Abstract
Background: Fever, leukocytosis, and large local reactions following the pneumococcal polysaccharide vaccine (PS23) have been described only in isolated case reports in the adult literature. Such atypical reactions can pose difficulty to providers when determining management. Patients experiencing this noninfectious reaction may receive unnecessary treatment if the diagnosis of robust inflammatory response to the PS23 vaccine is not considered.
Observations: This is a clinical case series of 5 adult patients who received the influenza and PS23 vaccines and experienced a cellulitis-like reaction, fever, and leukocytosis in the days following vaccination. Four of the five patients received the influenza and PS23 vaccines in the same arm. The patient who received the vaccines in opposite arms had the local findings in the arm that received the PS23 vaccine. All 5 patients sought care and 4 were admitted to the hospital for observation or treatment with intravenous antibiotics.
Conclusions: This case series highlights potential side effects of the PS23 vaccine that are not well described in the adult literature. Antibiotics were not helpful in treating these patients’ local and systemic symptoms. Patients with histories consistent with that highlighted in this case series may avoid antibiotics and hospitalization if their providers recognize these symptoms as a noninfectious reaction to the PS23 vaccine.
Introduction
The 23-valent pneumococcal polysaccharide vaccine (PS23) is a vaccine given to protect against infection caused by Streptococcus pneumoniae. Administration of PS23 is indicated in all persons over 64 y and those ages 2 y and older at risk for invasive pneumococcal disease.Citation1,Citation2 The advent of the pneumococcal vaccines has successfully reduced the incidence of invasive pneumococcal disease by 45%.Citation3 According to the Centers for Disease Control and Prevention, common adverse reactions reported with both the protein conjugated and polysaccharide pneumococcal vaccines are pain, redness, and swelling at the injection site, limitation of movement of the injected arm, fatigue, headache, fever, chills, decreased appetite, generalized muscle pain, and joint pain.Citation1,Citation4 There are several reviews detailing local reactions with and without systemic symptoms following PS23 vaccination in children.Citation5,Citation6 Although there are isolated case reports of systemic reactions to the PS23 vaccine in adults, there is a paucity of evidence detailing fever, leukocytosis, and cellulitis-like reaction within 24 h of the polysaccharide vaccine.Citation7-Citation10
We present a series of 5 adult patients who had systemic symptoms, leukocytosis, and large local reactions following administration of the combination of influenza and PS23 injections. These patients had such severe redness and swelling that treatment was initiated for presumed cellulitis. Prominent leukocytosis and systemic symptoms in combination with the severity of local inflammation has not been well described in adults following PS23 administration and can pose both a diagnostic and management dilemma for providers.
Cases
The 5 patients in this series ranged from ages 20 to 43 y with 3 females and 2 males (). These patients’ indications for the PS23 vaccine included asthma, smoking, splenectomy, and preparation for immunosuppressive therapy in rheumatoid arthritis. Patients 1, 3, 4, and 5 received the influenza and PS23 vaccines in the same arm. Patient 2 received the influenza and PS 23 vaccines in opposite arms. Patient 4 is the only patient who received an additional injection aside from the influenza and PS23 vaccines. She received the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine in the opposite arm.
Table 1. Demographics
All 5 patients developed fever greater than 101.5 °F within one day of receiving the influenza and PS23 vaccinations (). The highest temperature reported was 104.5 °F. In addition, all patients reported at least one other systemic symptom such as rigors or vomiting. All patients in this series experienced erythema, edema, and warmth of the pneumococcal and influenza injection sites. The local reaction expanded well beyond the immediate injection area by day 3–4 post-vaccination. Patient 2, who received the 2 vaccines in opposite arms, developed local erythema and edema at the site of the PS23 vaccine. The arm where the influenza vaccine was placed was normal. This suggests that the local reaction experienced by the patients who received the PS23 and influenza vaccines in the same arm was due to the PS23 vaccine rather than the influenza vaccine. demonstrates the local inflammation demonstrated by patient 4 and described in patients 1, 2, 3, and 5.
Table 2. Symptoms and labs
Figure 1. Left arm of patient 4 showing erythema of the upper arm where she received the PS23 and influenza vaccines 5 d prior. Patient’s consent was obtained to publish the picture.
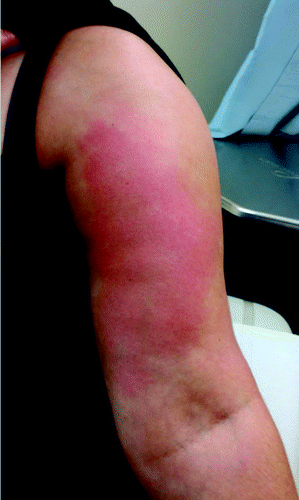
All 5 patients sought care within 2–3 d of vaccination and 4 were admitted to the hospital for observation, with or without treatment of presumed cellulitis. All patients had significant leukocytosis with white blood cell (WBC) counts ranging from 19 200 cells/ μL to 29 700 cells/ μL (median 22 000 cells/ μL) with a predominance of neutrophils. A c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were elevated in patients 2 and 5, but were not checked in the others. Three out of four admitted patients received full courses of antibiotics to cover for common skin flora as well as methicillin-resistant Staphylococcus aureus (). Patient 5, in particular, received several different antibiotics due to worsening of the skin erythema despite aggressive intravenous antibiotic therapy. Within 5–7 d, all patients experienced improvement in the skin findings and resolution of the systemic symptoms. In one patient, the local swelling and erythema at the injection site lasted about 2 wk.
Table 3. Treatment
From the records available, it appears that only patient 1 had received the PS23 vaccine prior to this reaction. The indication for prior PS23 vaccination was the patient’s asplenia. The date of this prior vaccination was not available as it occurred prior to the patient’s enrollment in the military healthcare system. No patients included in this series have received any subsequent pneumococcal vaccines.
Discussion
The 5 patients in this series all had leukocytosis, fever, and large local inflammatory reactions following influenza and PS23 vaccinations. Based on the known side effects of the PS23 vaccine, the lack of similar findings in patients who receive the influenza vaccine in isolation, and patient 2’s lack of influenza vaccine-associated local symptoms, we believe these reactions were due to the PS23 vaccine. Protracted fever and cellulitis-like reactions to the PS23 vaccine have been described in children, but only isolated case reports are available for adults.Citation7-Citation10 A systematic review of pediatric patients receiving the PS23 vaccine at the duPont Hospital for Children found a local reaction rate of 50% with higher likelihood in patients who had been previously vaccinated. That review also showed a systemic reaction rate of 1%.Citation6 To our knowledge, this is the first case series in adults detailing a febrile reaction with leukocytosis and local inflammation in response to the PS23 vaccine.
The impact of such reactions is not insignificant as 4 of 5 patients in this series were hospitalized for observation or treatment of presumed cellulitis. Similarly, the majority of patients were placed on at least one course of antibiotics, including several different agents in 2 cases. Such measures were ineffective in treating the patients in this series who were likely experiencing a noninfectious inflammatory response to the PS23 vaccination. This inflammatory response hypothesis is based on the timing of symptoms and leukocytosis. In addition, patients 2 and 5 had elevation of the nonspecific inflammatory markers ESR and CRP. It is important to note that the 5 patients in this series all experienced fever and systemic symptoms with local erythema within 24 h of PS23 administration, and this timing is not consistent with a secondary infection of the injection site. In addition, the patients who were treated with antibiotics did not improve as would be expected if these local reactions represented cellulitis infection.
It has been reported that local reactions are more common following repeat vaccination with the pneumococcal polysaccharide vaccine, but only one of the patients in our series had received the PS23 vaccine before.Citation11 Although the etiology of this reaction is unclear, one hypothesis based on these patients’ timing of symptoms and leukocytosis is that it is due to a robust cytokine response to the vaccine. Two of the five patients had nonspecific inflammatory markers drawn which were elevated. Because this is a retrospective review, specific cytokine analysis for these patients is not available. Further research is needed to help clarify the role of such underlying inflammatory mechanisms.
This type of reaction should be considered in patients with a similar constellation of symptoms following the PS23 vaccine, especially when such symptoms persist or worsen despite appropriate antibiotics. Providers should be aware that such PS23 related reactions can occur, allowing timely diagnosis and appropriate management. The timing of onset of symptoms and lack of resolution of symptoms in those who received antibiotics supports that these symptoms were not infectious. Patients with histories consistent with that highlighted in this series may benefit from symptomatic treatment with oral anti-inflammatories, and may avoid hospitalization and antibiotics if their symptoms are recognized as a noninfectious response to the PS23 vaccine.
Methods
We performed a search of the Vaccine Healthcare Centers Network database, called the Vaccine Adverse Event Clinical System (VAECS), after a patient presented with systemic symptoms and a cellulitis-like rash following the influenza and PS23 vaccines. VAECS is a secure, web based application that allows nurse case managers to document vaccine related adverse reactions, incident reports, and exemptions for Department of Defense affiliated vaccinees. A search was performed for systemic reactions to the PS23 vaccine, and 117 cases were identified. Nine cases reported leukocytosis, fever, and local reactions following the PS23. Four of the nine cases had either received multiple other vaccines, may have already been ill, or did not have labs available, so were not included in this series. Five patients were identified with similar histories following the co-administration of the influenza and PS23 vaccines and are included in this series.
| Abbreviations: | ||
| PS23 | = | pneumococcal polysaccharide vaccine |
| VAECS | = | Vaccine Adverse Event Clinical System |
| Tdap | = | tetanus, diphtheria, and acellular pertussis |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Drs K.A.E. and T.A.B. wrote and edited the manuscript. L.L.D. provided the data from the vaccine database and edited the manuscript. L.L.D. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. L.C.C. reviewed and edited the manuscript. The authors have no conflicts of interest or financial interests to disclose. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Navy, Department of Defense, or U.S. Government.
References
- Pneumococcal Polysaccharide VIS. Centers for Disease Control and Prevention; October 6, 2009. http://www.cdc.gov/vaccines/hcp/vis/vis-statements/ppv.pdf. Accessed Oct 11, 2013.
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:RR-8 1 - 24; PMID: 9132580
- Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59:1102 - 6; PMID: 20814406
- Centers for Disease Control and Prevention (CDC). Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep 2012; 61:394 - 5; PMID: 22647745
- Huang DT, Chiu NC, Chi H, Huang FY. Protracted fever with cellulitis-like reaction in pneumococcal polysaccharide-vaccinated children. Pediatr Infect Dis J 2008; 27:937 - 9; http://dx.doi.org/10.1097/INF.0b013e3181734fb6; PMID: 18776824
- Yousef E, Mannan S. Systemic reaction to pneumococcal vaccine: how common in pediatrics?. Allergy Asthma Proc 2008; 29:397 - 9; http://dx.doi.org/10.2500/aap.2008.29.3144; PMID: 18702888
- Gabor EP, Seeman M. Acute febrile systemic reaction to polyvalent pneumococcal vaccine. JAMA 1979; 242:2208 - 9; http://dx.doi.org/10.1001/jama.1979.03300200038020; PMID: 40046
- Hasan S, Yousef M, Shridharani S. Severe febrile systemic reaction to pneumococcal vaccine. J Natl Med Assoc 2005; 97:284 - 5; PMID: 15712794
- Lee A, Goyal R, Shan HY. Severe protracted fever following pneumococcal vaccine. Am J Med Sci 2006; 332:351 - 3; http://dx.doi.org/10.1097/00000441-200612000-00008; PMID: 17170626
- Diaz-Brito V, Gomes MH, Figueiredo-Dias P, Mota-Miranda A. [Leukemoid reaction and fever after polyvalent polysaccharide pneumococcal vaccination]. Acta Med Port 2007; 20:233 - 7; PMID: 17868533
- Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, Lewis LS, Carlone G, DeStefano F, Holder P, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999; 281:243 - 8; http://dx.doi.org/10.1001/jama.281.3.243; PMID: 9918479
