Abstract
In the clinical trials of the quadrivalent human papillomavirus (qHPV) vaccine, antibodies were measured by a competitive Luminex immunoassay (HPV-4 cLIA). A nine-valent HPV (9vHPV) vaccine targeting the types in the qHPV vaccine (HPV6/11/16/18), as well as 5 of the next most frequent HPV types found in cervical cancers worldwide (HPV31/33/45/52/58) is under development. To support the 9vHPV vaccine program, a nine-multiplexed cLIA (HPV-9 cLIA) was developed. Antibody titers were determined in a competitive format, where type-specific phycoerythrin (PE)-labeled, neutralizing mAbs (mAbs-PE) compete with an individual’s serum antibodies for binding to conformationally sensitive, neutralizing epitopes on the VLPs. Neutralizing antibody levels were quantitated against a reference standard - a pool of sera from 6 Rhesus macaques that were immunized with the 9vHPV vaccine. Specificity of the mAbs was assessed by measuring their individual binding capacities to the type-specific and non-type-specific VLPs at alternative concentrations of the mAbs. Antibody assignments to the HPV-9 cLIA reference standard for HPV6/11/16/18 were determined to provide for a measure of consistency in serostatus assignment between the HPV-4 and HPV-9 cLIAs. Antibody assignments to the HPV-9 reference standard for HPV31/33/45/52/58 were obtained by calibration to HPV11 using a direct binding IgG assay. For each HPV VLP type, the cross-reactivity of the mAb-PEs in the HPV-9 cLIA was <1% (i.e., the mAb-PEs result in <1% non-specific binding). The antibody concentrations assigned to the HPV-9 cLIA reference standard for types 6/11/16/18/31/33/45/52/58 were 3,817, 2,889, 23,061, 5,271, 3,942, 2,672, 1,489, 1274, and 2263 mMU/mL, respectively.
Keywords: :
The quadrivalent human papillomavirus (HPV) type 6/11/16/18 (abbreviated as qHPV) vaccine (Gardasil®) has been marketed since 2006 and is widely used and recommended for the prevention of HPV6, 11, 16, and 18- related disease. An investigational nine-valent HPV (abbreviated as 9vHPV) vaccine targeting HPV6/11/16/18, as well as 5 of the next most frequent HPV types found in cervical cancers worldwide (HPV31/33/45/52/58)Citation1 is currently under development (Merck, V503, NCT00543543). The qHPV and 9vHPV vaccines are composed of virus-like-particles (VLPs), which are made by expressing the L1 major capsid protein of the respective-HPV-types in eukaryotic cells.
Vaccination with L1 VLPs induces a broad polyclonal antibody response directed against conformational and linear epitopes displayed on the VLP surface.Citation2-Citation4 In the clinical trials of the qHPV vaccine, antibodies to the L1 VLPs were measured by an HPV-4 competitive Luminex immunoassay (cLIA).Citation5,Citation6 This type-specific multiplexed assay measures the competition for antibody binding to a single neutralizing epitope for each VLP. Subsequently, a multiplexed HPV-9 cLIA was developed for use in the clinical development program of the investigational 9vHPV vaccine. Here we describe the specificity of the 9 type-specific monoclonal antibodies (mAbs) used in the HPV-9 cLIA, as well as the assignment of antibody values for the HPV-9 cLIA reference standard.
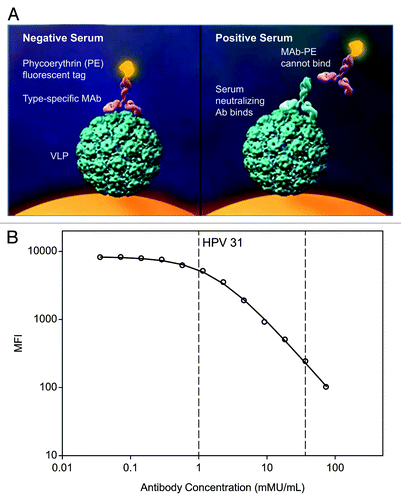
The HPV-9 cLIA is a competitive immunoassay that measures HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 specific antibodies to neutralizing epitopes on VLPs from a single serum sample. The assay is an extension of the previously developed and validated HPV-4 cLIA which was used in the clinical development of the qHPV vaccine (Gardasil).Citation5,Citation6 The HPV-9 cLIA uses yeast-derived VLPs that have been coupled to a set of 9 distinct fluorescent Luminex microspheres. The type-specific HPV-VLP antibody responses are associated with specific Luminex microspheres that are identified by their distinct red and infrared fluorescent dye spectral properties on the Luminex100 (or equivalent). Antibody titers are determined in a competitive format, where known, type-specific phycoerythrin (PE)-labeled, neutralizing monoclonal antibodies (mAbs-PE) compete with an individual’s serum antibodies for binding to conformationally sensitive, neutralizing epitopes on the VLPs (). The fluorescent signals from bound HPV-type-specific mAbs-PE are inversely proportional to the subject’s neutralizing antibody titers. For each genotype, relative inhibition of mAb-PE binding is compared with a reference standard using a four-parameter logistic curve fit. A representative reference standard curve for HPV type 31 illustrating the competitive format of the HPV-9 cLIA is shown in . Similar curves are obtained for each of the other 8 HPV types. The reference standard used in the HPV-4 cLIA was created using individual African Green Monkeys immunized with HPV type 6, 11, 16, or 18 VLPs on day 0 and weeks 8 and 24. The reference standard used in the HPV-9 cLIA consists of a pool of sera from 6 Rhesus macaques that were immunized on day 0, week 8, and week 24 with the investigational 9vHPV vaccine. The sera used in both reference standards were collected 1 month after the third dose of the vaccine. The HPV type-specific antibody levels of the reference standards are expressed as milliMerck Units per milliliter (mMU/mL).
Figure 1. (A) HPV-9 cLIA measures antibody concentration in a competitive format. Antibodies in test sera compete with type-specific mAbs for binding to neutralizing epitopes on each type-specific VLP coupled to Luminex microspheres. (B) Representative reference standard curve for HPV type 31 illustrating the competitive format of the HPV-9 cLIA. The circles denote the observed MFIs; the solid line shows the fitted curve; and the vertical dashed lines indicate the HPV type 31 lower and upper limits of quantitation. Similar curves are obtained for the other 8 HPV types.
HPV types are organized into genera and species on the basis of homology in the sequence of the L1 (major capsid protein) gene.Citation7 HPV type 16 is the prototype of the A9 species, which includes 6 cancer-causing HPV types (16/31/33/35/52/58). HPV type 18 is the prototype of the A7 species, which includes 5 cancer-causing HPV types (18/39/45/59/66). There can be a high degree of L1 sequence homology among members of the same family. For example, HPV types 18 and 45 share 88% amino acid homology. Therefore, the particular neutralizing epitope and relevant mAb used for HPV type 18 in the HPV-4 cLIA (18.J4), was selected for its analytical specificity, that is, its ability to distinguish HPV type 18 antibody responses from responses to other HPV types that are phylogenetically related to HPV type 18, such as HPV type 45. The HPV-9 cLIA uses the same 4 mAbs as that used in the HPV-4 cLIA for HPV types 6, 11, 16 and 18: H6.M48, K11.B2, H16.V5, and H18.J4.Citation3,Citation8,Citation9 As few mAbs exist for the rarer oncogenic types, Merck generated panels of mAbs to the HPV L1 VLPs of HPV types 31, 33, 45, 52, and 58. The generation and characterization of the panels of mAbs to these HPV types, as well as the selection of the pairs of antibodies that were of high affinity, type-specific, and recognized conformation-dependent neutralizing epitopes, has been previously described.Citation10
To determine the specificity of the mAbs used in the HPV-9 cLIA, each of the mAb-PEs was tested at 3 different concentrations: 10 μg/mL, 1.0 μg/mL, and 0.1 μg/mL, against the set of VLP coupled microspheres for all 9 HPV types. The mAb-PE concentrations employed for clinical testing in the HPV-9 cLIA ranged between 0.05 and 0.3 μg/mL. In this experiment, the binding capacity of the mAb-PEs to the alternative VLP-coupled microspheres was assessed in terms of the fluorescent signals from the bound mAb-PEs. For the assays to be considered non-cross-reactive (i.e., specific), each of the type-specific samples must exhibit a high Median Fluorescence Intensity (MFI) response against its corresponding VLP type, and a low response when tested against each of the other 8 VLP types.
The resulting MFIs at each combination of mAb-PE and VLP are provided in . For each HPV VLP type, the MFI response at 10 μg/mL of a non-type-specific mAb was >10-fold less than the MFI response at 0.1 μg/mL of the type-specific mAb. Therefore, cross-reactivity of the mAb-PEs in the HPV-9 cLIA can conservatively be reported as <1% (i.e., the mAb-PEs result in <1% non-specific binding). The highest degree of observed cross-reactivity was between H31.5D10.E4 and the HPV type 58 VLP, HPV types which share 77% homology of the L1 gene (). When tested against the HPV type 58 VLP, 0.1 μg/mL of H31.5D10.E4 resulted in a MFI of 50. Interestingly, the converse result was not evidenced as the degree of cross-reactivity between H58.6E11.F4 and the type 31 VLP was far less. When tested against the HPV type 33 VLP, 0.1 μg/mL of H58.6E11.F4 resulted in a MFI of 3.
Table 1. Median fluorescence intensities by HPV VLP and mAb-PE type
To assign antibody concentrations to the HPV-9 cLIA reference standard for the original qHPV vaccine types (i.e., 6, 11, 16, and 18), 320 samples from quadrivalent vaccine and non-vaccine recipients were tested in both the original HPV-4 cLIA and the new HPV-9 cLIA. For this stage of testing, nominal assignments of antibody concentrations were applied to the HPV-9 cLIA reference standard, and antibody levels to the qHPV types were determined for each sample in both the HPV-4 and HPV-9 cLIAs. For each serotype, the resulting HPV-4 cLIA and HPV-9 cLIA test sample results were compared using errors-in-variables regression on the natural log transformed responses, and the linear statistical relationship between the 2 sets of results was estimated.Citation11 Based on the estimated linear statistical relationship, antibody concentrations to the HPV-9 cLIA reference standard were then assigned such that the 2 assays resulted in perfect agreement at the HPV-4 cLIA serostatus cutoffs of 20, 16, 20, and 24 mMU/mL for HPV types 6, 11, 16, and 18, respectively.
The antibody assignments to the HPV-9 cLIA reference standard that yielded comparable titers at the serostatus cutoffs between the HPV-9 cLIA and HPV-4 cLIA were 3,817 mMU/mL, 2889 mMU/mL, 23 061 mMU/mL, and 5271 mMU/mL for types 6, 11, 16, and 18, respectively. Test sample antibody concentrations were re-determined using these assignments, and the resulting HPV-9 cLIA concentrations were plotted against their corresponding measures obtained in the HPV-4 cLIA (). The line of perfect concordance (i.e., the 45° line), as well as the line depicting the fitted linear relationship obtained through the errors-in-variables regression, are also shown in . Test sample serostatus assignments between the 2 assays are cross-tabulated in . For each of the 4 HPV types, the agreement rate in serostatus assignment between the 2 assays exceeded 94% (range = 94% to 99%) and there was no statistical evidence of imbalance in the discordant assignments between assays.
Figure 2. Comparison of HPV-4 cLIA and HPV-9 cLIA antibody titers to assign antibody values to the HPV-9 reference standard for HPV6, 11, 16, and 18.
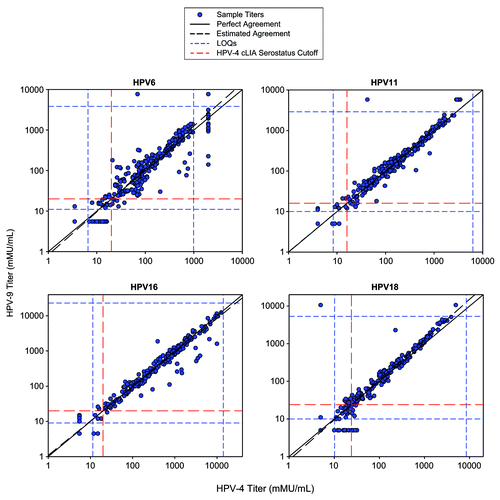
Figure 3. Cross-tabulation of test sample serostatus assignment between the HPV-4 and HPV-9 cLIAs and percent difference estimates for specific titer levels within the quantifiable range of the HPV-4 cLIA. The purpose of the evaluation was to determine the most appropriate assignment of antibody values for the HPV-9 cLIA reference standard. *Serostatus cutoff for the HPV-4 cLIA.
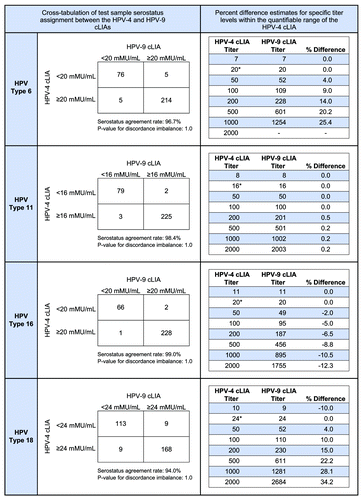
Though the concentrations were assigned to ensure comparability at the serotstatus cutoffs, the 2 assays yielded fairly comparable titers throughout the response range, with the magnitude of the difference directly related to the distance from the cutoff. For the test sample set included in this evaluation, the HPV-9 cLIA titers were, on average, 9.8% higher (90% CI: 2.1, 18.0), 0.3% higher (90% Cl: −3.1, 3.7), 8.4% lower (90% CI: −13.1, −3.3), and 12.0% higher (90% CI: 7.4, 16.7%) than the HPV-4 cLIA titers for types 6, 11, 16, and 18, respectively.
Antibody assignments to the HPV-9 cLIA reference standard for types 31, 33, 45, 52, and 58 were obtained by calibration to HPV type 11 in the HPV-9 cLIA reference standard using an antibody assignment of 2889 mMU/L. Since a true neutralization assay for HPV does not exist, the ‘gold standard’ for measuring neutralizing antibody titers is the athymic mouse xenograft system.Citation12 HPV type 11 was chosen as the calibrator for the additional HPV types as it is the HPV type that has been shown to have a positive correlation between the level of HPV 11 L1 VLP-specific IgG in animals immunized with HPV 11 virions and neutralization of HPV 11 in the athymic mouse xenograft model.Citation13 The calibration was performed using a direct-binding 9-plex VLP-specific IgG assay following the methodology of Concepcion and Frasch.Citation14 The HPV-9 cLIA reference standard was tested in 30 runs performed by 2 analysts across 6 d. Within each run, the HPV-9 cLIA reference standard was tested in a 2-fold dilution series consisting of 24 dilutions and starting at a dilution of 1:2, with each dilution tested in duplicate. Within each run, the antibody concentration for each the 5 new types was individually determined relative to HPV type 11 using the parameter estimates from a fitted parallel-line four-parameter logistic regression model. In each case, the legitimacy of fitting a common slope model was assessed and determined valid. The antibody concentration estimates were highly consistent across the 30 runs as the geometric coefficient of variation was under 10.0% for each serotype. The antibody concentrations assigned to the HPV-9 cLIA reference standard were the averages of the 30 estimates, being 3942, 2,672, 1489, 1274, and 2263 mMU/mL for types 31, 33, 45, 52, and 58, respectively.
In summary, a HPV-9 cLIA was developed for use in large Phase 3 studies of an investigational 9vHPV vaccine. Because an international 9-valent reference standard was not available, a 9-valent monkey reference standard (232005–107 [07FM]) was created for use in vaccine immunogenicity studies. Antibody assignments to the HPV-9 cLIA reference standard for HPV types 6, 11, 16, and 18 were determined to provide for a measure of consistency in serostatus assignment between the HPV-4 and the HPV-9 cLIAs. To determine antibody assignments for HPV types 31, 33, 45, 52, and 58, experiments were performed to calibrate the new types in the HPV-9 cLIA reference standard to HPV type 11 in the HPV-9 cLIA reference standard using the HPV type 11 antibody assignment.
We also evaluated the specificity of the monoclonal antibodies for HPV VLP types 6, 11, 16, 18, 31, 33, 45, 52, and 58 for use in the HPV-9 cLIA. The non-specific binding of the mAb-PEs in the HPV-9 cLIA was less than 1.0%. The highest degree of cross-reactivity observed was between H31.5D10.E4 and the HPV type 58 VLP which share 77% homology. Noting that the concentration of the HPV type 31 mAb-PE used for clinical testing is 0.05 μg/mL, and that in these experiments the 0.1 μg/mL concentration of the HPV type 31 mAb-PE resulted in an HPV type 58 response of only 50 MFI, the degree of cross-reactivity exhibited between the HPV type 31 mAb-PE and the HPV type 58 VLP is not of consequence when considered in the context of clinical testing. This confirms that the relevant mAbs selected for the HPV-9 cLIA have high analytical specificity, that is, they are able to distinguish among the HPV types that are contained in the 9vHPV vaccine.
Vaccination against HPV offers the opportunity to effectively prevent infection and disease caused by HPV. The 9vHPV vaccine has recently been reported to be safe and highly efficacious against the original 4 HPV types in the qHPV as well as the additional 5 types.Citation15 If vaccination programs are effectively implemented, up to 90% of invasive cervical cancer cases worldwide could be prevented, in addition to the majority of pre-cancerous lesions.Citation16
| Abbreviations: | ||
| cLIA | = | competitive Luminex immunoassay |
| GMT | = | geometric mean titer |
| HPV | = | human papillomavirus |
| mMU | = | milliMerck Units |
| HPV | = | Human Papillomavirus |
| MFI | = | median fluorescence intensity |
| 9vHPV | = | nine-valent human papillomavirus |
| PE | = | phycoerythrin |
| mAb | = | monoclonal antibody |
| qHPV | = | quadrivalent human papillomavirus |
| VLP | = | virus-like particles |
Disclosure of Potential Conflicts of interest
All authors are current (C.R., J.A., A.L., S.V., A.S.) or former (M.B., T.G., E.H., K.M., R.H.) employees of Merck and hold stock/stock options.
Acknowledgments
This study was funded by Merck. The authors thank Heather L Sings and Karyn Davis (Merck) for assistance in the preparation of this manuscript.
References
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Vol 100, A Review of Human Carcinogens. Part B: Biological Agents. Lyon, France: International Agency for Research on Cancer; 2011. International Agency for Research on Cancer. 2013.
- Orozco JJ, Carter JJ, Koutsky LA, Galloway DA. Humoral immune response recognizes a complex set of epitopes on human papillomavirus type 6 l1 capsomers. J Virol 2005; 79:9503 - 14; http://dx.doi.org/10.1128/JVI.79.15.9503-9514.2005; PMID: 16014913
- Christensen ND, Dillner J, Eklund C, Carter JJ, Wipf GC, Reed CA, Cladel NM, Galloway DA. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 1996; 223:174 - 84; http://dx.doi.org/10.1006/viro.1996.0466; PMID: 8806551
- Carter JJ, Wipf GC, Madeleine MM, Schwartz SM, Koutsky LA, Galloway DA. Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J Virol 2006; 80:4664 - 72; http://dx.doi.org/10.1128/JVI.80.10.4664-4672.2006; PMID: 16641259
- Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12:959 - 69; PMID: 16085914
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol 2003; 10:108 - 15; PMID: 12522048
- de Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324:17 - 27; http://dx.doi.org/10.1016/j.virol.2004.03.033; PMID: 15183049
- Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz GS. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 1996; 224:477 - 86; http://dx.doi.org/10.1006/viro.1996.0554; PMID: 8874508
- Christensen ND, Kreider JW, Cladel NM, Patrick SD, Welsh PA. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol 1990; 64:5678 - 81; PMID: 2170694
- Brown MJ, Seitx H, Towne V, Muller M, Finnefrock AC. Development of neutralizing monoclonal antibodies for oncogenic HPV types 31, 33, 45, 52, and 58. Clin Vaccine Immunol 2014; 21:587 - 93; http://dx.doi.org/10.1128/CVI.00773-13; PMID: 24574536
- Tan CY, Iglewicz B. Measurement-methods comparisions and linear statistical relationship. Technometrics 1999; 41:192 - 201; http://dx.doi.org/10.1080/00401706.1999.10485668
- Kreider JW, Howett MK, Wolfe SA, Bartlett GL, Zaino RJ, Sedlacek T, Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature 1985; 317:639 - 41; http://dx.doi.org/10.1038/317639a0; PMID: 2997616
- Bryan JT, Jansen KU, Lowe RS, Fife KH, McClowry T, Glass D, Brown DR. Human papillomavirus type 11 neutralization in the athymic mouse xenograft system: correlation with virus-like particle IgG concentration. J Med Virol 1997; 53:185 - 8; http://dx.doi.org/10.1002/(SICI)1096-9071(199711)53:3<185::AID-JMV1>3.0.CO;2-4; PMID: 9365880
- Concepcion N, Frasch CE. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin Diagn Lab Immunol 1998; 5:199 - 204; PMID: 9521143
- Joura E. Efficacy and Immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16- to 26-year-old women. EUROGIN 2013, November 3-6.Available at http://www.eurogin.com/2013/index.php/abstracts/eurogin-2013-abstracts. 2014.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, Bosch FX, de Sanjosé S. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer 2012; 7:38; http://dx.doi.org/10.1186/1750-9378-7-38; PMID: 23273245
