aged children (5–16 y), preschoolers (2–5 y), and infants (8–24 mo). The last dose of inoculation was given on 23 November 2012 and was followed by continuous clinical observation until 30 December 2012. Three subjects were lost to follow-up, which resulted in a final total of 297 subjects for analysis ().
Figure 1. Summary of the trial design. * A dose:3.50 ± 0.25 logCCID50/ml, B dose:4.25 ± 0.25 logCCID50/ml, C dose:5.00 ± 0.25 logCCID50/ml. (A) Adult: 16 y-old~ < 60 y-old. (B) Child: 5 y-old~ < 16 y-old. (C) Preschool: 2 y-old~ < 5 y-old. (D) Infant: 8 mo-old~ < 2 y-old.
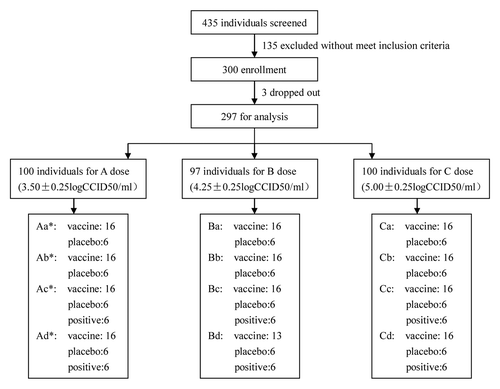
Table 1. The description of baseline level before immunization
Immunogenicity
To evaluate immunogenicity of this F-genptype mumps vaccine, this phase I trial was conducted as a randomized, double blind, controlled and multistep study to ensure its reliability. Additionally, the currently licensed vaccine (S79 live attenuated vaccine) was added as a positive control to the preschooler and infant groups (except for the placebo control group). Immunogenicity was primarily analyzed based upon the results from intervention C in the experimental group, according to intent, as intervention A and intervention B were designed for safety analysis. Neutralizing antibodies against the SP-A virus and the S79 virus and the antibody detected by ELISA before and after vaccination were used, in parallel, as the criteria for determining immunogenicity. The cross-neutralization assay showed that immunization with the F-genotype attenuated mumps vaccine could induce an antibody response in different individual populations (). In the adult group without the positive control, the vaccine immunization induced a 31% seroconversion rate for the ELISA antibody in the experiment group, a 56% rate for the anti-SP neutralizing antibody, and a 25% rate for the anti-S79 neutralizing antibody (). In the school-aged child group without the positive control, there was a slightly lower seroconversion rate for the ELISA antibody and 50% and 44% seroconversion rates for the anti-SP-A and anti-S79 neutralizing antibodies, respectively, compared with the placebo control group (intervention D) (). In the preschooler group, despite the lower induced seroconversion rate for the ELISA antibody in the experimental group compared with the positive control group (intervention E), the seroconversion rates for the anti-SP-A and anti-S79 neutralizing antibodies in the experimental group were higher than those in the positive control group ().
Figure 2. Seroconversion rates of serum 28 d after vaccination in C dose, placebo and control groups. Containing individuals (A) 16–60 y old, (B) 5–16 y old, (C) 2–5 y old and (D) 8–24 mo old. Detection of antibody, including the neutralizing antibodies against SP-A virus and S79 virus, and the antibody detected by ELISA. The genotype A vaccine control group was added in the 2–5-y-old and 8–24-mo-old groups. Seroconversion definition: ELISA:An antibody titer > 12 U/ml was considered seropositive. Neutralizing antibody: An antibody titer > 1:4 was considered seropositive.
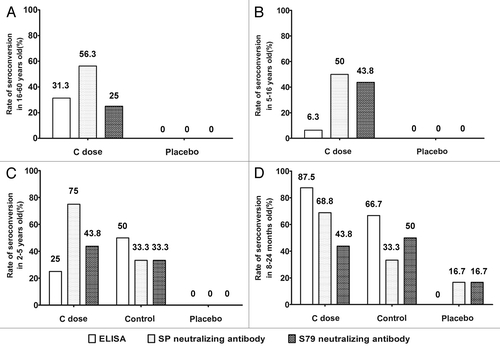
In the infant group, there was a higher seroconversion rate for the ELISA antibody in the experimental group compared with that in the positive control group, and the seroconversion rates for the anti-SP-A and the anti-S79 neutralizing antibodies in the experimental group were higher than those in the positive control group (). The comparative analysis of GMTs between the experimental and positive control groups demonstrated a difference (). The first GMTs for the ELISA antibodies in the experimental groups of all ages (120.1 [95% CI:86.2–154.0], 210.3 [95% CI:187.7- 232.8], 60.0 [95% CI:14.6 - 105.4] and 123.6 [95% CI:59.1–188.1]) were similar to those of the positive control group. The second GMTs for the anti-F-genotype virus neutralizing antibodies in the experimental groups of all ages (14.1 [95% CI: 8.6–22.9], 14.1 [95% CI: 8.3–23.8], 10.8 [95% CI: 5.1–23.2] and 8.7 [95% CI: 3.9–11.3]) were higher than those in the positive control group. However, the GMTs for the anti-A-genotype virus neutralizing antibodies in all experimental groups (5.4 [95% CI: 3.7–7.9], 15.3 [95% CI: 10.3–22.7], 7.3 [95% CI: 4.0–13.6] and 6.4 [95% CI: 3.4–12.1]) were similar to those in the positive control group.
Table 2. Immunogenicity of mumps attenuated vaccine(GMT,95%CI)
Virus shedding from vaccine-immunized individuals
To understand the biological characterization of the isolated SP-A virus exhibited by immunization with the new mumps vaccine, we performed viral nucleic acid detection in throat swabs collected at days 0, 4, 7, 10, 14, and 28 post-vaccination from all of the vaccine-immunized individuals. Negative results were obtained at all of the tested time points as compared with positive control, in which, the digital viral genome was capable of being identified in detection and qunantitation by the qRT-PCR (data not shown), implying no virus shedding from the vaccine-immunized individuals.
Safety assessment
The safety evaluation of all 3 doses of the experimental groups, the positive control group and the placebo control group showed similar AEs: 39% (25 in 64), 42% (27 in 64), 35% (23 in 64), 38% (14 in 36) and 40% (18 in 72), respectively, with no statistical significance in intervention A, B, or C of the experimental groups or the positive control(intervention E) and the placebo control (intervention D) groups (). A slightly higher rate of AE was reported for pain, redness and scleroma in intervention C than other groups. The ratios of the grade III AEs in all 3 doses of the experimental, positive control and placebo control groups were as follows: 1.56% (1 in 64), 0% (0 in 64), 1.56% (1 in 64), 5.56% (2 in 36) and 1.39% (1 in 72). The AEs in all of the groups were typically characterized by fever and cough (), with no significant differences noted among the groups (). The cumulative local AEs were mainly mild pain, local swelling and induration at the injection site, with a slightly higher frequency in the C-dose group than in the positive control group (); however, none of the AEs were more severe than a grade III. Furthermore, no SAE was reported throughout the trial, indicating that the vaccine is relatively safe.
Table 3. Adverse events and serious adverse events among subjects receiving vaccine or placebo or control (number of cases and occurrence
Discussion
Several studies based on the occurrence of unexpected outbreaks of mumps in the vaccinated population during the 1990s to the 2000s in the United Kingdom and North America compared several mumps vaccine strains for their immunogenicities and protective efficiencies.Citation22,Citation23 The various data suggested that the existing strains showed differences in immunogenicity, especially in the serological test results. Studies about JL vaccine once showed 95% effectiveness in US 1960s, but a reduced 62% in Switzerland in 1990s,Citation24,Citation25 as while Finland eliminated indigenous mumps in 1996 by JL-containing MMR.Citation26 Urabe vaccine was reported the effectiveness of 95% and 54% in different areas respectively and also showed varied rate of aseptic meningitis.Citation27,Citation28 However, observation of Rubini vaccine once showed an effectiveness of –55% and a very good safety.Citation28 Collectedly, much evidences obtained in clinical observation tendentiously implied a possibility that the epidemiological data for the safety and protective efficiency of the vaccines were pivotal.Citation28,Citation29
The primary goal of this trial was to evaluate the safety and immunogenicity of a new attenuated mumps vaccine that was developed with a virus strain of the F genotype. Based upon the fact that a mumps vaccine prepared from Urabe strain was recalled in Europe due to a higher rate of serious adverse events (SAEs) than that from JL strain,Citation12 this study was designed as a randomized, double blind, controlled and multi-step trial with the following schedule: adults → school children → preschoolers → infants, and A dose → B dose → C dose with continuous clinical safety observation after inoculation. The identification of all of the solicited and unsolicited AEs showed no significant differences among the different doses of this vaccine and the positive control, which was named S79 originated from JL strain and licensed in China in the 1990s,Citation12 and placebo control groups. There were no reports of distinctive differences of systematic AEs among the different groups in the trial. A slightly higher rate of AEs was reported for the C-dose vaccine group than for the other groups, but no adverse event was higher than a grade III. These data, as well as the fact that no SAEs were reported throughout the trial, suggest a favorable safety profile for the new mumps vaccine in this trial.
It has been shown that the mumps vaccine used as positive control in this trial exhibits a sero-conversion rate of 54.3–88.2% among the varied GMT values against A-genotype strain in vast areas of China.Citation14,Citation30 The immunogenicity analysis of this candidate vaccine strain showed higher seroconversion rates and GMTs of the anti-F-genotype virus-neutralizing antibody in the experiment groups with different ages than that in the positive control group, while the anti-A-genotype neutralizing antibody was similar in both the experiment group and the positive control group. These data suggest a similar rate of efficacy for this attenuated mumps vaccine strain as that of the A-genotype strain vaccine currently being used, and is likely to propose for the first time the presence of genotype-specific immune response induced in mumps vaccine immunization.
The previous data suggested that the efficiency of the immunogenicity and clinical protection of the mumps vaccine were related directly to the schedule of vaccination. In this regard, most of the failure of the vaccine might be attributable to primary vaccine failure, and the receipt of 2 vaccine doses might confer 5 times the level of antibodies.Citation31-Citation33 In our current work, a slightly lower seroconversion rate in young subjects than in old subjects who might have probably infected by virus or vaccinated with mumps vaccine before the immunization implied that the priming would potentially affect the seroconversion rate. These data conclusively suggested that a schedule of 2 doses of the vaccine should be used in the next phase II trial. However, the induction of the anti-F-genotype virus-neutralizing antibody implied its specificity against the current epidemic of mumps in China.
In the study described here, the trial of a new mumps attenuated vaccine prepared from F genotype strain was reported, and the results demonstrated the genotype-specific immunity induced by the mumps vaccine immunization. However, we failed to conduct a general evaluation of the clinical efficiency of this new vaccine due to lack of the data of epidemiological protective effect in immunized populations. The following issues need to be explored and analyzed in further works: the efficacy of the study in large populations, the appropriate immunization schedule, and the slightly higher occurrence of local swelling and induration at the injection site in all 3 vaccine dose groups.
Materials and Methods
Vaccines and placebos
The SP-A viral strain of the F-genotype attenuated mumps vaccine was isolated from individuals in a mumps outbreak and was subsequently adapted by a cloning screen to grow on human diploid cells (KMB17 strain) for gradual attenuation in the Institute of Medical Biology, Chinese Academy of Medical Sciences (IMB, CAMS). The attenuation of the vaccine candidate was evaluated with the indicators of pathogenicity and neurovirulence in rhesus monkeys, as described in our previous work.Citation34 Three doses of the vaccine candidate were manufactured in a lyophilized manner: 3.5 ± 0.25 logCCID50 (intervention A) (Lot number: 20110901), 4.3 ± 0.25 logCCID50 (intervention B) (Lot number: 20110902), and 5.0 ± 0.25 logCCID50 (intervention C) (Lot number: 20110903). The placebo (intervention D) (Lot number: 20110904) was identical to the vaccine except that it did not contain the mumps virus. The vials containing either vaccine or placebo were visibly indistinguishable. Both vaccine and placebo were prepared in a GMP-compliant facility and were tested by the National Institutes for Food and Drug Control (NIFDC) prior to the study. A commercial, live attenuated mumps vaccine containing the genotype A strain named S79 was licensed by Zhejiang Weixin Biological Products Ltd. Corp (Lot number: 20110528–1) in China in the 1990s, and was used as a positive control (intervention E) in this trial for a parallel comparison of immunogenicity and safety evaluation with this new SP-A vaccine candidate.
Trial design and participants
A randomized, controlled and observer-blind trial was designed by Hebei Provincial Center for Disease Control and Prevention (HBCDC) and the Institute of Medical Biology, Chinese Academy of Medical Sciences (IMB, CAMS); the trial was performed in Dingxing County, Hebei Province, China. An independent Data and Safety Monitoring Board (DSMB) was constructed to review and confirm all safety data generated during the study period.
Healthy individuals were recruited for this study using the following criteria: aged 8 mo to 60 y, could provide written consent (the written consent of 5–16-y, 2–5-y and 8–24-mo age groups was signed by the parent, and the written consent of the 16–60-y age group was signed by the subject), and had not had a mumps vaccination within the preceding six months. Individuals were considered ineligible if they had a concurrent acute or chronic infectious disease at the time of enrollment, had taken immunosuppressive drugs or products within the preceding two months, had concomitant administration of other licensed vaccines within the preceding 7 d, had a history of allergic reaction to any vaccine, had fever (defined as axillary temperature > 37 °C), were in a gestational/lactation period, or had a plan for pregnancy within 2 mo. Eligible participants were stratified into 4 age groups: 16–60 y, 5–16 y, 2–5 y and 8–24 mo. In each age group, the participants were assigned to receive intervention A, B, C, D or E using block randomization methods (). The interventions A, B, C, D, and E were designed for safety assessment, and the interventions C, D, and E were designed for immunogenicity assessment. A dose-escalating design was applied. The entire study was performed starting from the lowest dose in the adult group. Dose escalation to the next arm was dependent upon demonstrating the safety of the lower dose through Day 14. Each participant received one dose of vaccine, placebo, or positive control, and participants and staff were blinded to the group assignments within each arm.
Safety assessment
After injection, all participants were kept at the study site for 30 min for the measurement of temperature and the observation of immediate adverse events (AEs). The body temperatures were measured by an axillary thermometer. Blood samples and urine samples were collected from each participant for safety laboratory analyses (routine urine, liver and kidney function and complete blood count) prior to vaccination and 4 d post-vaccination. Follow-up for each participant was at 4, 7, 10, 14, and 28 d for the evaluation of systemic and local adverse events, and the results showed the sum of the AEs of the five safety time points. The participants, or their guardians, were required to complete a diary on the vaccination day and for the 28 d following the day of vaccination, in which solicited, unsolicited and serious adverse events (SAEs) were to be recorded. If adverse reactions were still present after 28 d, the participants were instructed to continue to monitor these reactions until they resolved. Blood samples were taken before the vaccination and on day 28 (range 25–31) after the vaccination. No concomitant drug/vaccine use was allowed during the study, except for the necessary use to treat SAEs.
Detection of virus shedding
To study the shedding of the vaccine strain, throat swabs were collected from each participant at 0, 4, 7, 10, 14, and 28 d after vaccination. The mumps RNA was detected by routine qRT-PCR amplificationCitation35 using a Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany) on RNA extracts from throat swabs diluted in 500 μl of PBS. The qRT-PCR amplification was performed using primers (forward primer (HN14–33): 5′-AACTCTTCAC AATATCAGAC-3′, reverse primer (HN141–160): 5′-CACCTAAAGT GACAATGACT-3′) and a probe (HN71–100) (5′-FAM-CTGACAAGAA GACATTCCGA ACCTGCTTCC-TAMRA3′). The RNA was extracted from 5.5 log CCID50/ml of mumps virus and was followed by dilution of 10 times to 10−4 as the series of positive control, and the clear amplified DNA band in sample of 10−4 was identified to be effective in the same qRT-PCR.
Serology
Blood samples were obtained at 0 and 28 d for the immunogenicity assays. After coagulation, the serum was separated, frozen, and stored at the study site at −20 °C until it was shipped to the National Institutes for Food and Drug Control. The cross-neutralization assay using SP-A strain of F genotype and S79 strain of A genotype was applied to determine the neutralizing antibody titer, according to previously described methods.Citation36 The sera were diluted in series, and the neutralization assay was performed on Vero cells using diluted 500 CCID50 SP-A and S79 viruses for incubation at 37 °C over 7 d for CPE observation. There is no known protective level of neutralizing antibody (antibody titer) for mumps, and there are no other immune parameters that correlate with protection from the mumps disease. Some researchers report that protective efficacy field studies have shown that mumps virus-neutralizing antibody titers as low as 1:2 provide protection against mumps,Citation37 and mumps virus-neutralizing antibody titers are often low (less than or equal to 1:8).Citation36 Therefore, considering the above factors, the concentration of one-half of the lower limits of detection and quantittation (LLOQ) is used as the standardard assigned to a negative sample for the purpose of calculating GMTs. For comparison purposes, the ELISA assay was also performed using a mumps IgG ELISA reagent kit (Demeditec Corp., demum01), in which, the IgG measured by the purified mumps antigen included the neutralizing antibody against mumps virus and the antibody interacting with the virus. An antibody titer >12 U/ml was considered seropositive, which is qualified by the ELISA kit manufacturer.
Statistical analysis
All data were double entered into custom-made data entry programs (Epidata 3.1). The data management programs included range and consistency checks. SAS software (SAS Institute Inc., Cary) was used for statistical analysis. Student’s t test or the Mann Whitney U test (when data were not normally distributed) was used for dimensional outcomes, and the chi square or Fisher’s exact test (when data were sparse) was used for dichotomous outcomes; all tests were two-tailed. In statistical analyses of antibody titers, the titers were logarithmically converted to allow an assessment of the GMTs.
Ethics
The study was approved by the institutional review board (IRB) in HBCDC and registered at www.cliniclatrials.gov (NCT01712906). Written informed consent was obtained from each participant or the participant’s parent/guardian following an explanation of the purpose and potential risks of the study.
Additional material
Download Zip (1.7 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding Statement
This work was supported by the National Major Special Technological Program (No. 2013ZX09101016), National High-Tech R&D Program (No. 2012AA02A404) and Application Foundation Project of Yunnan Province (No. 2013FZ129).
Author Contributions
Conceived and designed the experiments: Q.H.L., Y.L. (Yan Liang), J.C.M., C.G.L., Y.L.Z., and J.Z.W. Performed the experiments: Q.L., Y.L. (Yan Liang), Y.G.C., L.D.L., Y.L. (Yun Liao), Y.Z., L.J., Y.C.C., W.D., H.L., X.Y.C., N.M., and D.D. Analyzed the data: Q.L., Y.L. (Yan Liang), J.C.M., C.G.L., L.D.L., and X.Y.W. Contributed reagents/materials/analysis tools: L.J., Z.P.X., P.F.C., Q.Y.J., and J.J.W. Wrote the paper: Q.L., Y.L. (Yan Liang), Y.Z., and X.Y.W.
References
- Mühlemann K. The molecular epidemiology of mumps virus. Infect Genet Evol 2004; 4:215 - 9; http://dx.doi.org/10.1016/j.meegid.2004.02.003; PMID: 15450201
- Hviid A, Rubin S, Mühlemann K. Mumps. Lancet 2008; 371:932 - 44; http://dx.doi.org/10.1016/S0140-6736(08)60419-5; PMID: 18342688
- Centers for Disease Control and Prevention (CDC). Notice to readers: updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the control and elimination of mumps. MMWR Morb Mortal Wkly Rep 2006; 55:629 - 30; PMID: 16761359
- Control CfD. Mumps vaccine. MMWR Morb Mortal Wkly Rep 1977; 26:393 - 4
- Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1998; 47:RR-8 1 - 57; PMID: 9639369
- Jajosky RA, Hall PA, Adams DA, Dawkins FJ, Sharp P, Anderson WJ, Aponte JJ, Jones GF, Nitschke DA, Worsham CA, et al, Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases--United States, 2004. MMWR Morb Mortal Wkly Rep 2006; 53:1 - 79; PMID: 16775578
- Control CfD. The statutory reporting of infectious diseases. 2002.
- Centers for Disease Control and Prevention (CDC). Update: mumps outbreak - New York and New Jersey, June 2009-January 2010. MMWR Morb Mortal Wkly Rep 2010; 59:125 - 9; PMID: 20150887
- Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ 2011; 183:1014 - 20; http://dx.doi.org/10.1503/cmaj.101371; PMID: 21576295
- Gemmill IM. Mumps vaccine: is it time to re-evaluate our approach?. CMAJ 2006; 175:491 - 2; http://dx.doi.org/10.1503/cmaj.060900; PMID: 16940268
- Ogbuanu IU, Kutty PK, Hudson JM, Blog D, Abedi GR, Goodell S, Lawler J, McLean HQ, Pollock L, Rausch-Phung E, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012; 130:e1567 - 74; http://dx.doi.org/10.1542/peds.2012-0177; PMID: 23129075
- Yang W, Xu W, Xu F, Chen Z. Sequence research on S79 strain of mumps virus during the successive passaging. Chinese Journal of Vaccines and Immunization 2007; 13:150 - 7
- Chen Z. Mumps virus and vaccine. Chinese Journal of Vaccies and Immunization 2004; 10:120 - 3
- Liu S, Huang Y, Chen X. The Immune Effects Analysis of Mumps Vaccine Preparated with Different Strain. S China J Prev Med 2005; 31:39 - 42
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O’Keefe AL, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580 - 9; http://dx.doi.org/10.1056/NEJMoa0706589; PMID: 18403766
- Santos CL, Ishida MA, Foster PG, Sallum MA, Benega MA, Borges DB, Corrêa KO, Constantino CR, Afzal MA, Paiva TM. Detection of a new mumps virus genotype during parotitis epidemic of 2006-2007 in the state of São Paulo, Brazil. J Med Virol 2008; 80:323 - 9; http://dx.doi.org/10.1002/jmv.21068; PMID: 18098149
- Johansson B, Tecle T, Orvell C. Proposed criteria for classification of new genotypes of mumps virus. Scand J Infect Dis 2002; 34:355 - 7; http://dx.doi.org/10.1080/00365540110080043; PMID: 12069019
- Wu L, Bai Z, Li Y, Rima BK, Afzal MA. Wild type mumps viruses circulating in China establish a new genotype. Vaccine 1998; 16:281 - 5; http://dx.doi.org/10.1016/S0264-410X(97)00173-4; PMID: 9607043
- Rubin SA, Link MA, Sauder CJ, Zhang C, Ngo L, Rima BK, Duprex WP. Recent mumps outbreaks in vaccinated populations: no evidence of immune escape. J Virol 2012; 86:615 - 20; http://dx.doi.org/10.1128/JVI.06125-11; PMID: 22072778
- Peltola H, Kulkarni PS, Kapre SV, Paunio M, Jadhav SS, Dhere RM. Mumps outbreaks in Canada and the United States: time for new thinking on mumps vaccines. Clin Infect Dis 2007; 45:459 - 66; http://dx.doi.org/10.1086/520028; PMID: 17638194
- Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ 1999; 77:3 - 14; PMID: 10063655
- Kulkarni-Kale U, Ojha J, Manjari GS, Deobagkar DD, Mallya AD, Dhere RM, Kapre SV. Mapping antigenic diversity and strain specificity of mumps virus: a bioinformatics approach. Virology 2007; 359:436 - 46; http://dx.doi.org/10.1016/j.virol.2006.09.040; PMID: 17081582
- Schwarzer S, Reibel S, Lang AB, Struck MM, Finkel B, Gerike E, Tischer A, Gassner M, Glück R, Stück B, et al. Safety and characterization of the immune response engendered by two combined measles, mumps and rubella vaccines. Vaccine 1998; 16:298 - 304; http://dx.doi.org/10.1016/S0264-410X(97)00174-6; PMID: 9607046
- Hilleman MR, Weibel RE, Buynak EB, Stokes J Jr., Whitman JE Jr.. Live attenuated mumps-virus vaccine. IV. Protective efficacy as measured in a field evaluation. N Engl J Med 1967; 276:252 - 8; http://dx.doi.org/10.1056/NEJM196702022760502; PMID: 6016061
- Chamot E, Toscani L, Egger P, Germann D, Bourquin C. [Estimation of the efficacy of three strains of mumps vaccines during an epidemic of mumps in the Geneva canton (Switzerland)]. Rev Epidemiol Sante Publique 1998; 46:100 - 7; PMID: 9592852
- Peltola H, Davidkin I, Paunio M, Valle M, Leinikki P, Heinonen OP. Mumps and rubella eliminated from Finland. JAMA 2000; 284:2643 - 7; http://dx.doi.org/10.1001/jama.284.20.2643; PMID: 11086376
- Vesikari T, André FE, Simoen E, Florent G, Ala-Laurila EL, Heikkinen A, Kuusinen H, Terho A. Comparison of the Urabe Am 9-Schwarz and Jeryl Lynn-Moraten combinations of mumps-measles vaccines in young children. Acta Paediatr Scand 1983; 72:41 - 6; http://dx.doi.org/10.1111/j.1651-2227.1983.tb09661.x; PMID: 6344551
- Ong G, Goh KT, Ma S, Chew SK. Comparative efficacy of Rubini, Jeryl-Lynn and Urabe mumps vaccine in an Asian population. J Infect 2005; 51:294 - 8; http://dx.doi.org/10.1016/j.jinf.2004.10.001; PMID: 16291282
- Schlegel M, Osterwalder JJ, Galeazzi RL, Vernazza PL. Comparative efficacy of three mumps vaccines during disease outbreak in Eastern Switzerland: cohort study. BMJ 1999; 319:352; http://dx.doi.org/10.1136/bmj.319.7206.352; PMID: 10435956
- Wang S, Gu Z, Zhang Y, Liu H, Xie G. Comparison of HI antibody titer of local monovalent mumps vaccine and the imported MMR vaccine in previously immunized pupil. Progress in Microbiology and Immunology 1998; 26:51 - 2
- Lewis JE, Chernesky MA, Rawls ML, Rawls WE. Epidemic of mumps in a partially immune population. Can Med Assoc J 1979; 121:751 - 4; PMID: 519614
- Harling R, White JM, Ramsay ME, Macsween KF, van den Bosch C. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine 2005; 23:4070 - 4; http://dx.doi.org/10.1016/j.vaccine.2004.10.020; PMID: 15950329
- Hersh BS, Fine PE, Kent WK, Cochi SL, Kahn LH, Zell ER, Hays PL, Wood CL. Mumps outbreak in a highly vaccinated population. J Pediatr 1991; 119:187 - 93; http://dx.doi.org/10.1016/S0022-3476(05)80726-7; PMID: 1861205
- Liang Y, Ma S, Liu L, Zhao H, Wang L, Jiang L, Xie Z, Dong C, Li Q. Identification and development of a promising novel mumps vaccine candidate strain. Microbes Infect 2010; 12:1178 - 87; http://dx.doi.org/10.1016/j.micinf.2010.08.004; PMID: 20800105
- Boddicker JD, Rota PA, Kreman T, Wangeman A, Lowe L, Hummel KB, Thompson R, Bellini WJ, Pentella M, Desjardin LE. Real-time reverse transcription-PCR assay for detection of mumps virus RNA in clinical specimens. J Clin Microbiol 2007; 45:2902 - 8; http://dx.doi.org/10.1128/JCM.00614-07; PMID: 17652480
- Mauldin J, Carbone K, Hsu H, Yolken R, Rubin S. Mumps virus-specific antibody titers from pre-vaccine era sera: comparison of the plaque reduction neutralization assay and enzyme immunoassays. J Clin Microbiol 2005; 43:4847 - 51; http://dx.doi.org/10.1128/JCM.43.9.4847-4851.2005; PMID: 16145156
- Weibel RE, Buynak EB, Whitman JE Jr., Leagus MB, Stokes J Jr., Hilleman MR. Jeryl Lynn strain live mumps virus vaccine. Durable immunity for three years following vaccination. JAMA 1969; 207:1667 - 70; http://dx.doi.org/10.1001/jama.1969.03150220083008; PMID: 5818350
