Abstract
Particle replication in non-wetting templates (PRINT) is a novel nanoparticle platform that provides compositional flexibility with the ability to specify size and shape in formulating vaccines. The PRINT platform also offers manufacturing and cost advantages over traditional particle technologies. Across multiple antigen and adjuvant formulations, robust antibody and cellular responses have been achieved using PRINT particles in mouse models. Preclinical studies applying PRINT technology in the disease areas of influenza, malaria, and pneumonia are described in this commentary. The proof of principle studies pave the way toward significant cost-effective solutions to global vaccine supply needs.
Introduction
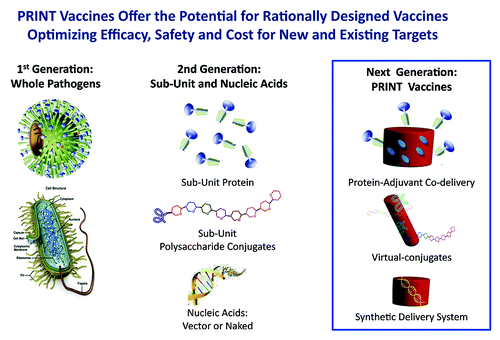
The mammalian immune system is exquisitely designed to recognize and respond to the plethora of sizes and shapes of pathogens.Citation1 As illustrated in , the first generation of vaccines included live, attenuated or killed whole organism vaccines that were very effective in combating common threats.Citation2 In parallel to advances in molecular and cellular biology, and to deal with adverse events associated with those first generation vaccines, the next generation focused on designing vaccines composed of specific subunits of pathogens. However, in many instances in order to obtain adequate efficacy it has been necessary to use adjuvants and multiple doses to improve the immune response and maintain long-lived immunity.Citation3
Now, further advances in our understanding of the immune system combined with advances in nanotechnology, make it possible to mimic size, shape, and antigenic composition of pathogens in vaccine particles to regain the advantages of the early vaccines while retaining the safety and manufacturing advantages of well-defined formulations (). While nanotechnology systems such as dendrimer, micelle, polymeric nanoparticles, liposomes, nanoemulsions, spray drying, and virus-like particlesCitation1 have been used for some time, each of these have limitations. The limitations include low levels of entrapped antigen, the potential for damage of three-dimensional antigen structures, prolonged exposure to organic solvents in processing, polydispersity, manufacturing challenges, batch-to-batch variability, and shape restrictions.Citation4 Particle replication in non-wetting templates (PRINT)Citation5 is a novel nanoparticle fabrication technology that has the potential to overcome the limitations of the other methods while maintaining precise control of particle composition, size, shape, and surface properties, and as such it is well-suited for creating the next generation of engineered synthetic vaccines.
PRINT (Particle Replication in Non-Wetting Templates) Platform
The PRINT process begins with a particle matrix solution that is cast as a film on a plastic sheet that serves to deliver a uniform antigen-adjuvant mixture to an elastomeric mold that contains nanosized cavities corresponding to the size and shape of the desired final particles. The nanosized cavities in the mold are produced from a photolithographically etched master template. A sandwich of the delivery sheet and the mold are brought together which enables capillary forces to fill the mold’s cavities with the desired composition without coating the space between the molds. Depending on the particle composition, particles are then solidified by a vitrification, crystallization or gelation process. To harvest the particles, a filled mold is brought into a contact with a high-energy adhesive film that removes particles from the mold. Particles are collected from the harvesting film using a solvent that dissolves the adhesive layer.Citation5 The PRINT process is now automated in a high throughput, low cost cGMP manufacturing process that leverages roll-to-roll techniques used for decades in the films and printing industry.
Particle sizes from 55 to 10 000 nm can be produced independent of matrix composition to explore the optimal delivery of antigens and immunostimulants. The use of photolithography developed in the electronics industry to create the molds allows the generation of particles that have unique and highly consistent size, shape, and structure.
A key feature of the PRINT technology is the ability to maintain uniform particle size and shape while providing formulation flexibility of particle compositions. Active components can be varied widely as well and include oligonucleotides,Citation9-Citation11 RNA,Citation12,Citation13 polysaccharides, proteins,Citation6,Citation13,Citation14 and small moleculesCitation11,Citation15-Citation17 that can serve as antigens or immunomodulators. The particle matrix can be composed entirely of active components or selective inert materials such as poly(lactic-co-glycolic) acid (PLGA),Citation6 or polyethylene glycol (PEG) hydrogelsCitation7 that serve as bulking agents to reduce costs. As will be shown in the examples below, it is possible to formulate multiple materials together within the same particle, including poorly miscible compounds. For vaccine applications, special attention has been paid to the development of the PRINT process to ensure compatibility between target antigens and matrix components, thus preventing damage and denaturation of three-dimensional antigen structures critical for humoral immune response. Particle surface chemistry and charge can be manipulated without affecting particle shape and size, using the PRINT technology. Incorporation of cationic components into PRINT particles allows the adsorption of negatively charged nucleic acid, polysaccharide, and protein antigens to the particle surface.Citation6,Citation18 This strategy allows encapsulation of antigens and adjuvants in the particle matrix as well as use of particle surface to deliver complex mixes of antigens and immunodulators to cells. In addition, positively charged nanoparticles are taken up by cells more easily due to the negative charge of the cells.Citation18
Careful consideration of particle size, shape, surface charge, and antigenic composition allows for the development of particles that more closely resemble native pathogenic viruses and bacteria in contrast to subunit vaccines. The ability to produce vaccines that better mimic pathogenic targets in a multifaceted way provides the opportunity to advance the field of vaccinology beyond subunit-based and live attenuated vaccines.
Examples of Vaccine Development Using PRINT Nanofabrication Platform
Development of a nanoparticle-based influenza vaccine using PRINT technology
While there are various effective vaccines for influenza, improvements are needed in the protection of elderly and newborn populations and in rapidly responding to pandemic outbreaks.Citation19 In considering how PRINT technology could be used to address these needs, various formulations of influenza virus hemagglutinin (HA) antigenCitation19 were used to rapidly screen a variety of particle sizes and shapes to find a combination with the strongest immune response (). HA preparations were added to cationic PEG particles of different sizes and allowed to absorb through electrostatic interactions. The objective was to use electrostatic binding of the antigen to the particles rather than chemical conjugation to ensure integrity of the antigens and avoid the inefficiency of conjugation.
Figure 2. Antigen adsorbed on PRINT particles. Recombinant H3 HA was adsorbed on 1 × 1 μm and 1 × 3 μm cylinders (where first number is a diameter and second number is length in micrometers) and 1 × 1 × 10 μm, 2 × 2 × 2 μm, 2 × 2 × 6 μm rods (where first, second, and third number correspond to height, width, and length, in micrometers). Particles along with a 2 μg dose of soluble H3 HA protein were injected intramuscularly into BALB/c mice followed by IgG antibody level measurement on day 21 after injection.
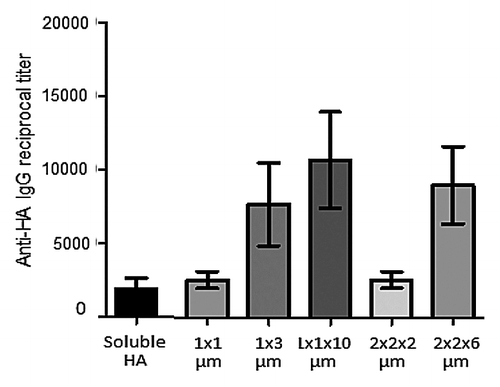
In preliminary mouse studies the HA antigen adsorbed on asymmetric (oblong) shaped 1 × 3 μm, 1 × 1× 10 μm, and 2 × 2 × 6 μm PRINT particles showed greater levels of IgG antibody than dose-matched soluble antigen and symmetric particles (). While there were many options for the matrix materials, PLGA and cationic cholesterol derivatives were chosen for later formulations in order to limit any safety concerns and streamline the regulatory process in the later stages of vaccine development. Further screening efforts resulted in the selection of a lead particle size of 80 × 80 × 320 nm that could be filter-sterilized.Citation6
Co-delivery of malaria CSP antigen and TLR 7/8 adjuvant to achieve induction of T cell mediated immunity
Development of a broadly protective malaria vaccine remains a significant challenge. To explore the potential of PRINT technology to contribute to the solution, particles were designed to efficiently co-deliver circumsporozoite surface protein (CSP) with toll-like receptor 7/8 (TLR 7/8) ligand with the goal of maximizing the T-cell mediated response.Citation20
Cylindrical 80 × 80 × 320 nm PLGA particles were produced with a proprietary TLR 7/8 ligand encapsulated in the particles. Cholesteryl 3β-N-(dimethylaminoethyl)carbamate hydrochloride (DC-Chol) was incorporated in the particle matrix to give the particles a net positive charge that would effectively adsorb the negatively charged CSP on the surface and not change the CSP antigenic determinants. As shown in , the resulting vaccine-enhanced antibody response is seen after one injection. Moreover, stronger antigen-specific CD4+ and CD8+ T-cell responses were seen in antigen-adjuvant PRINT particle systems as compared with soluble protein or soluble protein mixed with adjuvant materials ().
Figure 3. CSP antigen and TLR 7/8 adjuvant co-delivery using PRINT particles. 80 × 80 × 320 nm PLGA-DC Chol particles with a proprietary TLR 7/8 ligand encapsulated in the particle matrix and CSP protein adsorbed on the surface of the particle were injected intramuscularly into BALB/c mice. CSP, matched dose of soluble protein control; CSP+TLR7/8, matched dose of soluble protein control mixed with TLR7/8 ligand; PRINT CSP TLR7/8, PRINT particle designed for co-delivery of CSP and TLR7/8 ligand. (A) Anti-CSP IgG serum antibody levels were evaluated 21 d after a single injection. (B) Cellular responses were evaluated 7 d post-immunization for IFN-γ, IL-2, and TNFα by re-stimulating splenocytes with CSP antigen. Average % of cytokine-producing CD4+ and CD8+ cells is shown. Response for PRINT particle containing CSP alone without adjuvant was at baseline and is not shown.
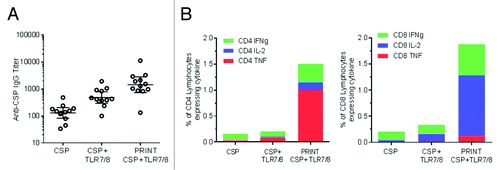
Clinical trials with RTS,S vaccine have demonstrated that increased protective efficacy is associated with higher anti-CSP antibody titers and increased CSP-specific cellular immune responses.Citation20 Our proof of concept data suggest that co-delivery of TLR 7/8 adjuvant with the CSP antigen on the same particle may enhance both antibody and cellular CD8+ and CD4+ T-cell responses. Even though the cellular and antibody responses with soluble antigen/adjuvant combinations eventually reached the same levels achieved with PRINT formulations after multiple doses (data not shown), the enhanced response seen after a single injection with PRINT- may be an advantage, offering the potential for earlier protection, cost savings, and better patient compliance.
Development of novel pneumococcal vaccine using PRINT technology
Licensed pneumococcal conjugate vaccines containing pneumococcal polysaccharide-protein carrier conjugates are very efficient in protecting infants from invasive pneumococcal disease.Citation21 Challenges in the production of traditional conjugate vaccines have hampered strain coverage, higher manufacturing costs and to unmet world-wide demand. Specific issues include polysaccharide activation complexities, downstream manufacturing inefficiencies, and complicated quality control which result low-yield and variable process.Citation22,Citation23 PRINT particle-based design strategies bring together the polysaccharide and carrier protein in close proximity without traditional conjugation. This strategy offers the potential for significant manufacturing and cost advantages over traditional conjugate vaccines, opening up novel product opportunities for a variety of bacterial diseases.
A key advantage of PRINT technology is its flexibility to design and package various pathogen associated molecular features and determinants of immunogenicity in nanoparticles of different size, shape, composition and surface organization. Recent studies have demonstrated that size, shape, and geometry of nanoparticles can possess remarkably different binding tendencies and facilitate receptor mediated recognition, biodistribution, and cellular uptake by antigen presenting cells influencing the outcome of the biological response.Citation24-Citation27
To investigate the role of particle size and shape on immunogenicity, PRINT particle designs of different size and shape were evaluated in generating anti-polysaccharide immune response to pneumococcal polysaccharide type 14 (PnPs14) antigen. In particular, PRINT particles of 2 different sizes (200 × 200 nm cylinders, 1 × 1 µm cylinders) and shape (1 × 3 µm disc) encapsulating PnPs14 antigen were investigated in mice (). Assembly of various PRINT particles involved simple encapsulation of PnPs14 in a chicken ovalbumin (OVA) protein matrix which was crosslinked with glutaraldehyde and served the dual role as a carrier protein and particle delivery matrix. Mice were inoculated with 3 dose-matched PRINT formulations with equivalent PnPs14 antigen loading per particle mass and polysaccharide specific immunogenicity was compared with that of licensed pneumococcal vaccine. Serum analysis after 3 vaccine doses demonstrated that smaller 200 × 200 nm particles which consist of more particles per dose on a total mass basis showed superior in vivo performance in inducing PnPs14-specific IgG response over bigger size (1 × 1 µm cylinders) and shape (1 × 3 µm disc) particles with equivalent PnPs14 antigen loading per total particle mass . Furthermore, 200 × 200 nm sized PRINT particles elicited PnPs14-specific antibody response comparable to a licensed pneumococcal vaccine (). The enhanced immunogenicity that has been observed also included robust antibody responses following a single immunization (unpublished results).
Figure 4. Anti-polysaccharide ELISA immune responses to pneumococcal polysaccharide type 14 (PnPs14). (A) PnPs14 was encapsulated in 0.2 × 0.2 μm, 1 × 1 μm cylinders (where first number is a diameter and second number is length in micrometers) and 1 × 3 μm disks (where first number is height and second number is a diameter, in micrometers) consisting of crosslinked chicken ovalbumin (OVA) protein matrix. (B) BALB/c mice were injected intramuscularly on days 1, 29, and 57. Anti-PnPs14 IgG levels were measured on day 71. Blank Protein particles, OVA protein particles without PnPs14; Sol. Protein+PS14, soluble mix of OVA protein and PnPs14; Prevnar 13®, commercial pneumococcal 13-valent conjugate vaccine; PRINT Protein/PS14 Particles, particles with PnPs14 encapsulated in OVA protein matrix, as described in (A).
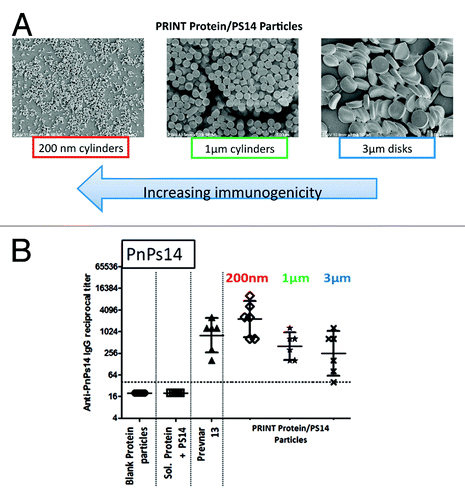
The data shown above reinforce the importance of particle design on modulation of immunogenicity. Continued optimization of particle designs should enable dose-sparing, expanded serotypes coverage, and utilization of pneumococcal protein antigens leading to improved humoral and cellular responses.
Discussion and Future Prospects
Synthetic nanoparticle-based vaccines are likely to play a key role in achieving protection and therapy against pandemics, emerging pathogens, chronic infections, and cancer ().Citation1 Examples shown above demonstrate that PRINT-based vaccines are capable of inducing robust antibody immunity against proteins () and polysaccharides () as well as T-cell mediated immunity involving antigen-specific CD4+ and CD8+ T-cells (). By leveraging fabrication methodologies, the PRINT vaccine platform provides flexibility and scalability to independently control shape, size, composition, surface chemistry and charge, porosity, and modulus for development of successful vaccine delivery agents. Continued efforts to refine particle parameters listed above should lead to superior performing PRINT vaccine formulations. In addition to compositional flexibility the PRINT technology offers several manufacturing advantages including precise engineering of sterile-filterable, monodisperse, and scalable vaccine formulations under cGMP conditions in a cost efficient manner.
The PRINT process has clear advantages in not altering the functionality of proteins, even when particles are formulated from pure protein.Citation6,Citation8,Citation11,Citation13,Citation14 This allowed functionalization of PRINT particles with specific antibodies for targeted delivery.Citation28 Overall compatibility of PRINT process with immunoglobulinsCitation11,Citation28 suggests that PRINT particles can be used to deliver pathogen-specific antibodies for passive immunity applications or enhance delivery of the current antibody therapies used for immunosuppression.
PRINT vaccines generally have not required aluminum salts for enhanced immune response while inducing both humoral and cell-mediated response (–). Formulation of vaccines with alum adjuvants can be a major limitation for vaccines where balanced antibody and cell-mediated immune response is desirable, as alum biases the response toward B-cell antibody response.Citation29 In addition, extracellular proteins are taken up by dendritic cells (DC) and processed through MHC class II pathway, while uptake of PRINT nanoparticles by DC engages both MHC class I as well as MHC class II pathwaysCitation1,Citation30 which, importantly, leads to activation of both antigen-specific CD4+ and CD8+ T-cells. As such, PRINT-based vaccines provide a way to elicit a multipronged immune response against challenging pathogens.
Another emerging use of PRINT technology with potential to contribute to vaccine delivery is microneedle patches. Microneedle patches are arrays of micrometer-sized projections for minimally-invasive drug delivery into the skin. PRINT process can easily be adapted to create an array of discrete microneedles of the desired chemical composition attached to a flexible, water-soluble substrate.Citation31 This application of PRINT can be particularly useful for skin delivery of vaccines.Citation27
In summary, the field of nanotechnology is becoming mature enough to contribute powerfully to vaccinology. Technology platforms, such as PRINT, provide vaccine developers with new strategies and tools for the prevention and treatment of human disease.
| Abbreviations: | ||
| CDAP | = | 1-cyano-4-dimethylaminopyridinium tetrafluoroborate |
| cGMP | = | current good manufacturing practice |
| CSP | = | circumsporozoite protein |
| DC | = | dendritic cells |
| DC Chol | = | cholesteryl 3β-N-(dimethylaminoethyl)carbamate hydrochloride |
| HA | = | hemagglutinin |
| MHC | = | major histocompatibility complex |
| OVA | = | chicken ovalbumin |
| PEG | = | polyethylene glycol |
| PLGA | = | poly(lactic-co-glycolic) acid |
| PnPs14 | = | pneumococcal polysaccharide type 14 |
| = | particle replication in non-wetting templates | |
| TLR | = | toll-like receptor |
Disclosure of Potential Conflicts of Interest
The authors of this manuscript are current employees of Liquidia Technologies, Inc. that develops PRINT nanofabrication platform for various commercial applications.
Acknowledgments
The authors would like to thank Liquidia’s immunology and particle teams and Peter Berglund and Andrew Murphy for their contributions to these data.
Additional Information
Introduction to PRINT platform and video of PRINT process can be found at http://liquidia.com/ProductPlatform.html
References
- Smith DM, Simon JK, Baker JR Jr.. Applications of nanotechnology for immunology. Nat Rev Immunol 2013; 13:592 - 605; http://dx.doi.org/10.1038/nri3488; PMID: 23883969
- Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nat Rev Microbiol 2011; 9:889 - 93; http://dx.doi.org/10.1038/nrmicro2668; PMID: 21963800
- Levine MM, Sztein MB. Vaccine development strategies for improving immunization: the role of modern immunology. Nat Immunol 2004; 5:460 - 4; http://dx.doi.org/10.1038/ni0504-460; PMID: 15116108
- Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater 2013; 12:978 - 90; http://dx.doi.org/10.1038/nmat3775; PMID: 24150416
- Perry JL, Herlihy KP, Napier ME, Desimone JM. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc Chem Res 2011; 44:990 - 8; http://dx.doi.org/10.1021/ar2000315; PMID: 21809808
- Galloway AL, Murphy A, DeSimone JM, Di J, Herrmann JP, Hunter ME, Kindig JP, Malinoski FJ, Rumley MA, Stoltz DM, et al. Development of a nanoparticle-based influenza vaccine using the PRINT technology. Nanomedicine 2013; 9:523 - 31; http://dx.doi.org/10.1016/j.nano.2012.11.001; PMID: 23178283
- Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev 2006; 35:1095 - 104; http://dx.doi.org/10.1039/b600913c; PMID: 17057838
- Kelly JY, DeSimone JM. Shape-specific, monodisperse nano-molding of protein particles. J Am Chem Soc 2008; 130:5438 - 9; http://dx.doi.org/10.1021/ja8014428; PMID: 18376832
- Dunn SS, Tian S, Blake S, Wang J, Galloway AL, Murphy A, Pohlhaus PD, Rolland JP, Napier ME, DeSimone JM. Reductively responsive siRNA-conjugated hydrogel nanoparticles for gene silencing. J Am Chem Soc 2012; 134:7423 - 30; http://dx.doi.org/10.1021/ja300174v; PMID: 22475061
- Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett 2012; 12:287 - 92; http://dx.doi.org/10.1021/nl2035354; PMID: 22165988
- Garcia A, Mack P, Williams S, Fromen C, Shen T, Tully J, Pillai J, Kuehl P, Napier M, DeSimone JM, et al. Microfabricated Engineered Particle Systems for Respiratory Drug Delivery and Other Pharmaceutical Applications. J Drug Deliv. 2012; 2012:941243
- Xu J, Luft JC, Yi X, Tian S, Owens G, Wang J, Johnson A, Berglund P, Smith J, Napier ME, et al. RNA replicon delivery via lipid-complexed PRINT protein particles. Mol Pharm 2013; 10:3366 - 74; http://dx.doi.org/10.1021/mp400190z; PMID: 23924216
- Xu J, Wang J, Luft JC, Tian S, Owens G Jr., Pandya AA, Berglund P, Pohlhaus P, Maynor BW, Smith J, et al. Rendering protein-based particles transiently insoluble for therapeutic applications. J Am Chem Soc 2012; 134:8774 - 7; http://dx.doi.org/10.1021/ja302363r; PMID: 22568387
- Chen K, Merkel TJ, Pandya A, Napier ME, Luft JC, Daniel W, Sheiko S, DeSimone JM. Low modulus biomimetic microgel particles with high loading of hemoglobin. Biomacromolecules 2012; 13:2748 - 59; http://dx.doi.org/10.1021/bm3007242; PMID: 22852860
- Chu KS, Schorzman AN, Finniss MC, Bowerman CJ, Peng L, Luft JC, Madden AJ, Wang AZ, Zamboni WC, DeSimone JM. Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials 2013; 34:8424 - 9; http://dx.doi.org/10.1016/j.biomaterials.2013.07.038; PMID: 23899444
- Chu KS, Hasan W, Rawal S, Walsh MD, Enlow EM, Luft JC, Bridges AS, Kuijer JL, Napier ME, Zamboni WC, et al. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine 2013; 9:686 - 93; http://dx.doi.org/10.1016/j.nano.2012.11.008; PMID: 23219874
- Parrott MC, Finniss M, Luft JC, Pandya A, Gullapalli A, Napier ME, DeSimone JM. Incorporation and controlled release of silyl ether prodrugs from PRINT nanoparticles. J Am Chem Soc 2012; 134:7978 - 82; http://dx.doi.org/10.1021/ja301710z; PMID: 22545784
- Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv Healthc Mater 2013; 2:72 - 94; http://dx.doi.org/10.1002/adhm.201200268; PMID: 23225517
- Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol 2006; 7:449 - 55; http://dx.doi.org/10.1038/ni1343; PMID: 16622432
- Regules JA, Cummings JF, Ockenhouse CF. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines 2011; 10:589 - 99; http://dx.doi.org/10.1586/erv.11.57; PMID: 21604980
- Black SB, Shinefield HR, Hansen J, Elvin L, Laufer D, Malinoski F. Postlicensure evaluation of the effectiveness of seven valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2001; 20:1105 - 7
- Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations?. Nat Rev Drug Discov 2010; 9:308 - 24; http://dx.doi.org/10.1038/nrd3012; PMID: 20357803
- Frasch CE. Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine 2009; 27:6468 - 70; http://dx.doi.org/10.1016/j.vaccine.2009.06.013; PMID: 19555714
- Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012; 4:148rv9
- Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 2009; 26:244 - 9; http://dx.doi.org/10.1007/s11095-008-9626-z; PMID: 18548338
- Roberts RA, Shen T, Allen IC, Hasan W, DeSimone JM, Ting JP. Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles. PLoS One 2013; 8:e62115; http://dx.doi.org/10.1371/journal.pone.0062115; PMID: 23593509
- Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 2012; 64:1547 - 68; http://dx.doi.org/10.1016/j.addr.2012.04.005; PMID: 22575858
- Wang J, Tian S, Petros RA, Napier ME, Desimone JM. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J Am Chem Soc 2010; 132:11306 - 13; http://dx.doi.org/10.1021/ja1043177; PMID: 20698697
- Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 2004; 304:1808 - 10; http://dx.doi.org/10.1126/science.1089926; PMID: 15205534
- Jones SW, Roberts RA, Robbins GR, Perry JL, Kai MP, Chen K, Bo T, Napier ME, Ting JPY, Desimone JM, et al. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest 2013; 123:3061 - 73; http://dx.doi.org/10.1172/JCI66895; PMID: 23778144
- Moga KA, Bickford LR, Geil RD, Dunn SS, Pandya AA, Wang Y, Fain JH, Archuleta CF, O’Neill AT, Desimone JM. Rapidly-dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Adv Mater 2013; 25:5060 - 6; http://dx.doi.org/10.1002/adma.201300526; PMID: 23893866