Abstract
In recipients primed with acellular pertussis diphtheria-tetanus combined vaccine (DTaP) an increased incidence of severe local reactions with extensive redness/swelling has been reported for each subsequent dose of diphtheria-tetanus based combination vaccine given as a booster. This has been attributed to residual active pertussis toxin (PT) in the primary vaccine. In this study, we investigated the possible contribution of the A-subunit enzymatic activity and the B-oligomer carbohydrate binding activity of residual PT in DTaP to local reactions in a murine model using Japanese DTaP batches produced before and after the introduction of a test for reversion of pertussis toxoid to toxin. Residual PT activity was correlated with the B-oligomer carbohydrate binding activity. The in vivo mouse footpad swelling model assay indicated that the B-oligomer carbohydrate binding activity and possibly other factors were associated with intensified sensitization to local reaction following diphtheria toxoid booster.
Introduction
Acellular pertussis vaccine (aP) in combination with diphtheria (D) and tetanus (T) toxoids (DTaP) was developed successfully in Japan and has been used there since 1981.Citation1 It has proven to be clinically safe and effective and along with whole-cell pertussis vaccine (wP) combined with D and T toxoids (DTwP) is recommended by WHO for pediatric immunisation.Citation2-Citation4 Although the rates of adverse reactions to DTaP are lower than for DTwP, DTaPs have been reported to cause occasional rather severe local reactions to booster doses of D and T combined toxoids (DTd, approximately one-fifth antigen content of the DTaP primary dose) given at 11–12 y of age in Japan.Citation5,Citation6 The rates of local reactions increased with each subsequent booster dose of DT based combination vaccines and extensive local redness/swelling have also been reported for booster doses in DTaP-based schedules in other countries.Citation7-Citation9
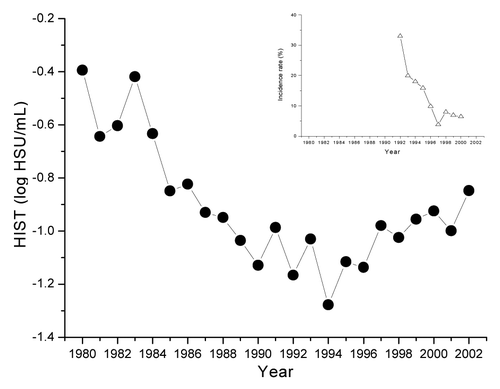
In Japan in the 1980s it emerged that pertussis toxoid (PTd) in DTaP could revert to toxicity during long-term storage.Citation10 Accordingly, following extensive investigation of this issue, the detoxification process was improved by manufacturers and the levels of residual histamine sensitizing (HS) activity in Japanese DTaPs began to decline from 1985 onwards. This finally resulted in the revision of the Japanese Minimum Requirements for Biological ProductsCitation11 in 1991. The histamine sensitization test (HIST) for reversion of PTd to active pertussis toxin (PT) was implemented on DTaP incubated at 37 ◦C for 4 wk (accelerated reversion test) and also the specified upper limit for residual HS activity was revised from 0.8 to 0.4 HS units (HSU)/mL.Citation12 Subsequently, in a clinical surveillance studyCitation13 performed during 1992–2000 it was observed that the changes in annual mean residual HS activity in DTaP lots were associated with decreased annual incidence rates of local reactions following boosting with DTd in adolescents (). Thus, adolescents who received the primary DTaP immunizations before 1985 and were boosted with DTd in 1992–1993 showed a higher incidence of local reaction (measured as size of area of redness at the injection sites) in comparison with those receiving the DTaP primary immunizations after 1985 followed by the DTd booster after 1993.
Figure 1. Change in annual mean residual HS activity of DTaP batches and local reactions to the booster dose with DTd. Annual geometric means of HS activity (●) of DTaP batches used for primary immunization were determined shortly after them being manufactured. Detoxification processes for aP antigens were revised from the mid-1980s without changing other specifications for DTaP. Local reactions following DTd booster in adolescents at the age of 11–12 y* were monitored from 1992 to 2000 in cohorts from Hisayama-cho, Fukuoka, Japan.Citation13 The number of adolescents (in brackets) who received the booster in these cohorts from 1992 to 2000 were 1992 (368), 1993 (986), 1994 (1170), 1995 (175), 1996 (150), 1997 (56), 1998 (310), 1999 (313), and 2000 (146). (△) is the percentage of local reaction incidence at the injection sites determined by size of area of redness of ≥5 cm in diameter observed 2 d after the booster; *The vaccination program in Japan was temporarily suspended due to 2 cases of severe adverse events after DTwP vaccination in 1975 and resumed 3 mo later to start at 2 y of age until revision of the immunization schedule in 1994 to start at 3 mo of age. Therefore all children in this surveillance study were immunized with DTaP at 2 y of age with the subsequent 3 doses at 3 to 8 wk intervals with an additional dose 12 to 18 mo later (primary immunization).
PT is a member of the AB5 family of bacterial toxins, having an A-subunit named S1, which is an ADP-ribosyltransferase that targets the α-subunit of some GTP-binding regulatory proteins.Citation14,Citation15 The B-oligomer (B-subunit) of subunits S2 through to S5 is required for cell targeting and cytosolic entry of S1.Citation16,Citation17 PT is an important protective antigen and in its detoxified form, PTd is included as a component in all types of aP vaccines. Although detoxified, the possibility of partial reversion to toxic PT activity of aP is recognized and monitoring for PT residual toxicity by the HIST is regarded as an essential part of the safety control of aP-containing vaccines and is required by regulatory authorities.Citation11,Citation18,Citation19
Previous investigation performed in an animal model showed that excess residual active PT as indicated by residual HS activity in DTaP could cause intensified sensitization to subsequent severe local reaction to a booster dose of D toxoid (Dd) without augmenting IgG and IgE responses, suggesting a role for cell mediated immunity.Citation20 In the present study, to elucidate the role of subunits of PT in relation to local reactions, the activity of A- and B-subunits of PT in Japanese DTaP batches produced before and after 1990 were investigated using the mouse footpad swelling (MFS) model and established in vitro A- and B-subunits assay systems.Citation21-Citation25
Results
Relationship of ADP-ribosyltransferase activity to HS and sensitizing activities to MFS reaction
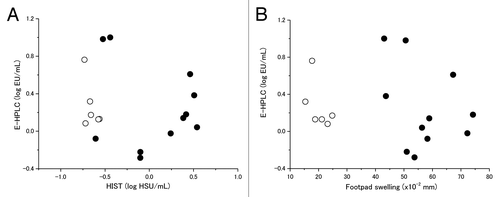
Eleven and 6 batches of DTaP produced before and after 1990, respectively, were assayed for enzymatic activity by enzyme coupled-high performance liquid chromatography (E-HPLC). They were also assayed for HS activity in mice and sensitizing activity to MFS and the results are represented in . Batch to batch variations in these assays were similar for vaccine batches made before and after 1990. Much reduced HS activities were seen in batches produced after 1990 in comparison with earlier ones. The difference between batches produced before and after 1990 was even more evident in the sensitizing activity to MFS compared with HS activity. Mean swelling (×10−2 mm) for batches produced before 1990 was 57.2 (×10−2mm) and that for batches produced after 1990 was 20.2 (×10−2 mm) (P < 0.0001). However, similar levels of ADP-ribosyltransferase activity were observed for batches produced both before and after 1990, although considerable batch to batch variations were seen (). The results indicate that the enzymatic activity in those vaccines was not directly proportional to the observed decrease in both the HS activity and the sensitizing activity to MFS for the products manufactured after 1990. Furthermore, they also suggest that the change in the detoxification process after 1990 probably had a limited effect on the A-subunit of PT.
Figure 2. Relationship of residual enzymatic activity of S1 as measured by E-HPLC to (A) HIST and (B) sensitizing activity to mouse foot swelling (MFS) to Dd booster. Vaccine produced after 1990 differ to those before 1990 only in strengthened detoxification procedure for aP antigens and no change was made to other specifications. (●) DTaP (n = 11) produced before 1990; (○) DTaP (n = 6) produced after 1990.
Carbohydrate binding activities of PT B-oligomer detected using different antibodies
Carbohydrate binding activities of PT B-oligomer using fetuin ligand in DTaP batches produced before and after 1990 were measured by detecting fetuin-bound PT using either a polyclonal antibody (pAb) against PT or monoclonal antibodies (Mabs) against S2&3 and S4 subunits of PT, respectively. Although large variations in binding activities were observed between these batches (), the overall results indicated that the vaccines produced after 1990 showed significantly lower binding activities than those produced before 1990 (P < 0.05) ().
Figure 3. Comparison of carbohydrate binding, enzymatic and HS activities in DTaP made before (open bar, n = 11) and after (gray bar, n = 17) 1990. Values inside the bars represent the lowest and highest activities. Bracketed numbers outside of bars represent fold difference between before and after 1990. *Indicate statistically significant at 5% level. # log binding activity unit (BU/mL) for pAb, Mab S2&3, and Mab S4 binding assay; log enzymatic activity unit (EU/mL) for E-HPLC assay and log HSU/mL for HIST (see Methods Section).
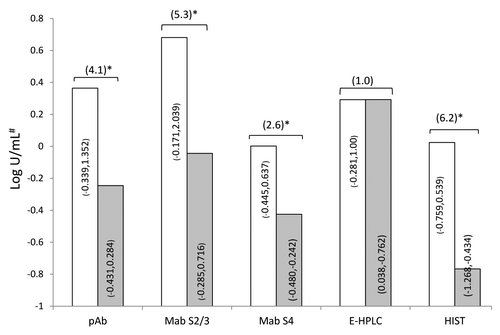
In the HIST, the mean HS activity of batches produced before 1990 was 0.024 log HSU/mL and that of batches produced after 1990 was -0.767 log HSU/mL, an approximately 6-fold difference (P = 0.0013) (). In the fetuin-binding assay, all the antibodies detected carbohydrate binding activity differences between the 2 groups of DTaP vaccines, but the ratio of differences detected by Mab S4 was far lower than those detected using pAb or Mab S2&3. The ratio of difference in binding activities between vaccines produced before and after 1990 using different detecting antibody () showed a ranking of Mab 2&3 (5.3) followed by pAb (4.1) and Mab S4 (2.6). This may be explained by the different efficiency of each detecting antibody e.g., they could show different abilities to distinguish between the 2 groups of vaccines on one hand, and on the other hand the changes to the detoxification procedure 1990 may have had greater impact on subunits 2&3 than subunit 4.
Carbohydrate binding activities and sensitizing activity to MFS of DTaP produced before and after 1990
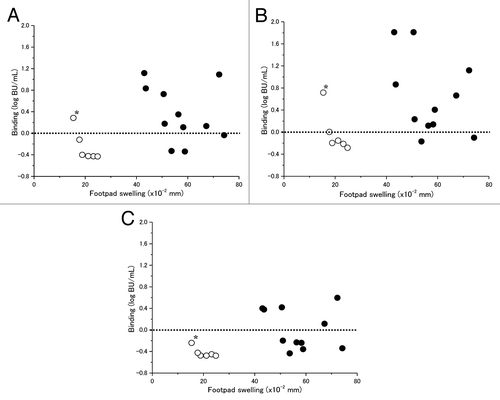
DTaP batches produced before 1990 (11 batches) showed significant sensitization (>40 × 10−2 mm reaction) to MFS, while those produced after 1990 (6 batches) showed far less sensitization (<30 × 10−2 mm reaction) (). Although no significant correlation could be seen between the sensitizing activities to MFS and carbohydrate binding activities using any of the detection antibodies, most vaccine batches produced before 1990 showed higher carbohydrate binding activity units (BU) with values between 1 BU/mL (0 log BU/mL) up to approximately 13 BU/mL (1.11 log BU/mL) and 65 BU/mL (1.81 log BU/mL) respectively when pAb and Mab 2&3 were used as detecting antibodies, while binding activities detected for most of the batches produced after 1990 were at or less than 0.76 BU/mL (-0.119 log BU/mL) except for one outlying batch (). In the present study there was no significant sensitizing activity to MFS observed if residual B-subunit binding activity of PTd was below 0.76 BU/mL (–0.119 log BU/mL) (upper value detected by pAb except for an outlying value) or 1 BU/mL (0 log BU/mL) (upper value detected by Mab S2&3 except for an outlying value) detected by pAb or Mab S2&3, respectively. The results suggest that the higher carbohydrate binding activity of PTd in DTaP batches made before 1990 was related to the observed reactogenicity in the mouse model.
Figure 4. Relationships between sensitizing activity to footpad swelling and B-subunit carbohydrate binding activities to fetuin ligand detected using various antibodies: (A) pAb, (B) Mab S2&3, and (C) Mab S4. (●) DTaP (n = 11) produced before 1990; (○) DTaP (n = 6) produced after 1990 which differ from those produced before 1990 only in strengthened detoxification procedure for aP antigens. *Outlier (see Statistical analysis section under Materials and Methods).
Discussion
In general, DTaPs have proved less reactogenic and safer than DTwPs.Citation1-Citation3,Citation26 However, severe local swelling has been regarded as a safety problem for booster immunizations with DTaP and DTd. Although the mechanisms of the reactogenicity remain unknown, residual PT activity of DTaPs for immunization was shown to play a role in the enhanced sensitization of mice to the DTd booster related hind paw swelling.Citation20 We attempted to investigate further the possible contribution of the enzymatic activity (A-subunit) and carbohydrate binding activity (B-oligomer) of PT to the enhanced local reaction in a mouse model.
Various chemical treatments have been used by manufacturers to detoxify PT. Although formaldehyde has been the only detoxification reagent used by all manufacturers in Japan,Citation27 different detoxification conditions e.g., formaldehyde concentration, incubation period, or temperature could result in different amino acid side-chain modifications and changes in conformational and linear epitope binding patterns for the resulting PTd.Citation28,Citation29 This may reflect the variation in residual activities observed among these vaccines made both before and after 1990 from different manufacturers. Furthermore, although DTaP batches used in this study contained either aluminum hydroxide or aluminum phosphate (≤0.3mg Al/mL) according to manufacturer, these aluminum gels were reported not to be the major cause of injection site inflammation at the primary dose and also showed no sensitizing effect to DTd boosters.Citation20,Citation30
In general, it is noted that the detoxification procedure after 1990 had significant impact on B-subunit binding activity ( and ) while the A-subunit enzymatic activity hardly changed ( and ) when compared with earlier products. Therefore, the reduced residual PT activities observed in the in vivo assays for samples produced after 1990 were probably largely due to the reduction in B-subunit binding activities detected, whether by reduced binding to fetuin ligand or reduction/modification of epitopes recognized by the detection antibodies.
Results from this study also suggest that the choice of detection antibody may impact on the detection efficiency of carbohydrate binding activity of chemically detoxified PTd. While the B-oligomer of PT has 4 distinct subunits, all amino acid residues involved in binding activities have thus far been mapped to the S2 and S3 subunits of the B-pentamer.Citation31-Citation35 Multiple binding sites have been identified on S2 and S3 subunits.Citation36 In the present study, a pAb and different Mabs to different subunits in B-oligomer were used to detect the PT molecule bound to fetuin ligand. While there were no significant differences in binding results between pAb and Mab S2&3 as detection antibodies, significant differences were observed between binding activities assessed using Mab S2&3 and Mab S4 (P = 0.0007), or pAb and Mab S4 (P = 0.039) (). Although all the antibodies were able to detect a difference in binding activities between the samples produced before and after 1990, pAb and Mab S2&3 showed the biggest differential binding activities for products made before and after 1990 (). This lower sensitivity of Mab S4 in differentiating products made before and after 1990 suggests that the S4 epitope on the PTd molecule may have been less affected by the change in detoxification process after 1990 than the epitopes recognized by Mab S2&3. Since the exact mechanisms and sites of action of the different toxoiding reactions on the PT molecule have not yet been defined, using pAb as the detection antibody may be more appropriate for detecting possible structural changes by recognizing a wider range of epitopes on PTd molecules than a particular Mab. In any case, if a Mab is used in preference to pAb, Mab to subunits 2&3 would be more appropriate.
Although significant differences between the vaccines produced before and after 1990 were shown in residual HS activity and also in the sensitizing activity to MFS, enzymatic activity did not differ between these 2 groups. On the other hand, higher carbohydrate binding activities were seen for the vaccines produced before 1990 than those after 1990 and in most cases the binding activity showed a positive relationship to the sensitization activity to MFS.
Cell mediated immunity is reported to be a potential cause of local reactions to booster vaccination.Citation37 Although the precise mechanisms and the role of each A- or B-subunit for reactivity in vivo are unknown, B-subunit of PT was reported to enhance both Th1 and Th2 immune responses to co-administered antigens.Citation38 Our results suggest that B-oligomer binding activity may play a role in priming the intensification of the MFS and similarly the immune response intensified by B-subunit may result in intensified sensitization to booster doses. However, conclusive correlation of the binding activity to MFS was not established in the present study which suggests other unknown mechanisms via holotoxin activity could also play a role in the priming for intensified MFS to a co-administered antigen.Citation20 Although residual enzymatic activity of PT alone showed no direct relevance to the sensitization to MFS, the higher residual carbohydrate binding activity of PT could promote the entry of the A-subunit into cells in vivo and thus holotoxin activity could also contribute to the intensified MFS observed for the vaccines produced before 1990.
Materials and Methods
Materials
The PT used was a freeze-dried reference preparation (National Institute for Biological Standards and Control (NIBSC), 90/518).Citation39 Japanese reference pertussis vaccine for toxicity test lot 2 (reference vaccine) with a known HS activity (48 HSU/vial) was used as the reference preparation for HIST.
A total of 28 batches of DTaP from 6 manufacturer sources in Japan were used in this study, which included vaccine batches produced in the 1980s (n = 11), before the implementation of a stricter regulation on HS activity for residual PT, and manufactured in the 1990s (n = 17), after the implementation of the regulation. Aluminum hydroxide or aluminum phosphate was contained as the adjuvant in the vaccines. While pertussis antigen formulations in these vaccines varied depending on the source manufacturer,Citation1,Citation27 contents of D and T toxoids were almost the same for all the products. Those produced after 1990 differed from before 1990 only in strengthened detoxification procedure for pertussis antigens e.g., employing higher formalin concentration, longer period, or higher temperature of treatment etc. After obtaining, all vaccine samples were stored at 4–8 °C throughout.
The PT enzyme substrate, fluorescein-tagged Gαi3C20 peptide, F-VFDAVTDVIIKNNLKECGLY-COOH (F-Gαi3C20) was custom-synthesized by AnaSpec Inc. and was reported to have >95% purity. Polyclonal anti-PT antibody (pAb) (NIBSC, 97/572), monoclonal antibodies (Mabs) to S2&3 subunits (NIBSC, 99/534), and S4 subunits (NIBSC, 99/554) were from NIBSC. Peroxidase labeled anti-sheep IgG (A3415) and peroxidase labeled anti-mouse IgG (A0168) was obtained from Sigma. All other chemicals, unless specified otherwise, were of analytical grade and purchased from either Sigma-Aldrich or VWR-BDH.
E-HPLC coupled assay
The determination of ADP-ribosyltransferase activity of PT in vaccines was performed as previously described.Citation23,Citation40 The enzyme activity was expressed as enzymatic activity units (EU) where 1 EU equals to fluorescence produced by 1 µg of PT (90/518) under identical assay and analysis conditions. All assays were performed in duplicate and met the in house assay validity criteria.Citation40
Carbohydrate binding assay
The carbohydrate binding activity of PT in vaccines using bovine fetuin as carbohydrate coat was performed as described by Gomez et al.Citation24 Apart from polyclonal antibody (1/10 000 dilution), Mab to S2&S3 and Mab to S4 were also used as the detecting antibody at 1/500 dilution. All samples were assayed in duplicate. PT (90/518) used in the assay is for calibration purpose for comparing differences between vaccine products in binding activity units (BU). BU is arbitrary units representing the binding activity in vaccine products. The potency of PT binding activity in vaccine relative to 90/518 was calculated using a parallel line analysis and was expressed as arbitrary binding activity units (BU) where 1 BU equals to optical density produced by 1 µg of PT (90/518) under identical assay and analysis conditions.
Temperature method of HIST
The HIST by temperature measurement was performed as described in previous publications.Citation11,Citation41 The HS activity in the vaccines was calculated relative to that of the reference vaccine using a parallel line analysis and was expressed as HSU.
Mouse footpad swelling (MFS) model
An animal model in which mice are primed with DTaP and boosted (challenged) with Dd was used to assess the sensitizing activity to intensified local reaction (swelling size) as described by Yamamoto et al.Citation20 In brief, BALB/c female mice were injected intramuscularly with an immunizing vaccine (DTaP) twice at a one-month interval. They were injected intraperitoneally with indomethacin daily from 3 d before challenge and their right and left hind paws were injected subcutaneously with a 50 µL dose of Dd (diluted to 30 Lf/mL with saline containing 0.15 mg Al/ mL of Al(OH)3 gel) and saline respectively 14 d after the last immunization of vaccine. Thickness of right and left hind paws was measured until 48 h after the challenge with Dd and the maximum difference between the thickness of right and left paws was taken as the swelling reaction.
Statistical analysis
Analysis of the parallel line assays was performed by Finney method.Citation42 Significance and validity tests were made at a level of P = 0.05 and confidence intervals were expressed at 95% probability level unless otherwise stated. Comparison of groups was made by t test to calculate P value for null hypothesis.
The outlier was detected by calculating F value using s2 for all batches in a group and s2 for batches excluding the most deviated one from the analysis. If the F value was significant, the most deviated value was considered an outlier.
Ethical statements
All animal work was performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Infectious Diseases. The procedures were approved by the Institutional Committee on Animal Care and Use.
| Abbreviations: | ||
| aP | = | acellular pertussis vaccine |
| D | = | diphtheria |
| Dd | = | D toxoid |
| DTd | = | D and T combined toxoids |
| DTaP | = | acellular pertussis diphtheria-tetanus combined vaccine |
| DTwP | = | whole-cell pertussis vaccine combined with D and T toxoids |
| E-HPLC | = | enzyme coupled-high performance liquid chromatography |
| HS | = | histamine sensitizing |
| HIST | = | histamine sensitization test |
| Mab | = | monoclonal antibody |
| MFS | = | mouse footpad swelling |
| NIBSC | = | National Institute for Biological Standards and Control |
| pAb | = | polyclonal antibody |
| PT | = | pertussis toxin |
| PTd | = | pertussis toxoid |
| T | = | tetanus |
| wP | = | whole-cell pertussis vaccine |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Grant-in-Aid from the Ministry of Health, Labour and Welfare, Japan.
References
- Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet 1984; 1:122 - 6; http://dx.doi.org/10.1016/S0140-6736(84)90061-8; PMID: 6140441
- Kimura M, Hikino N. Results with a new DTP vaccine in Japan. Dev Biol Stand 1985; 61:545 - 61; PMID: 2872131
- Kimura M, Kuno-Sakai H. Experiences with acellular pertussis vaccine in Japan and epidemiology of pertussis. Tokai J Exp Clin Med 1987; 12:263 - 73; PMID: 3508651
- Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec 2010; 85:385 - 400; PMID: 20939150
- Kimura M, Kuno-Sakai H. Pertussis vaccines in Japan. Acta Paediatr Jpn 1988; 30:143 - 53; http://dx.doi.org/10.1111/j.1442-200X.1988.tb02512.x; PMID: 3149848
- Kamiya H, Nii R. Overview of currently available Japanese acellular pertussis vaccines and future problems. Tokai J Exp Clin Med 1988; 13:Suppl 45 - 9; PMID: 3273618
- Gold MS, Noonan S, Osbourn M, Precepa S, Kempe AE. Local reactions after the fourth dose of acellular pertussis vaccine in South Australia. Med J Aust 2003; 179:191 - 4; PMID: 12914508
- Skowronski DM, Remple VP, Macnabb J, Pielak K, Patrick DM, Halperin SA, Scheifele D. Injection-site reactions to booster doses of acellular pertussis vaccine: rate, severity, and anticipated impact. Pediatrics 2003; 112:e453; http://dx.doi.org/10.1542/peds.112.6.e453; PMID: 14654644
- Rennels MB, Deloria MA, Pichichero ME, Losonsky GA, Englund JA, Meade BD, Anderson EL, Steinhoff MC, Edwards KM. Extensive swelling after booster doses of acellular pertussis-tetanus-diphtheria vaccines. Pediatrics 2000; 105:e12; http://dx.doi.org/10.1542/peds.105.1.e12; PMID: 10617749
- Iwasa S, Ishida S, Asakawa S, Akama K. A curious histamine-sensitizing activity shown by the newly-developed Japanese acellular pertussis vaccine. Dev Biol Stand 1985; 61:453 - 60; PMID: 3835082
- Ministry of Health, Labour and Welfare, Japanese Government. Minimum Requirements for Biological Products; 2004. Available from: http://www.niid.go.jp/niid/ja/mrbp.html
- Horiuchi Y, Takahashi M, Konda T, Ochiai M, Yamamoto A, Kataoka M, Toyoizumi H, Arakawa Y. Quality control of diphtheria tetanus acellular pertussis combined (DTaP) vaccines in Japan. Jpn J Infect Dis 2001; 54:167 - 80; PMID: 11754154
- Okada K, Ueda K, Morokuma K, Fukushige J, Miyazaki C. Comparison of antibody titers in eleven- to twelve-year old Japanese school children six years after administration of acellular and whole cell pertussis vaccines combined with diphtheria-tetanus toxoids. Pediatr Infect Dis J 1998; 17:1167 - 9; http://dx.doi.org/10.1097/00006454-199812000-00016; PMID: 9877371
- Tamura M, Nogimori K, Murai S, Yajima M, Ito K, Katada T, Ui M, Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 1982; 21:5516 - 22; http://dx.doi.org/10.1021/bi00265a021; PMID: 6293544
- Katada T, Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A 1982; 79:3129 - 33; http://dx.doi.org/10.1073/pnas.79.10.3129; PMID: 6954463
- Tamura M, Nogimori K, Yajima M, Ase K, Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem 1983; 258:6756 - 61; PMID: 6343381
- Ui M. In: Moss J, Vaughan M, editors. ADP-ribosylating toxins and G protein. Washington, D.C.: American Society for Microbiology; 1990. p. 45-77.
- World Health Organization. Recommendations to assure the quality, safety and efficacy of acellular pertussis vaccines; 2013. Technical Report Series 979, Annex 4.
- European Pharmacopoeia. Suppl. 7.8, Methods of Analysis 07/2013:20633: Residual pertussis toxin and irreversibility of pertussis toxoid. Strasbourg: Council of Europe; 2013.
- Yamamoto A, Nagata N, Ochiai M, Kataoka M, Toyoizumi H, Okada K, Horiuchi Y. Enhanced sensitisation of mice with diphtheria tetanus acellular pertussis vaccine to local swelling reaction to the booster immunisation. Vaccine 2002; 20:3088 - 94; http://dx.doi.org/10.1016/S0264-410X(02)00290-6; PMID: 12163259
- Finck-Barbançon V, Barbieri JT. ADP-ribosylation of alpha i3C20 by the S1 subunit and deletion peptides of S1 of pertussis toxin. Biochemistry 1995; 34:1070 - 5; http://dx.doi.org/10.1021/bi00003a043; PMID: 7827022
- Cyr T, Menzies AJ, Calver J, Whitehouse LW. A quantitative analysis for the ADP-ribosylation activity of pertussis toxin: an enzymatic-HPLC coupled assay applicable to formulated whole cell and acellular pertussis vaccine products. Biologicals 2001; 29:81 - 95; http://dx.doi.org/10.1006/biol.2001.0280; PMID: 11580213
- Yuen CT, Canthaboo C, Menzies JA, Cyr T, Whitehouse LW, Jones C, Corbel MJ, Xing D. Detection of residual pertussis toxin in vaccines using a modified ribosylation assay. Vaccine 2002; 21:44 - 52; http://dx.doi.org/10.1016/S0264-410X(02)00446-2; PMID: 12443661
- Gomez SR, Xing DKL, Corbel MJ, Coote J, Parton R, Yuen C-T. Development of a carbohydrate binding assay for the B-oligomer of pertussis toxin and toxoid. Anal Biochem 2006; 356:244 - 53; http://dx.doi.org/10.1016/j.ab.2006.05.012; PMID: 16782039
- Yuen CT, Horiuchi Y, Asokanathan C, Cook S, Douglas-Bardsley A, Ochiai M, Corbel M, Xing D. An in vitro assay system as a potential replacement for the histamine sensitisation test for acellular pertussis based combination vaccines. Vaccine 2010; 28:3714 - 21; http://dx.doi.org/10.1016/j.vaccine.2010.03.009; PMID: 20307594
- Kimura M, Kuno-Sakai H. Developments in pertussis immunisation in Japan. Lancet 1990; 336:30 - 2; http://dx.doi.org/10.1016/0140-6736(90)91530-N; PMID: 1973217
- Sato Y, Sato H. Further characterization of Japanese acellular pertussis vaccine prepared in 1988 by 6 Japanese manufacturers. Tokai J Exp Clin Med 1988; 13:Suppl 79 - 88; PMID: 2908530
- Burns DL, Kenimer JG, Manclark CR. Role of the A subunit of pertussis toxin in alteration of Chinese hamster ovary cell morphology. Infect Immun 1987; 55:24 - 8; PMID: 3539804
- Ibsen PH. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine 1996; 14:359 - 68; http://dx.doi.org/10.1016/0264-410X(95)00230-X; PMID: 8735545
- Kataoka M, Yamamoto A, Ochiai M, Harashima A, Nagata N, Hasegawa H, Kurata T, Horiuchi Y. Comparison of acellular pertussis-based combination vaccines by Japanese control tests for toxicities and laboratory models for local reaction. Vaccine 2009; 27:1881 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.01.095; PMID: 19368767
- Francotte M, Locht C, Feron C, Capiau C, De Wilde M. Monoclonal antibodies specific for pertussis toxin subunits and identification of the haptoglobin-binding site. In: Lerner RA, Ginsberg H, Chanock RM, Brown F, editors. Vaccines 89. Plainview, NY: Cold Spring Harbor Laboratory Press; 1989. p. 243-7.
- Loosmore S, Zealey G, Cockle S, Boux H, Chong P, Yacoob R, Klein M. Characterization of pertussis toxin analogs containing mutations in B-oligomer subunits. Infect Immun 1993; 61:2316 - 24; PMID: 8500874
- Stein PE, Boodhoo A, Armstrong GD, Heerze LD, Cockle SA, Klein MH, Read RJ. Structure of a pertussis toxin-sugar complex as a model for receptor binding. Nat Struct Biol 1994; 1:591 - 6; http://dx.doi.org/10.1038/nsb0994-591; PMID: 7634099
- Lobet Y, Feron C, Dequesne G, Simoen E, Hauser P, Locht C. Site-specific alterations in the B oligomer that affect receptor-binding activities and mitogenicity of pertussis toxin. J Exp Med 1993; 177:79 - 87; http://dx.doi.org/10.1084/jem.177.1.79; PMID: 8418210
- Saukkonen K, Burnette WN, Mar VL, Masure HR, Tuomanen EI. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci U S A 1992; 89:118 - 22; http://dx.doi.org/10.1073/pnas.89.1.118; PMID: 1729677
- Millen SH, Lewallen DM, Herr AB, Iyer SS, Weiss AA. Identification and characterization of the carbohydrate ligands recognized by pertussis toxin via a glycan microarray and surface plasmon resonance. Biochemistry 2010; 49:5954 - 67; http://dx.doi.org/10.1021/bi100474z; PMID: 20515023
- Scheifele DW, Ochnio JJ, Halperin SA. Cellular immunity as a potential cause of local reactions to booster vaccination with diphtheria and tetanus toxoids and acellular pertussis antigens. Pediatr Infect Dis J 2009; 28:985 - 9; http://dx.doi.org/10.1097/INF.0b013e3181a9cc2a; PMID: 19755930
- Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol 1998; 10:651 - 62; http://dx.doi.org/10.1093/intimm/10.5.651; PMID: 9645613
- Xing D, Das RG, Newland P, Corbel M. Comparison of the bioactivity of reference preparations for assaying Bordetella pertussis toxin activity in vaccines by the histamine sensitisation and Chinese hamster ovary-cell tests: assessment of validity of expression of activity in terms of protein concentration. Vaccine 2002; 20:3535 - 42; http://dx.doi.org/10.1016/S0264-410X(02)00338-9; PMID: 12297399
- Gomez SR, Yuen CT, Asokanathan C, Douglas-Bardsley A, Corbel MJ, Coote JG, Parton R, Xing DK. ADP-ribosylation activity in pertussis vaccines and its relationship to the in vivo histamine-sensitisation test. Vaccine 2007; 25:3311 - 8; http://dx.doi.org/10.1016/j.vaccine.2007.01.009; PMID: 17287049
- Ishida S, Kurokawa M, Asakawa S, Iwasa S. A sensitive assay method for the histamine-sensitizing factor using change in rectal temperature of mice after histamine challenge as a response. J Biol Stand 1979; 7:21 - 9; http://dx.doi.org/10.1016/S0092-1157(79)80034-7; PMID: 489619
- Finney DJ. Statistical methods in biological assay. 3rd ed. London: Charles Griffin Co. Ltd; 1978.
