Abstract
This open-label study was designed to assess immunogenicity and safety of 13-valent pneumococcal conjugate vaccine (PCV13) when administered to Japanese adults aged ≥50 years not previously vaccinated with 23-valent pneumococcal polysaccharide vaccine and to compare this Japanese study population with similar study populations in the United States (US; 50–64 years age group) and European Union (EU; ≥65 years age group). Functional antibody immune responses were measured by opsonophagocytic activity assays. Immune responses in both Japanese age groups showed significant pre/postvaccination fold rises for each serotype. In the Japanese 50–64 years age group, immune responses for the majority of serotypes were significantly lower than in the ≥65 years Japanese age group and generally lower than in the 50–64 years age group in the US study. Immune responses in the Japanese ≥65 years age group were significantly higher for the majority of serotypes compared with the ≥65 years age group in the EU study. The safety profiles across age groups and studies were generally similar. In conclusion, PCV13 elicited robust immune responses in the Japanese study population. The unanticipated higher immune responses observed in the older age group in the Japanese study are of interest and of potential benefit given the higher incidence of pneumococcal disease in older adults. PCV13 was well tolerated and safe.
Introduction
Diseases caused by Streptococcus pneumoniae are a major public health problem affecting all age groups worldwide. Adults aged >50 y, particularly those aged >65 y or with certain underlying medical conditions, are at increased risk for developing pneumococcal disease.Citation1,Citation2 In Japan, S. pneumoniae is the most commonly detected causative agent of community-acquired pneumonia in adults, with mortality particularly high in infants and the elderly.Citation3,Citation4 Treatment of pneumococcal infections is becoming more difficult because of the increased prevalence of antibiotic-resistant S. pneumoniae strains.Citation5 In Japan, a rapid increase in multidrug-resistant S. pneumoniae strains has also been observed.Citation6 Vaccination is now considered an important preventive strategy.
In Japan, a 23-valent pneumococcal polysaccharide vaccine (PPSV23) is available for adults, but vaccination is not widespread. The 13-valent pneumococcal conjugate vaccine (PCV13), which has been licensed for use in adults aged ≥50 y in the United States, European Union, and many other countries has not, to date, been licensed in Japan. In contrast to PPSV23, PCV13 is manufactured by conjugating the capsular saccharides of S. pneumoniae to an immunogenic protein carrier (CRM197; a nontoxic diphtheria toxin cross-reactive material). This converts the T-cell–independent response of the unconjugated vaccine to a T-cell–dependent immune response. T cells provide the signals required for the generation of B-cell memory.Citation7,Citation8 Thus, PCV13 has the potential for eliciting a memory response on subsequent natural exposure if required.
The aim of this study was to assess the immunogenicity and safety of PCV13 when administered to Japanese subjects who have not previously been vaccinated with PPSV23 in 2 age groups (≥65 y and 50–64 y) and to compare each age group with similar study populations in the United StatesCitation9 (US; age 50–64 y) and the European UnionCitation10 (EU; age ≥65 y).
Results
Baseline characteristics and disposition of subjects
A total of 271 Japanese subjects were enrolled at 2 sites; 1 subject was considered not eligible, and 269 subjects completed the study. In the ≥65 y age group, from 137 enrolled subjects (site 1, n = 68; site 2, n = 69), 3 were withdrawn (1 at investigator request before vaccination, 1 was lost to follow-up, and 1 did not have postvaccination blood drawn within the prescribed time windows) and were not included in the evaluable immunogenicity population (n = 134). In the 50–64 y age group, 134 subjects were enrolled (site 1, n = 68; site 2, n = 66) and completed the study; all were included in the evaluable immunogenicity population (n = 134). Of the 268 evaluable subjects, the mean age was 70.5 y (52.2% female) and 57.5 y (56.7% female) in the ≥65 y and 50–64 y age groups, respectively.
A history of medical conditions was more common in the ≥65 y compared with the 50–64 y age groups (49.3% and 26.9%, respectively). The most common conditions were metabolic and nutritional disorders (18.4% and 6.0%, respectively), including diabetes mellitus (6.6% and 1.5% of subjects, respectively) and hyperlipidemia (11.8% and 4.5% of subjects, respectively); gastrointestinal disorders (8.8% and 2.2% of subjects, respectively); vascular disorders (21.3% and 8.2%, respectively) such as hypertension (19.9% and 8.2%, respectively); and cardiac disorders (6.6% and 0.7% of subjects, respectively). Fewer subjects in the ≥65 y age group had ever smoked compared with subjects in the 50–64 y age group (38% and 50.0%, respectively).
Immune responses for each age group in the Japanese study
For the overall population, opsonophagocytic activity (OPA) assay titer geometric mean fold rises (GMFRs) ranged from 11.5 (serotype 3) to 137.2 (serotype 7F). For the 2 age groups, GMFRs ranged from 7.3–99.9 and 13.6–192.5 for the 50–64 y and ≥65 y groups, respectively (Table S1).
OPA assay geometric means titers (GMTs) 1 mo after vaccination in the 50–64 y age group were lower than in the ≥65 y age group for all serotypes, and statistically significantly lower for 8 of 13 serotypes (). In both age groups, a high proportion of adults (≥85.8%) achieved OPA titers of at least the lowest limit of quantitation (≥LLOQ) with no significant differences between groups for 12 of 13 serotypes; the exception was serotype 7F, which was significantly lower in the 50–64 y age group ().
Table 1. Pneumococcal OPA GMTs and subjects achieving OPA titers ≥ LLOQ by age group in the Japanese study (evaluable immunogenicity population)
Immune response of the 50–64 y age group across studies
OPA GMTs in the Japanese study for 50–64 y age group were generally lower than in the US study with a similar age group and significantly lower for 6 of 13 serotypes; serotype 14 was statistically significantly higher in the Japanese study (). However, generally similar responses were observed for proportions of subjects achieving OPA titers ≥ LLOQ in the Japanese study (range: 85.8–99.2%) and US study (range: 84.8–98.4%); except for serotype 14 which was statistically significantly higher in the Japanese study compared with the US study (98.5% and 89.8%, respectively) (Table S2).
Table 2. Serotype pneumococcal OPA GMTs for age groups 50–64 y and ≥65 y across studies (evaluable immunogenicity population)
Post hoc analysis of the 50–64 y age group in the Japanese study compared with the US study identified no factors that might influence the immune response, including gender and smoking habits (data not shown).
Immune response of the ≥65 y age group across studies
The OPA GMTs in the Japanese study for the ≥65 y age group were higher for all serotypes and statistically significantly higher for 11 of 13 serotypes compared with the EU study (). In addition, proportions of adults achieving OPA titers ≥ LLOQ in the Japanese study were higher (range: 89.5–99.3%) than in the EU study (range: 76.9–95.6%) for all serotypes and were statistically significantly higher for 7 of 13 serotypes (Table S2).
Post hoc analyses of diphtheria antitoxin antibodies in the Japanese study
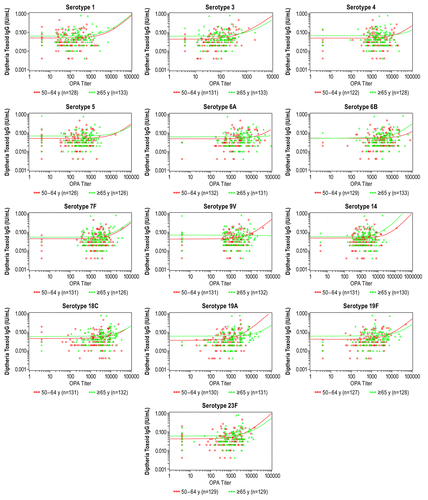
Post hoc analysis of prevaccination diphtheria antitoxin antibodies for each age group showed lower antibody concentrations in the 50–64 y age group compared with the ≥65 y age group (geometric mean concentrations [GMCs] 0.03 μg/mL and 0.04 μg/mL, respectively) and significantly lower postvaccination responses (GMCs 0.10 μg/mL and 0.34 μg/mL, respectively). The percentage of responders achieving 0.01 IU/mL was >95% in both groups (prevaccination: 95.5% and 99.2%; and postvaccination: 97.0% and 100%, respectively). The percentage of responders achieving 0.1 IU/mL was low in both groups (prevaccination: 13.5% and 18.8%; and postvaccination: 44.4% and 75.2%, respectively) (Table S3). The graphical review of potential correlation by scatter plots and regression analyses showed no clear correlation between prevaccination diphtheria antitoxin levels and postvaccination serotype-specific pneumococcal responses ().
Figure 1. Scatter plots and regression analysis between prevaccination diphtheria toxoid IgG concentrations and postvaccination pneumococcal OPA titers by serotype and age group (evaluable immunogenicity population). IgG, immunoglobulin G; OPA, opsonophagocytic activity.
Safety comparisons across studies
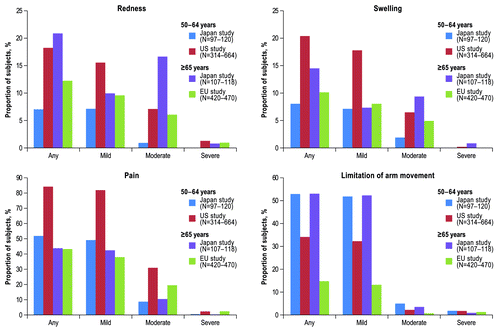
In the Japanese study, the majority of local reactions were mild or moderate in severity and overall were generally similar in incidence between the 2 age groups; exceptions were any redness, moderate redness, and moderate swelling, which were significantly lower (P < 0.05) in the 50–64 y age group compared with the ≥65 y age group (). In the 50–64 y age group, a statistically significantly lower (P < 0.05) percentage of subjects in the Japanese study reported any redness, swelling, and pain, compared with the US study (). In the ≥65 y age group, a statistically significantly higher (P < 0.05) percentage of subjects in the Japanese study reported any local reaction, redness, and limitation of arm movement compared with the EU study ().
Figure 2. Subjects reporting local reactions within 14 d after vaccination by age group (safety population)*. *Severity of local reactions were categorized as follows: redness and swelling, mild = 2.5–5.0 cm, moderate = 5.1–10.0 cm, and severe >10.0 cm; pain, mild = awareness of symptom but easily tolerated, moderate = discomfort enough to cause interference with usual activity, and severe = incapacitating with inability to do usual activity; limitation of arm movement, mild = some limitation, moderate = unable to move above head but able to move above shoulder, and severe = unable to move above shoulder. Abbreviation: EU, European Union.
In the Japanese study, systemic events were similar across age groups, with the exception of fatigue and headache, which were significantly higher (P < 0.05) in the 50–64 y age group compared with the ≥65 y age group (). In the 50–64 y age group, the incidence of all systemic events, fatigue, headache, new muscle pain, any aggravated muscle pain, new joint pain, and aggravated joint pain were significantly lower (P < 0.001) in the Japanese study compared with the US study (). In the ≥65 y age group, the incidence of all systemic events in the Japanese study was similar to that in the EU study, with the exception of headache (which was significantly lower [P = 0.019] in the Japanese study) and rash (which was significantly higher [P = 0.049] in the Japanese study).
Table 3. Subjects reporting systemic events within 14 d after vaccination by age group (safety population)
There were no deaths, discontinuations due to adverse events (AEs), or vaccine-related serious AEs (SAEs) in the Japanese study. There were no statistically significant differences in the occurrence of SAEs between age groups in the Japanese study or when compared with similar age groups in the US or EU studies. Details of these data are available elsewhere.Citation11
Discussion
PCV13 elicited a robust immune response for all 13 serotypes in both age groups in the Japanese study, as measured by OPA functional antibody response. OPA responses are thought to contribute to vaccine-induced protection against pneumococcal disease.Citation12 Surprisingly, immune responses in the 50–64 y age group were lower than in the ≥65 y age group. This was in contrast to observations in the US and EU comparator adult studies, which showed, as expected, generally higher immune responses in younger subjects than in older subjects.Citation9,Citation10 Furthermore, the immune responses in the 50–64 y age group in the Japanese study were generally statistically significantly lower than those in subjects of a similar age in the US study, although percentages of responders achieving OPA titers ≥ LLOQ were generally similar between studies for this age group. In addition, the immune responses in the ≥65 y age group were significantly higher for the majority of serotypes in the Japanese study compared with those observed in subjects of similar age in the EU study. The rationale for the responses in the Japanese study in both age groups remains unclear. The potential impact of the S. pneumoniae strains circulating in the communities was of interest as the majority of strains were serotypes included in PCV13 and are known to cause IPD globally.Citation6,Citation13,Citation14 At the time of the studies, PCV7 was licensed for children in the US and EU but not in Japan, leading to potential differences in exposure to pneumococcal strains between the study populations. These differences were most likely reflected by the prevaccination antibody levels. Theoretically, these preexisting antibody levels might serve as a marker for priming and may be associated with increases in antibodies due to immunologic memory.Citation15 On the contrary, high preexisting antibody levels may reduce immune responses.Citation16 This study, however, did not provide convincing evidence to support either of these theories. Prevaccination antibody levels within the Japanese study were similar across the age groups and generally similar across studies, with some exceptions. For example, there were significantly higher prevaccination antibody levels in the Japanese study for serotypes 6A, 6B, 7F, 9V, 14, and 18C compared with the US study and for serotypes 6A, 6B, and 9V compared with the EU study (Pfizer data on file). However, there was no consistent pattern of response postvaccination, suggesting an impact of the prevaccination antibody levels in either age group.
Higher immune responses in Asian populations after administration of PCV13 compared with US and EU populations have been observed in infant PCV13 studies, which is consistent with what was observed with the higher immune responses of the ≥65 y age group in the Japanese study compared with the EU studyCitation17,Citation18 and what we anticipated, but did not observe, for the 50–64 y age group. Post hoc analyses of the 50–64 y age group in the Japanese study compared with the US study identified no notable influencing factors that could explain the lower immune response in the Japanese study, including age, gender, and smoking habits. A few studies have shown that baseline diphtheria levels due to prior exposure to toxigenic strains of Corynebacterium diphtheriae may impact CRM197-based vaccine antibody responses. This is thought to be caused by cross-reactive T-cell priming by the diphtheria antigen with the CRM197-based vaccine.Citation19-Citation22 In the Japanese study, baseline levels of diphtheria antitoxin antibodies were significantly lower in the 50–64 y age group, as were the majority of pneumococcal responses compared with the ≥65 y age group. However, there was no clear correlation (based on scatter plots and regression analysis) observed between prevaccination diphtheria antitoxin titers and postvaccination pneumococcal responses in both age groups. Lower diphtheria antitoxin levels in the younger age groups 50–64 y of age are thought to be due to missed booster vaccinations, whereby relatively higher diphtheria antitoxin levels observed in elderly adults aged ≥65 y may be explained by past infections following diphtheria epidemics during and shortly after World War II.Citation23,Citation24
Multiple factors are known to influence the immune response across populations. For adults, these include genetic differences between populations;Citation25 regional, cultural, or ethnic differences including nutritional factors (eg, fish consumption with n-3 fatty acids);Citation26 comorbidities; immunosenescence;Citation27 coadministration of influenza vaccine;Citation10 gender;Citation28 and smoking habits.Citation29
One potential explanation and a limitation of this study was that it was conducted at 2 sites only, which may have introduced bias. There was no imbalance in recruitment of the age groups across the 2 Japanese sites, and to our knowledge, there were no geographic or environmental influencing factors. For all the studies presented here, the eligibility criteria were the same, suggesting the study populations were similar and included generally healthy adults of similar age and gender. No subjects received influenza vaccine within 14 d of vaccination. An assessment of the impact of ethnicity was not possible as too few Asian subjects participated in the US or EU studies to allow for comparison with the Japanese study populations; thus, there is no clear explanation for the unanticipated Japanese immune responses, particularly in the younger age group of subjects aged 50–64 y.
Local reactions in the Japanese study were mainly mild or moderate but were generally more commonly observed in the ≥65 y age group, mirroring the higher immune responses observed in this age group compared with the younger age group. Overall, systemic events in the Japanese study were generally similar or lower across age groups and studies. There were no deaths, discontinuations due to AEs, or vaccine-related SAEs in the Japanese study.
In conclusion, PCV13 elicited a robust immune response in Japanese subjects in both age groups and was well tolerated and safe. The higher immune responses in the older age group in the Japanese study population are of interest and of potential benefit, given the higher incidence of pneumococcal disease in older adults.
Materials and Methods
Trial design
This was an open-label, multicenter study conducted from October 30, 2007, to January 22, 2008, at 2 medical clinics in Fukuoka and Tokyo, Japan. The Institutional Review Board of Kyushu Clinical Pharmacology Research Clinic 2-13-16, Jigyo, Chuo-ku, Fukuoka approved the study.
Participants
Healthy Japanese men and women aged ≥50 y were eligible for the study. Participants were ineligible if they had a history of S. pneumoniae infection within the previous 5 y; were previously vaccinated with any pneumococcal vaccine or vaccinated with diphtheria-containing vaccine within 6 mo of study vaccine; received influenza vaccine less than 14 d prior to vaccination; received blood products or immunoglobulins (Igs) within the previous 6 mo; known or suspected immunodeficiency or suppression (eg, for cancer, human immunodeficiency virus, or autoimmune disease); severe chronic illness with pulmonary, renal, or cardiac failure; evidence of severe cognitive impairment; or were residents in a nursing home or other long-term care facility. Participants with stable underlying diseases (i.e., disease not requiring significant change in therapy or hospitalization over 12 wk prior to study vaccination) such as cardiovascular disease, chronic pulmonary disease, renal disease, and diabetes that did not meet the exclusion criteria were eligible.
Vaccines and administration
PCV13 contains pneumococcal capsular polysaccharides of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F (containing 2.2 µg of each saccharide except for 4.4 µg of serotype 6B) individually conjugated to CRM197, 0.85% sodium chloride, 0.02% polysorbate 80, and 0.125 mg aluminum phosphate per 0.5-mL dose. PCV13 was manufactured by Wyeth, a Pfizer company (Lot number 7-5095-005A). A single dose of PCV13 was administered by intramuscular injection into the deltoid muscle of either arm.
Immunogenicity assessments
Two 10-mL blood samples were taken; one immediately prior to, and one approximately 1 mo after vaccination. Functional OPA was measured using serotype-specific validated microcolony OPA assays. OPA titers were defined as the interpolated reciprocal serum dilution that results in complement-mediated killing of 50% of the bacteria in the assay. To quantify functional antibody titers, the LLOQ was determined for each serotype-specific OPA assay during assay validation. The limit of detection (LOD) for each serotype-specific assay is a titer of 8, titers below the LLOQ were set to 0.5*LOD, a titer of 4. OPA assay procedures were based on previously described methods.Citation30,Citation31 OPA assays for the comparator studies were performed in the same laboratories using identical procedures.
In a post hoc analysis, standard enzyme-linked immunosorbent assay (ELISA) methods were used on available blood samples for determining diphtheria toxoid IgG antibody concentrations (IU/mL).
Safety assessments
Local reactions (redness, swelling, pain, and limitation of arm movement) at the PCV13 injection site, and systemic events including fever (axillary temperature ≥37.5 °C), fatigue, headache, chills, rash, vomiting, decreased appetite, new and aggravated generalized muscle or joint pain, and the use of antipyretic and pain medications to treat symptoms, were recorded for 14 d in an electronic diary by the participants. Other AEs were collected by the investigator on the case report form in response to direct questioning of the subject on his/her health at each visit. The investigator assessed each AE for severity, for SAE criteria, and causality. Similar assessments were done for the comparators studies.
Statistical methods
Sample size
Sample size estimation was based on the proportion of subjects with certain OPA titers for the pneumococcal serotypes from Wyeth study 6097A1-508.Citation24 The study was sized to allow for estimation of the proportion of subjects achieving ≥2-fold rise with ±8.0% precision for the serotype with the responder rate (72.0% for 19F serotype). Assuming a dropout rate of ≤11%, 135 subjects per age group (270 subjects in total) were to be enrolled to ensure that 120 subjects per age group were evaluable.
Analysis populations
The evaluable immunogenicity population included eligible subjects who had ≥1 valid and determinate assay result and had no major protocol violation. The safety population included all vaccinated subjects.
Immunogenicity analyses
Serotype-specific OPA titers were logarithmically transformed for analysis and GMTs calculated; two-sided 95% confidence intervals (CIs) were constructed. For the GMTs, 95% CIs were constructed by back transformation of the CIs for the mean of the logarithmically transformed assay results computed using the Student t distribution. GMFRs for the 13 serotypes from prevaccination to 1 mo postvaccination were also computed using the logarithmically transformed assay results and two-sided 95% CIs constructed. For the comparison of OPA GMTs, the GMT ratio was calculated by back transformation of the mean difference between groups on the logarithmic scale; two-sided 95% CIs for the ratio were constructed. There was no statistically significant difference in response between age groups if the 95% CIs of the GMT ratio contained 1. A statistically significantly higher response was declared if the lower limit of the 95% CI for the GMT ratio was >1.0 and a significantly lower response if the upper limit of the 95% CI for the GMT ratio was <1.0. For each serotype, the proportion of participants achieving an OPA titer ≥ LLOQ was computed along with exact two-sided 95% CIs; difference in proportions between groups and exact two-sided 95% CIs were constructed. There was no statistically significant difference in response between age groups if the 95% CIs of the difference between age groups contained 0. A statistically significantly higher response was declared if the lower limit of the two-sided 95% CI for the difference in proportions was >0 and a significantly lower response if the upper limit of the 95% CI for the difference in proportions was <0.
ELISA diphtheria toxoid IgG concentrations were analyzed in a similar manner in a post hoc analysis. To examine if prevaccination diphtheria toxoid IgG concentrations affected the immune response to PCV13, prevaccination diphtheria toxoid IgG concentrations were compared with postvaccination serotype-specific pneumococcal OPA titers by scatter plot and regression analysis in both age groups.
Safety analyses
The proportion of subjects with local reactions and systemic events reported on any day within the 14-d period after vaccination was summarized. Differences in the incidences of local reactions and systemic events between groups were determined, and corresponding exact two-sided 95% CIs and P values generated based on Chan and Zhang.Citation32 AEs were categorized according to the Medical Dictionary for Regulatory Activities (MedDRA) and were summarized for each age group separately. All summaries included the number and percentage of adults experiencing ≥1 event and the number of events. The incidence rates for each event for the age groups were compared using the Miettinen and Nurminen method.Citation33
| Abbreviations: | ||
| AE | = | adverse event |
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| EU | = | European Union |
| GMC | = | geometric mean concentrations |
| GMFRs | = | geometric mean fold rises |
| GMT | = | geometric means titers |
| Ig | = | immunoglobulin |
| LLOQ | = | lowest limit of quantitation |
| OPA | = | opsonophagocytic activity |
| PCV13 | = | 13-valent pneumococcal conjugate vaccine |
| PPSV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| SAE | = | serious adverse event |
Additional material
Download Zip (141.5 KB)Disclosure of Potential Conflicts of Interest
M.S. and S.I. have no relevant conflicts of interest to disclose. The remaining authors are all employees of Pfizer Inc.
Funding
This study was supported by Wyeth, which was acquired by Pfizer in October 2009.
Acknowledgments
This study was supported by Wyeth, which was acquired by Pfizer in October 2009. Wyeth, a Pfizer company, was involved in the study design, data collection, data analysis, data interpretation, and writing of the manuscript. Editorial assistance was provided by Rachel Spice, PhD, of Excerpta Medica and Nicole Gudleski O’Regan, PhD, of Complete Healthcare Communications, Inc., and was funded by Pfizer Inc.
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT00562354.
References
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83:373 - 84; PMID: 18927997
- Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010; 59:1102 - 6; PMID: 20814406
- Saito A, Kohno S, Matsushima T, Watanabe A, Oizumi K, Yamaguchi K, Oda H, Study Group. Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother 2006; 12:63 - 9; http://dx.doi.org/10.1007/s10156-005-0425-8; PMID: 16648944
- Ehara N, Fukushima K, Kakeya H, Mukae H, Akamatsu S, Kageyama A, Saito A, Kohno S. A novel method for rapid detection of Streptococcus pneumoniae antigen in sputum and its application in adult respiratory tract infections. J Med Microbiol 2008; 57:820 - 6; http://dx.doi.org/10.1099/jmm.0.47793-0; PMID: 18566139
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013; 1:CD000422; PMID: 23440780
- Chiba N, Morozumi M, Sunaoshi K, Takahashi S, Takano M, Komori T, Sunakawa K, Ubukata K, IPD Surveillance Study Group. Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol Infect 2010; 138:61 - 8; http://dx.doi.org/10.1017/S0950268809990239; PMID: 19538821
- Ada G. Vaccines and vaccination. N Engl J Med 2001; 345:1042 - 53; http://dx.doi.org/10.1056/NEJMra011223; PMID: 11586958
- Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408 - 16; http://dx.doi.org/10.1093/infdis/jis212; PMID: 22457293
- Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013; 31:3594 - 602; http://dx.doi.org/10.1016/j.vaccine.2013.04.084; PMID: 23688525
- Schwarz TF, Flamaing J, Rümke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥65 years. Vaccine 2011; 29:5195 - 202; http://dx.doi.org/10.1016/j.vaccine.2011.05.031; PMID: 21619909
- ClinicalTrials.gov. Study Evaluating 13-valent Pneumococcal Conjugate Vaccine in Healthy Japanese Adults Aged >= 50 Years. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00562354. Accessed February 10, 2014.
- Lee LH, Frasch CE, Falk LA, Klein DL, Deal CD. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine 2003; 21:2190 - 6; http://dx.doi.org/10.1016/S0264-410X(03)00025-2; PMID: 12706710
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al, Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32 - 41; http://dx.doi.org/10.1086/648593; PMID: 19947881
- World Health Organization. Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec 2007; 82:93 - 104; PMID: 17380597
- Klugman K, Black S, Dagan R, Malley R, Whitney C. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 6th ed. China: Saunders; 2012:504-541.
- Siegrist C-A. Vaccine immunology. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 5th ed. China: Saunders; 2008:17-36.
- Nunes MC, Madhi SA. Review on the immunogenicity and safety of PCV-13 in infants and toddlers. Expert Rev Vaccines 2011; 10:951 - 80; http://dx.doi.org/10.1586/erv.11.76; PMID: 21806394
- Kim DS, Shin SH, Lee HJ, Hong YJ, Lee SY, Choi KM, Oh CE, Kim KH, Juergens C, Patterson S, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given to Korean children receiving routine pediatric vaccines. Pediatr Infect Dis J 2013; 32:266 - 73; PMID: 23011012
- Peeters CC, Tenbergen-Meekes AM, Poolman JT, Beurret M, Zegers BJ, Rijkers GT. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun 1991; 59:3504 - 10; PMID: 1894357
- Barington T, Skettrup M, Juul L, Heilmann C. Non-epitope-specific suppression of the antibody response to Haemophilus influenzae type b conjugate vaccines by preimmunization with vaccine components. Infect Immun 1993; 61:432 - 8; PMID: 7678586
- Shelly MA, Pichichero ME, Treanor JJ. Low baseline antibody level to diphtheria is associated with poor response to conjugated pneumococcal vaccine in adults. Scand J Infect Dis 2001; 33:542 - 4; http://dx.doi.org/10.1080/00365540110026502; PMID: 11515767
- Stray-Pedersen A, Aaberge IS, Früh A, Abrahamsen TG. Pneumococcal conjugate vaccine followed by pneumococcal polysaccharide vaccine; immunogenicity in patients with ataxia-telangiectasia. Clin Exp Immunol 2005; 140:507 - 16; http://dx.doi.org/10.1111/j.1365-2249.2005.02791.x; PMID: 15932512
- National Institute of Infectious Diseases. Infectious Agents Surveillance Report: Diphtheria. Available at: http://idsc.nih.go.jp/iasr/19/224/inx224.html. Accessed February 10, 2014.
- Okada K, Ueda K, Morokuma K, Kino Y, Tokugawa K, Nishima S. Seroepidemiologic study on pertussis, diphtheria, and tetanus in the Fukuoka area of southern Japan: seroprevalence among persons 0-80 years old and vaccination program. Jpn J Infect Dis 2004; 57:67 - 71; PMID: 15118214
- Kollmann TR. Variation between Populations in the Innate Immune Response to Vaccine Adjuvants. Front Immunol 2013; 4:81; http://dx.doi.org/10.3389/fimmu.2013.00081; PMID: 23565115
- Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr 2002; 56:Suppl 3 S14 - 9; http://dx.doi.org/10.1038/sj.ejcn.1601478; PMID: 12142955
- Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9:185 - 94; http://dx.doi.org/10.1038/nri2508; PMID: 19240757
- Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 2008; 26:3551 - 5; http://dx.doi.org/10.1016/j.vaccine.2008.04.054; PMID: 18524433
- Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009; 9:377 - 84; http://dx.doi.org/10.1038/nri2530; PMID: 19330016
- Hu BT, Yu X, Jones TR, Kirch C, Harris S, Hildreth SW, Madore DV, Quataert SA. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin Diagn Lab Immunol 2005; 12:287 - 95; PMID: 15699424
- Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011; 29:7207 - 11; http://dx.doi.org/10.1016/j.vaccine.2011.06.056; PMID: 21689707
- Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics 1999; 55:1202 - 9; http://dx.doi.org/10.1111/j.0006-341X.1999.01202.x; PMID: 11315068
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985; 4:213 - 26; http://dx.doi.org/10.1002/sim.4780040211; PMID: 4023479
