Abstract
HPV-023 (NCT00518336; ClinicalTrial.gov) is a long-term follow-up of an initial double-blind, randomized (1:1), placebo-controlled study (HPV-001, NCT00689741) evaluating the efficacy against human papillomavirus (HPV)-16/18 infection and associated cyto-histopathological abnormalities, persistence of immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine. Among the women, aged 15–25 years, enrolled in HPV-001 and who participated in the follow-up study HPV-007 (NCT00120848), a subset of 437 women from five Brazilian centers participated in this 36-month long-term follow-up (HPV-023) for a total of 113 months (9.4 years). During HPV-023, anti-HPV-16/18 antibodies were measured annually by enzyme-linked immunosorbent assay (ELISA) and pseudovirion-based neutralisation assay (PBNA). Cervical samples were tested for HPV DNA every 6 months, and cyto-pathological examinations were performed annually. During HPV-023, no new HPV-16/18-associated infections and cyto-histopathological abnormalities occurred in the vaccine group. Vaccine efficacy (VE) against HPV-16/18 incident infection was 100% (95%CI: 66.1, 100). Over the 113 months (9.4 years), VE was 95.6% (86.2, 99.1; 3/50 cases in vaccine and placebo groups, respectively) against incident infection, 100% (84·1, 100; 0/21) against 6-month persistent infection (PI); 100% (61·4, 100; 0/10) against 12-month PI; 97·1% (82.5, 99.9; 1/30) against ≥ ASC-US; 95·0% (68.0, 99.9; 1/18) against ≥ LSIL; 100% (45.2, 100; 0/8) against CIN1+; and 100% (–128.1, 100; 0/3) against CIN2+ associated with HPV-16/18. All vaccinees remained seropositive to HPV-16/18, with antibody titers remaining several folds above natural infection levels, as measured by ELISA and PBNA. There were no safety concerns. To date, these data represent the longest follow-up reported for a licensed HPV vaccine.
Introduction
Persistent infection with human papillomavirus (HPV) has been clearly established as the necessary cause of cervical cancer.Citation1,Citation2 To date, at least 40 different HPV types are known to infect the genital mucosa, of which approximately 15 are associated with cervical cancer.Citation3-Citation6 Among these types, HPV-16 and HPV-18 are the most common and responsible for approximately 70% of cervical cancers.Citation5 HPV vaccines are now widely available and vaccination programs are being implemented. These vaccines must offer long-term protection as women remain at risk of HPV infection for as long as they are sexually active.Citation7 Evidence from clinical trials of the long-term efficacy against vaccine HPV types is of most importance, particularly with respect to maintaining confidence in mass vaccination programs. Extended follow-up studies of participants in these trials have provided evidence of sustained immune responses through to at least 100 mo (8.3 y) post initial vaccination.Citation8,Citation9
GlaxoSmithKline Vaccines (registered as GlaxoSmithKline Biologicals SA) has developed a prophylactic HPV vaccine based on L1 proteins of HPV-16 and HPV-18 formulated with AS04 (an adjuvant system comprised of 3-O-desacyl-4’-monophosphoryl lipid A [MPL, 50µg] absorbed on aluminum hydroxide [Al(OH)3, 500µg]): the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®*; GlaxoSmithKline) referred to in this paper as the “HPV-16/18 vaccine.” The HPV-16/18 vaccine has been designed to provide long-term protection against HPV-16/18 cervical infection through generation of a sustained immune response.Citation10-Citation12
An extensive clinical trial program has shown that the HPV-16/18 vaccine is highly immunogenic,Citation13-Citation15 efficacious against HPV-16/18 infection and associated cyto-histopathological abnormalities,Citation16–Citation18 and generally well tolerated.Citation19,Citation20 Results of a large multinational phase III efficacy study (HPV-008; NCT00122681) in women aged 15–25 y demonstrated high and sustained protection against cervical intraepithelial neoplasia (CIN) grade 2 and above (CIN2+), CIN3+, and persistent infection (6- and 12-mo definitions) associated with HPV-16/18.Citation17,Citation18 Significant vaccine efficacy (VE) against CIN2+ and CIN3+ irrespective of HPV DNA association in the lesion was shown, with a 93·2% reduction in CIN3+ independent of the HPV type in naïve women.Citation18 In this same study, cross-protection against non-vaccine oncogenic types (including HPV-31, -33, -45, and -51) elicited by the HPV-16/18 vaccine was also demonstrated.Citation6
An initial double-blind, randomized, multi-center vaccination study (HPV-001; NCT00689741) was started in 2001, followed for up to 27 mo, and then followed by a long-term follow-up study of the entire cohort up to 77 mo (6.4 y) post initial vaccination (HPV-007; NCT00120848). Results of the initial and follow-up studies were reported previously.Citation21-Citation23
HPV-007 was followed by an additional extension study (HPV-023; NCT00518336) in which participants from Brazil were invited to continue follow-up. HPV-023 was designed to evaluate the long-term VE, persistence of immunogenicity and safety of the HPV-16/18 vaccine. This study provides long-term data that were not available at time of licensure, representing up to 113 mo (9.4 y) of follow-up post initial vaccination.
The primary objective of HPV-023 was to evaluate long-term VE against incident cervical infection with HPV-16 and/or HPV-18 in young women who were previously uninfected with HPV-16 or HPV-18. Secondary objectives were to evaluate long-term VE against persistent infection (6- and 12-mo definitions), and cyto-histopathological abnormalities associated with HPV-16 and/or HPV-18; VE against incident and persistent infection, and cyto-histopathological abnormalities associated with non-vaccine oncogenic types. Other objectives included the evaluation of long-term vaccine immunogenicity and safety. Further and specific to this paper, mathematical modeling was developed in order to predict the long-term vaccine-induced HPV-16 and HPV-18 antibody titers up to 20 y post-vaccination.
*Cervarix® is a registered trademark of the GlaxoSmithKline group of companies.
Results
Of the 1113 women enrolled in HPV-001 (including 506 in Brazil), 776 continued into HPV-007 (448 in Brazil). Of the women from the Brazilian centers who were invited to participate in HPV-023, 437 agreed to continue, and 431 (98·6%) completed the study. A total of 399 women were included in the according-to-protocol (ATP) efficacy cohort and 304 in the ATP immunogenicity cohort. In summary, 85·2% of Brazilian women enrolled in HPV-001 completed HPV-023 ().
Figure 1. Flow of participants HPV-001: NCT00689741; HPV-007: NCT00120848; HPV-023: NCT00518336. ATP = according-to-protocol. The ATP cohort for primary analysis of efficacy included all women for whom differential treatment effect on efficacy was likely (i.e., those meeting all eligibility criteria in HPV-001, HPV-007 and HPV-023), complying with the procedures defined in the respective study protocol, and for whom data concerning efficacy endpoint measures were available. The ATP immunogenicity cohort included all evaluable women (i.e., those meeting all eligibility criteria, complying with the procedures defined in the protocol, and fulfilling requirements for analysis) for whom data concerning immunogenicity were available. M0 = Month 0 = time of randomization; M18 = Month 18 (18 mo after the first dose of vaccine); M33 = Month 33 (33 mo after the first dose); M76 = Month 76 (76 mo after the first dose); M77 = Month 77 (77 mo after the first dose); M113 = Month 113 (113 mo after the first dose).
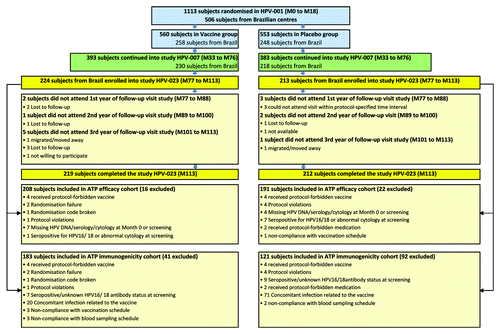
Demographic characteristics were similar between the ATP cohorts and the total vaccinated cohort (TVC), between both study groups in HPV-023, and between the Brazilian women enrolled in HPV-001 compared with those Brazilian women enrolled in HPV-023 (Table S1).Citation11 Mean age at HPV-023 study entry was 26.5 y (standard deviation [SD]: 3.1), and mean age at entry into HPV-001 was 19.9 y (3·0) for the Brazilian women entering HPV-023. The study population of HPV-023 was racially diverse with 57.7% being Caucasian. The mean follow-up time since first vaccination in HPV-001 was 107 mo (8·9 y [SD: 0.4]), with a maximum duration of 113 mo (9·4 y).
Efficacy against incident and persistent infection
Primary endpoint
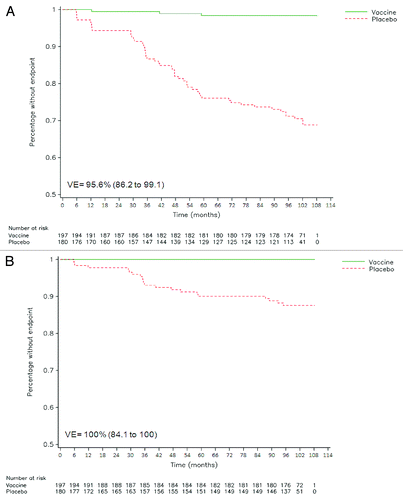
During the entire 36-mo period of HPV-023, no incident HPV-16/18 infection occurred in the vaccine group whereas nine cases occurred in the placebo group, resulting in 100% VE (95% CI: 66.1 to 100). Sustained VE against HPV-16/18 incident infection was also observed in the combined analysis (; ; Fig. S1).
Table 1. Vaccine efficacy against infection (incident and persistent) and cyto-histopathological abnormalities associated with HPV-16/18
Figure 2. Reverse cumulative distribution curves for HPV-16/18 incident infection (A) and HPV-16/18 6-mo persistent infection (B) in cervical samples (ATP efficacy cohort). Combined analysis of initial and follow-up studies (HPV-001/007/023). Vaccine = HPV-16/18 vaccine group. Placebo = placebo group. VE = vaccine efficacy, with 95% confidence interval.
Secondary endpoints
There were no cases of either 6- or 12-mo HPV-16/18 persistent infection in the vaccine group vs. four cases and one case, respectively, in the placebo group during the 36-mo follow-up. In the combined analysis, sustained VE was observed for both 6-mo (; Fig. S1) and 12-mo definitions of persistent infection with HPV-16/18 ().
VE against incident or persistent infection (6- and 12-mo definitions) associated with ‘any oncogenic’ HPV type could not be demonstrated during the 36-mo follow-up of HPV-023 or over the 113 mo of follow-up ().
Table 2. Vaccine efficacy against infection (incident and persistent) and cyto-histopathological abnormalities associated with any oncogenic HPV type§
In the combined analysis, VE was observed only for HPV-45 incident infection with six cases in the vaccine group and 18 cases in the placebo group (70·8% [23.2 to 90.5]). VE against HPV-31, HPV-33, and HPV-51 incident infection did not reach statistical significance, calculated at 40·4% (–27.2 to 72.9), 34.8% (–51.9 to 72.9), and 6.7% (–41.9 to 38.6), respectively.
Results from the TVC were consistent with the results obtained from the ATP cohort.
Efficacy against cyto-histopathological abnormalities
VE against atypical squamous cells of undetermined significance [ASC-US] or worse (≥ASC-US), ≥ low-grade squamous intraepithelial lesion (LSIL), CIN1+, or CIN2+ associated with HPV-16/18 could not be demonstrated in HPV-023. In the combined analysis, high and sustained VE was observed for ≥ASC-US, ≥LSIL, and CIN1+ associated with HPV-16/18 ().
VE against ≥ASC-US, ≥LSIL, CIN1+, or CIN2+ associated with “any oncogenic” HPV type was not demonstrated in HPV-023. In the combined analysis, VE was observed for ≥ASC-US, ≥LSIL, and CIN1+ associated with ‘any oncogenic’ HPV type ().
Sustained VE (combined analysis) was observed for ≥ASC-US associated with HPV-52 (45.4% [2.0 to 70.3]), and ≥LSIL associated with HPV-66 (71.9% [9.1 to 93.3]).
An exploratory analysis performed on cytopathological endpoints irrespective of HPV DNA association showed (combined analysis) an overall benefit of vaccination against ≥ASC-US (26.8% [0.3 to 46.5]) and ≥LSIL (32.8% [0.7 to 54.8]). There was no such benefit demonstrated for the histopathological endpoints.
Immunogenicity
All women in the vaccine group remained seropositive to both HPV-16 and HPV-18 up to 113 mo post-vaccination. High and sustained levels of IgG antibodies were observed, reaching a plateau approximately 18 mo after the first vaccine dose and remaining stable thereafter (). Compared with levels following natural infection, IgG levels in the vaccine group were 10.8-fold and 10.0-fold higher for HPV-16 and HPV-18, respectively.
Figure 3. Seropositivity rates and geometric mean titers for anti-HPV-16 (A) and anti-HPV-18 (B) antibodies, measured by ELISA (ATP immunogenicity cohort). ATP immunogenicity cohort = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). ELISA = enzyme-linked immunosorbent assay; HPV = HPV-16/18 vaccine group; Placebo = placebo group; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. EL.U/mL = ELISA units/mL. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008, NCT00122681) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29·8 EL.U/mL (95% CI: [28·5 to 31·0]) for HPV-16 and 22·6 EL.U/mL (95% CI: [21·6 to 23·6]) for HPV-18; measured by ELISA.Citation16
![Figure 3. Seropositivity rates and geometric mean titers for anti-HPV-16 (A) and anti-HPV-18 (B) antibodies, measured by ELISA (ATP immunogenicity cohort). ATP immunogenicity cohort = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). ELISA = enzyme-linked immunosorbent assay; HPV = HPV-16/18 vaccine group; Placebo = placebo group; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. EL.U/mL = ELISA units/mL. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008, NCT00122681) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29·8 EL.U/mL (95% CI: [28·5 to 31·0]) for HPV-16 and 22·6 EL.U/mL (95% CI: [21·6 to 23·6]) for HPV-18; measured by ELISA.Citation16](/cms/asset/68c22677-6605-4d91-a5ff-9efb44f81c82/khvi_a_10929532_f0003.gif)
In a subset of women from the vaccine group (n = 55), 100% were positive for neutralising antibodies against both HPV-16 and HPV-18, up to 113 mo post-vaccination, with the neutralising antibody kinetic patterns similar to those of the total IgG antibodies. Neutralising antibodies in the vaccine group were 7.7-fold and 4.0-fold higher for HPV-16 and HPV-18, respectively, compared with levels elicited by natural infection ().
Figure 4. Seropositivity rates and geometric mean titers for (A) anti-HPV-16 and (B) anti-HPV-18 antibodies, measured by PBNA (ATP immunogenicity cohort). ATP cohort for immunogenicity = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for a subset of the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). PBNA = Pseudovirion-Based Neutralisation Assay; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010, NCT00423046) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180·1 ED50 (95% CI: [153·3 to 211·4]) for HPV-16 and 137·3 ED50 (95% CI: [112·2 to 168·0]) for HPV-18; measured by PBNA).Citation24
![Figure 4. Seropositivity rates and geometric mean titers for (A) anti-HPV-16 and (B) anti-HPV-18 antibodies, measured by PBNA (ATP immunogenicity cohort). ATP cohort for immunogenicity = women who met all eligibility criteria (all had received 3 doses of vaccine or placebo), complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample. Data are shown for a subset of the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies. Histogram bars show the GMT and corresponding 95% Confidence intervals (CI). PBNA = Pseudovirion-Based Neutralisation Assay; PRE = pre-vaccination; PII = post dose II; PIII = post dose III; M = Month. Figures above the bars are the seropositivity rates for the corresponding timepoint. Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010, NCT00423046) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180·1 ED50 (95% CI: [153·3 to 211·4]) for HPV-16 and 137·3 ED50 (95% CI: [112·2 to 168·0]) for HPV-18; measured by PBNA).Citation24](/cms/asset/7d3e1bc5-993a-4aae-927e-d28d720271e1/khvi_a_10929532_f0004.gif)
Predicted long-term persistence of antibody responses
Using the data of up to 113 mo of follow-up, both the piece-wise and modified power-law models predicted geometric mean titers (GMTs) remain well above natural infection levels when the data are extrapolated to the 20-y time-point ( and ; ). The piece-wise model, which takes into account a decrease of antibody levels over time, predicts antibody GMTs to remain above natural infection levels for the duration of 32.3 y and 20.5 y for anti-HPV-16 and anti-HPV-18 antibodies, respectively (). Unlike with the modified power-law model, the GMTs predicted by the piece-wise model tend to increase with an increasing duration of follow-up and increased amount of measured data narrowing the gap between both piece-wise and modified power-law modeling predictions.
Figure 5. Anti-HPV-16 antibody responses predicted by the modified power-law (A), piece-wise model (B), and their comparison (C), up to 20 y. GMT = geometric mean titer; EL.U/mL = ELISA units/mL; Natural infection = mean antibody titers associated with natural infection were obtained from women enrolled in a Phase III efficacy study (HPV-008, NCT00122681).Citation16
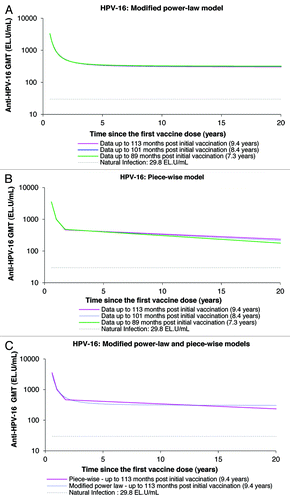
Figure 6. Anti-HPV-18 antibody responses predicted by the modified power-law (A), piece-wise model (B), and their comparison (C), up to 20 y. GMT = geometric mean titer; EL.U/mL = ELISA units/mL; Natural infection = mean antibody titers associated with natural infection were obtained from women enrolled in a Phase III efficacy study (HPV-008, NCT00122681).Citation16
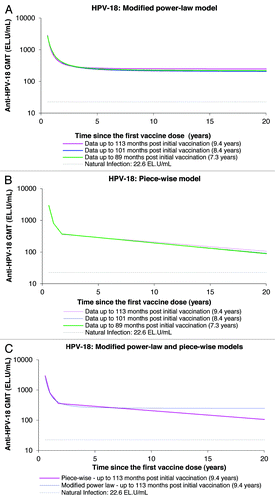
Table 3. Predicted anti-HPV-16 and -18 antibody responses by piece-wise and modified power-law models
Safety
The occurrences of medically significant adverse events (AEs) (listing those AEs with more than 1 case report in either the vaccine or placebo arm), serious adverse events (SAEs) (listing those SAEs with more than 1 case report in either the vaccine or placebo arm), new onset chronic diseases (NOCD), and new onset autoimmune diseases (NOAD) are described in . The most common medically significant AEs were reported in the vaccine group, with five (2.2%) cases each for gastritis, incomplete spontaneous abortion, depression, and hypertension, respectively, out of a total 224 women. The most common medically significant AE reported in the placebo group was genital herpes (three [1.4%] cases). None of the women in the vaccine group reported genital herpes. There were no withdrawals due to AEs, and no AE was considered to be possibly related to the study vaccine or placebo.
Table 4. Number and percentage of women reporting medically significant adverse events, serious adverse events, new onset chronic diseases (NOCD) and new onset autoimmune diseases (NOAD) between 77 mo and up to 113 mo post initial vaccination (36-mo follow-up; TVC)
Nine women reported at least one NOCD during HPV-023, six in the vaccine group (). Of the six events in the vaccine group, four were identified as a NOAD (hypothyroidism, rheumatoid arthritis, and vitiligo).
A total of 103 pregnancies were reported by 94 women during the 36-mo follow-up period of HPV-023 (). Eight women in the vaccine group reported a spontaneous abortion compared with just four women in the placebo group during HPV-023.
Table 5. Outcome of reported pregnancies reported during HPV-023 (36-mo follow-up, TVC)
Discussion
Principal findings
No breakthrough cases of infection or cyto-histopathological abnormalities associated with HPV-16/18 occurred in the vaccine group over the 36-mo period of HPV-023. This was associated with high and sustained levels of IgG (measured by enzyme-linked immunosorbent assay [ELISA]) and neutralising antibodies (measured by pseudovirion-based neutralisation assay [PBNA]) against HPV-16 and HPV-18, with vaccine-induced GMTs being well above titers associated with clearance of natural infection in other studies.Citation16,Citation24 Neutralising antibodies against vaccine types are likely to be a major basis of protection against HPV infection, and the correlation between the antibodies measured by ELISA and PBNA has been previously demonstrated.Citation25 Further, there was some indication in the results that the vaccine offered protection against other oncogenic types beyond HPV-16/18. However, few cases of incident or persistent infection or cyto-histopathological endpoints associated with HPV-31 and HPV-45, the two types most closely related to HPV-16 and HPV-18, respectively, and the most frequent types associated with cervical cancer after HPV-16 and HPV-18, were observed.Citation3 Finally, the safety profile was also clinically acceptable. All reported SAEs and pregnancy outcomes in this study were considered as unrelated to the vaccine.
These data add an additional 36 mo to those already collected from extended follow-up studies of the HPV-16/18 vaccine in a sub-set of participants assessed at 48 (4.5 y) and 77 (6.4 y) months post initial vaccination.Citation22,Citation23 This now provides a total of 113 mo (9.4 y) of follow-up evaluating the durability of the immune response elicited by the primary vaccination.
In addition, the predicted duration of anti-HPV-16 and HPV-18 antibody responses following vaccination were explored by mathematical modeling. Factors that can influence long-term immunity include the peak level of antibody response one month after the last vaccine dose, rates of B-cell decay and proliferation, B-cell immunologic memory, cell-mediated immunity, and individual variability.Citation26,Citation27 Based on data from the initial and follow-up studies, the results of the modeling predict that anti-HPV-16 and anti-HPV-18 antibody levels will decrease but will remain several folds higher than those associated with natural infection for at least 20 y post-vaccination. These results provide circumstantial evidence that, should a booster be needed, this need will not occur before a substantial amount of time has elapsed after vaccination, which is consistent with previous modeling results.Citation26 Note the study population in HPV-023 is different to that in DavidCitation26 with respect to size and geographic origin; as the modeling of the HPV-023 data was solely based on the Brazilian cohort. The limitations of mathematical evaluations have been discussed.Citation26 While the clinical relevance of long-term antibody persistence is being investigated, modeling of predicted GMTs is informative for clinicians and policy makers until these long-term observational data are available.Citation26
Strengths of study
To ensure consistency in the collection of efficacy, immunogenicity and safety data, the study design of HPV-023 closely followed the design of HPV-001 and HPV-007. Treatment allocation remained double-blinded throughout all studies. Further, we considered all endpoints, irrespective of causal HPV type. Histological diagnoses were determined by a panel of expert gynecological pathologists who were unaware of the women’s treatment or history of cervical disease. Women who reached an endpoint (virological, cyto-histopathological) for a specific HPV-type in a previous analysis of the preceding studies were censored from the analyses related to the same endpoint in HPV-023. As a result of the high VE, this occurred more often for women in the placebo group than in the vaccine group. Importantly, the combined analysis presented here was not affected by censoring, and therefore provides valuable information. Finally, this study represents the longest follow-up of a clinical trial evaluating the efficacy, immunogenicity and safety of a licensed HPV vaccine to date, gathering data over 113 mo post initial vaccination.
Comparison with other studies
These data are very much in line with previous follow-up studies of the HPV-16/18 vaccine, which indicate that the vaccine provides long-term immunogenicity and prevents HPV-16/18 infection and associated disease in vaccinated women.Citation8,Citation11,Citation28,Citation29 Another study has shown that the HPV-16/18 vaccine generates an anamnestic response (renewed rapid production of an antibody on a subsequent encounter with the same antigen), in women from a similar cohort who were seropositive, after a fourth dose of vaccine.Citation10
A larger sample size, than that recruited in this study, is needed for a full assessment of type-specific cross-protection, however other larger studies of the HPV-16/18 vaccine have shown that it offers substantial cross-protection against cervical infection and cyto-histopathological endpoints associated with various combinations of non-vaccine oncogenic types, including HPV-31, HPV-33, HPV-45, and HPV-51 individually.6,17,28
Regarding the assessment of safety, pooled safety analyses of HPV-16/18 vaccine have shown this vaccine to be generally well tolerated in women of all ages.Citation20,Citation30,Citation31
Limitations of study
This study presents results showing significant VE in the cumulative analysis across HPV-001/007/023 and confirms a high rate of VE against HPV-16/18 infection. It did not have power to show efficacy against more stringent outcomes (persistent infection, cytology and histology including CIN2+) during the immediate study (HPV-023) period due to the small number of events in the control arm. While the clear explanation for this is the small sample size, limited length of follow-up (36 mo) and with this, a limited number of events, this could also relate to the aging of the study population given that HPV infection is of higher incidence right after sexual debut and declines thereafter.
Therefore we undertook a combined analysis, as it provided a global overview of the vaccine protection up to 113 mo post-vaccination with high VE for all HPV-16/18 virological and most HPV-16/18 associated cyto-histopathological endpoints. Even for this combined analysis, the limited power also hampered any conclusions to be drawn on the long-term durability of protection elicited by the vaccine against non-vaccine types. As another limitation, no randomization occurred at the start of the follow-up study HPV-023. This might have an influence on the HPV-023 study analysis, as opposed to the combined analysis over the entire follow-up period up to 113 mo post-vaccination.
The safety data may suggest a non-significant signal toward more AEs occurring in the vaccine group, although there is no clinical pattern of increased incidence of (medically significant) AEs due to the vaccine since most reported events were only single events. However, again, as the power is low, these results may also be consistent with chance. A pooled analysis of safety data, including 57 580 subjects and 96 704 doses of HPV-16/18 vaccine, collected from 42 completed and on-going clinical studies, showed that the incidences and distribution of AEs were similar across the HPV-16/18 vaccinees and controls, with no new safety signals being identified.Citation30
Further, HPV-023 was not powered to assess pregnancy outcomes. However we present a descriptive table that summarizes the findings from the 36-mo follow-up, in women vaccinated nine years earlier. The results do call to attention the occurrence of twice as many spontaneous abortions in the HPV-16/18 vaccine arm compared with the control arm. This again could be a result of chance. A large pooled analysis of miscarriage outcomes in 3599 pregnant women vaccinated with HPV-16/18 vaccine or placebo from two multicenter phase III randomized trials, conducted by the National Cancer Institute, concluded that there was no overall effect of the HPV-16/18 vaccine on the risk of miscarriage (estimated risk of miscarriage being 11.5% and 10.2% in the HPV-16/18 group and control group, respectively).Citation32 Pregnancy outcomes monitored in post-marketing settings in women who inadvertently received the HPV-16/18 vaccine during pregnancy are in line with published reports for similar populations.Citation31
Conclusion
No breakthrough cases of HPV-16/18 infection or related cervical lesions occurred in the vaccinated cohort over the 36-mo study period. The HPV-16/18 AS04-adjuvanted vaccine also continued to provide high and sustained levels of IgG and neutralising antibodies against HPV-16 and HPV-18, with antibody titers remaining several folds above natural infection levels, up to 113 mo post-vaccination. This study represents the longest follow-up in a clinical trial setting of a licensed vaccine containing the two most frequently observed oncogenic types, HPV-16 and HPV-18, confirming the previous estimations by mathematical models.Citation26 These results should provide confidence in the duration of protection offered by HPV mass vaccination programs existing in a number of countries around the world.
Patients and Methods
Study design and participants
In 2001, healthy women aged 15–25 y were recruited from North America (USA, Canada) and Brazil into an initial double-blind, randomized, multi-center vaccination study (HPV-001; NCT00689741).Citation21 Eligible study participants were HPV-16 and HPV-18 seronegative by enzyme-linked immunosorbent assay (ELISA), HPV DNA-negative in the cervix by polymerase chain reaction (PCR) for 14 oncogenic types (HPV-16,-18,-31,-33,-35,-39,-45,-51,-52,-56,-58,-59,-66,-68), and had normal cervical cytology at baseline. Women were randomized (1:1) to receive three doses of either the HPV-16/18 vaccine or placebo (Al[OH]3) at 0, 1, and 6 mo as previously described.Citation21,Citation22
Women who had received all three doses of vaccine or placebo and whose treatment allocation had remained blinded were invited to take part in HPV-007 (NCT00120848).Citation23
Due to the high retention rate of subjects in the HPV-007 Brazilian cohort, women from five hospital-based Brazilian centers who had received all three doses of HPV-16/18 vaccine or placebo (Al[OH]3) at 0, 1, and 6 mo as previously described,Citation21,Citation22 and whose treatment allocation had remained blinded in both HPV-001 and HPV-007, were invited to take part in this long-term follow-up study, HPV-023.Citation11 HPV-023 started in November 2007 and lasted for three years. Results of the initial and follow-up studies have been previously reported.Citation21-Citation23
Intervention
No vaccine or placebo was administered in HPV-023. To ensure consistency in the collection of efficacy and safety data, the study design of HPV-023 closely followed the design of HPV-001 and HPV-007. Detailed methodologies of the initial and follow-up studies, including interim analyses of HPV-023, have been reported previously·8,11,21,23 Treatment allocation remained double-blinded throughout all studies.
Similar to HPV-001 and HPV-007, women continued to receive gynecological care according to Brazilian standards in HPV-023.
Women who received the placebo in HPV-001 and who have remained blinded throughout the studies have been offered a crossover vaccination course (0, 1, and 6 mo) with the HPV-16/18 vaccine at the completion of HPV-023. At the time of HPV-023 conduct, HPV vaccination was not routinely offered to women in the Brazilian Health System.
The follow-up evaluations performed in HPV-023 were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice Guidelines. The protocol and other materials were approved by the Independent Ethics Committee or Institutional Review Board of each study center and the National Committee of Ethics and Research. Written informed consent was obtained from all women before any study procedure was performed.
Main outcomes measures
Virology and cyto-histopathology
Gynaecological examinations were performed, cervical swabs were collected every six months for HPV-DNA typing, and cytology specimens collected every 12 mo (or at 6-mo intervals, if driven by clinical management algorithm). Methods were described previously.Citation21,Citation22
A broad spectrum PCR-system (SPF10-DEIA-LiPA25) was used to test cervical samples and biopsy material for 25 HPV types including the well-defined 14 oncogenic types.Citation33 SPF10-DEIA-positive specimens were tested by line probe assay and type-specific HPV-16 and HPV-18 PCR-DEIA as previously described.Citation21,Citation22
VE was calculated against the following clinical endpoints associated with oncogenic HPV types: incident infection, 6-mo and 12-mo persistent infection, cytological abnormalities (≥ASC-US), and histopathological lesions (CIN1+ and CIN2+). Definitions of these endpoints were described previously.Citation8,Citation11
Immunogenicity
Blood samples were collected at months 0, 7, 12, and 18 during HPV-001 and on a yearly basis during the follow-up studies (HPV-007 and HPV-023). As women were enrolled into the follow-up studies independently of the date of first vaccination, results from these studies are presented according to 6-mo intervals relative to the time of the first vaccination for each woman.
Antibody titers to HPV-16 and HPV-18 (total IgG) were measured by ELISA in all women as described previously.Citation21,Citation22 Neutralising antibody titers to HPV-16 and HPV-18 were assessed using the PBNA in a subset of women.Citation24,Citation34
Results are presented along with the GMTs of ‘cleared’ natural infection (i.e., women DNA-negative and seropositive at enrolment) obtained from Phase III studies HPV-008 and HPV-010 (NCT00423046) as benchmarks.Citation16,Citation24 The ratios between GMTs and natural infection levels were calculated for both HPV-16 and HPV-18 antibodies when measured either by ELISA or PBNA. For each calculation, the timepoint considered was the timepoint with the lowest GMT value between the timepoints M101–106 and M107–113.
Safety
SAEs, medically significant AEs (i.e., AEs or SAEs prompting emergency room or physician visits that were not related to common diseases) and NOCDs (e.g., NOADs, asthma, type I diabetes) were recorded. Pregnancies and their outcomes were also recorded.
Statistical methods
Two interim analyses were performed after one and two years of HPV-023 follow-up.Citation8,Citation11 The overall α value for all analyses was 0·05 (two-sided test). Alpha values were adjusted for the two interim analyses: for the first and second interim analyses α = 0·001 (two-sided), and for the final analysis, α = 0·049 (two-sided). Based on an estimated 6% cervical infection rate, minimum 80% VE, and a 10% discontinuation rate per year for women enrolled in the trial, the power at the end of the trial was estimated at 83%. Combined analyses (HPV-001/007/023) were descriptive.
VE was calculated against each of the clinical endpoints associated with HPV-16/18 and also considered all oncogenic HPV types, for the time period of HPV-023. The conditional exact method was used to estimate VE and exact 95% confidence intervals (CIs) around the rate ratio (ratio of the event rates in the vaccine group vs. placebo group). The calculation took into account the follow-up time of the women within each group (T[year]). VE was defined as one minus the rate ratio.
Primary analyses of efficacy were performed on the ATP efficacy cohort for virological endpoints (incident and persistent infection). Because of more limited number of events, primary efficacy analyses related to cyto-histopathological endpoints (≥ASC-US, ≥LSIL, CIN1+, and CIN2+) was performed on the TVC with efficacy results available (TVC-efficacy). The analyses of immunogenicity were performed on the ATP immunogenicity cohort. The primary safety analysis and the mathematical modeling analysis were performed on the TVC. The ATP immunogenicity and efficacy cohorts included women who met all eligibility criteria, complied with study procedures in the current and preceding studies, and had data available for at least one vaccine antibody blood sample (ATP immunogenicity cohort) or data available for the efficacy measure considered (ATP efficacy cohort). The TVC included women who were enrolled in the current study, had received at least one dose of study vaccine or placebo in the initial study and for whom end-point measures were available. All women enrolled in the initial study were randomized and received at least one dose of vaccine or placebo in accordance with the randomization schedule.Citation21
Women were not included in immunogenicity assessments if HPV infection was detected for the type under consideration during the study periods in order to exclude any influence of a natural infection on the immune response. Women were censored from efficacy assessments once a specific endpoint was met in the current or preceding studies.
Incidence rates were compared between groups using Fisher exact test. The null hypothesis was that the expected incidence rate during the considered period was similar in both groups.
In addition, a descriptive combined analysis was performed (for each virological and cyto-histopathological endpoint, VE estimates were calculated and 95% CI provided) for the total follow-up period, up to 113 mo post-vaccination. Time-to-event curves for HPV-16/18 incident infection and HPV-16/18 6-mo persistent infection, which include VE data from HPV-001/007/023, were generated for the ATP efficacy cohort and the TVC.
Furthermore, an exploratory analysis was performed for each cyto-histopathological endpoint, irrespective of HPV DNA association for the total follow-up period, up to 113 mo post-vaccination.
Safety data are presented for all three years of HPV-023 with data collected from end of HPV-007 up to the final visit (Month 36) in HPV-023.
Mathematical modeling
To assess the persistence of HPV-16 and HPV-18 vaccine-induced antibody responses, the individual antibody levels of each woman at each timepoint in HPV-023 (i.e., up to 7.3 y [89 mo], up to 8.4 y [101 mo] and up to 9.4 y [113 mo]), were retrospectively fitted into two different statistical mixed effects models, the modified power-law and piece-wise models, separately for both total anti-HPV-16 and anti-HPV-18 antibodies as previously described.Citation26,Citation27 The modified power-law model aims to describe and predict antibody titers (in logarithm) over time as a logarithmic function based on parameters such as the biological dynamic of B-cell turnover and the proportion of antibodies produced by memory B-cells. The piece-wise model describes and predicts the antibody titers (in logarithm) over time according to three linear functions and three breaking time points (Month 7-Month 12, Month 12-Month 21, and Month 21 onwards), and is also based on parameters reflecting the dynamic of B-cell turnover.Citation26
| Abbreviations: | ||
| AE | = | adverse event |
| ASC-US | = | atypical squamous cells of undetermined significance |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| CIN1+ | = | cervical intraepithelial neoplasia grade 1 and above |
| CIN2+ | = | cervical intraepithelial neoplasia grade 2 and above |
| CIN3+ | = | cervical intraepithelial neoplasia grade 3 and above |
| DNA | = | deoxyribonucleic acid |
| ELISA | = | enzyme-linked immunosorbent assay |
| EL.U/mL | = | ELISA units/mL |
| GMT | = | geometric mean titre |
| HSIL | = | high-grade squamous intraepithelial lesion |
| HPV | = | human papillomavirus |
| LSIL | = | low-grade squamous intraepithelial lesion |
| MPL | = | 3-O-desacyl-4’-monophosphoryl lipid A |
| NOAD | = | new onset auto-immune disease |
| NOCD | = | new onset chronic disease |
| PBNA | = | pseudovirion-based neutralisation assay |
| SAE | = | serious adverse event |
| SD | = | standard deviation |
| TVC | = | total vaccinated cohort |
| VE | = | vaccine efficacy |
Additional material
Download Zip (152.2 KB)Disclosure of Potential Conflicts of Interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that the Institutions of P.N., C.M.R.-M., N.D.C., J.T., and P.d.B. received funding and/or equipment/administrative support from the GlaxoSmithKline group of companies. P.N. received consulting fee/honorarium from the GlaxoSmithKline group of companies. P.N., N.D.C., and J.T. received support for travel to meetings from the GlaxoSmithKline group of companies. C.R.-M., N.D.C., J.T., and P.d.B. received fees for participation in advisory boards and/or lectures from the GlaxoSmithKline group of companies. T.Z., G.C., B.G., and D.D are employees of the GlaxoSmithKline group of companies. N.S. is a former employee of the GlaxoSmithKline group of companies. N.S., B.G., and D.D. hold stock options from the GlaxoSmithKline group of companies.
Acknowledgments
The authors would like to thank the study participants and the staff members of the sites of this study including Renata Robial, Cristina Helena Rama, Jean Carlos de Matos, Simone Pollini Goncalves, Círbia Campos Teixeira, Eliane Oliveira, Marta Francis Benevides Rehme, Vera Maria Araújo Garcia e Boza, Simone Alberttoni Souza, Marcia Glaser Gutierrez Dieckmann, Amanda Novaes, Roberta Musumecci, Renata Stringhini, and Bruna Baiense. Shamita Gupta provided scientific writing support for the study protocol. Ayline Yudhira provided central study coordination, and Annelies Vanneuville provided global study management (all from GlaxoSmithKline Vaccines). Laboratory work was provided by Wim Quint, Leen-Jan Van Doorn and Anco Molijn (DDL Diagnostic Laboratory, Rijswijk, The Netherlands); Ronald Luff, Marise Mc Neeley, Elaine Alt, Basem Iskaros, Anjali Limaye, Xiaolin Liu-Jarin, Christina Provenzano, and Barbara Winkler (Quest Diagnostics, Teterboro, NJ, USA); Ariane Meurée and Sylviane Poncelet (GlaxoSmithKline Vaccines). Catherine Streeton (Streeton Associates) provided medical writing services, and Jean-Michel Heine (Keyrus Biopharma) provided editorial assistance and manuscript coordination, both on behalf of GlaxoSmithKline Vaccines.
Authors and Sponsor Contributions
The trial was funded by GlaxoSmithKline Biologicals SA, who designed the study in collaboration with investigators, and coordinated collection, analysis, and interpretation of data. Investigators collected data for the trial and cared for the participants. All authors had full access to the trial data. All authors contributed to the study design, and/or analysis and/or interpretation of data, reviewed manuscript drafts, approved the final manuscript as submitted, and had final responsibility for the decision to submit for publication. All authors take the responsibility for the overall content of the manuscript. GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and publishing of the present publication. The manuscript was developed and coordinated by the authors in collaboration with an independent medical writer and a publication manager working on behalf of GlaxoSmithKline Vaccines.
Ethical approval
Studies were conducted in conformity with country or local requirements regarding ethics committee review, informed consent, and other statutes or regulations regarding the rights and welfare of human subjects participating in biomedical research. The protocol and other materials were approved by the Independent Ethics Committee or Institutional Review Board of each study center and the National Committee of Ethics and Research. Written informed consent was obtained from all participants before any study procedure was performed. The data presented in this manuscript do not contain any personally identifiable information.
Data sharing
No additional data available. The study protocol and result summaries are publicly available on http://www.gsk-clinicalstudyregister.com. The study has the GSK ID number 109625. Study information is also available on http://www.clinicaltrials.gov, with the clinical trial identifier NCT00518336.
Copyright Statement
Antoon Loomans, VP, General counsel, as authorized representative of GSK group of companies and the Corresponding Author, on behalf of all authors except those employed by the GSK group of companies, have the right to grant a worldwide license to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (1) publish, reproduce, distribute, display and store the Contribution, (2) translate the Contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the Contribution, (3) create any other derivative work(s) based on the Contribution, (4) to exploit all subsidiary rights in the Contribution, (5) the inclusion of electronic links from the Contribution to third party material wherever it may be located, and (6) license any third party to do any or all of the above. All proprietary rights other than copyright, e.g., patents and trademarks are reserved by the GlaxoSmithKline group of companies.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 9; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F; PMID: 10451482
- Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244 - 65; http://dx.doi.org/10.1136/jcp.55.4.244; PMID: 11919208
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJLM, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518 - 27; http://dx.doi.org/10.1056/NEJMoa021641; PMID: 12571259
- Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer 2009; 4:8; http://dx.doi.org/10.1186/1750-9378-4-8; PMID: 19486508
- de Sanjose S, Quint WGV, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, et al, Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048 - 56; http://dx.doi.org/10.1016/S1470-2045(10)70230-8; PMID: 20952254
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow S-N, Apter D, Kitchener H, et al, HPV PATRICIA Study Group. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100 - 10; http://dx.doi.org/10.1016/S1470-2045(11)70287-X; PMID: 22075170
- Castellsagué X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol 2009; 115:Suppl S15 - 23; http://dx.doi.org/10.1016/j.ygyno.2009.09.021; PMID: 19819540
- Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 2012; 8:390 - 7; http://dx.doi.org/10.4161/hv.18865; PMID: 22327492
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, Hughes JP, Hawes SE, Weiss NS, Koutsky LA. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239 - 43; http://dx.doi.org/10.1016/j.jcv.2011.12.009; PMID: 22209292
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006; 24:5937 - 49; http://dx.doi.org/10.1016/j.vaccine.2006.06.005; PMID: 16828940
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, Sanchez N, Schuind A. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247 - 55; http://dx.doi.org/10.1016/j.vaccine.2010.07.007; PMID: 20643092
- Moscicki AB, Wheeler CM, Romanowski B, Hedrick J, Gall S, Ferris D, Poncelet S, Zahaf T, Moris P, Geeraerts B, et al. Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012; 31:234 - 41; http://dx.doi.org/10.1016/j.vaccine.2012.09.037; PMID: 23063422
- Petäjä T, Pedersen C, Poder A, Strauss G, Catteau G, Thomas F, Lehtinen M, Descamps D. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int J Cancer 2011; 129:2147 - 57; http://dx.doi.org/10.1002/ijc.25887; PMID: 21190190
- Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Schulze K, Poncelet SM, Catteau G, Thomas F, Descamps D. Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years. Hum Vaccin 2011; 7:958 - 65; http://dx.doi.org/10.4161/hv.7.9.15999; PMID: 21892005
- Schwarz TF, Huang L-M, Medina DMR, Valencia A, Lin T-Y, Behre U, Catteau G, Thomas F, Descamps Dl. Four-Year follow-up of the immunogenicity and safety of the HPV-16/18 AS04-Adjuvanted vaccine when administered to adolescent girls aged 10–14 years. J Adol Hlth 2012; 50:187 - 94; http://dx.doi.org/10.1016/j.jadohealth.2011.11.004
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter DL, Kitchener HC, Castellsague X, et al, HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 70; http://dx.doi.org/10.1016/S0140-6736(07)60946-5; PMID: 17602732
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al, HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/10.1016/S0140-6736(09)61248-4; PMID: 19586656
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al, HPV PATRICIA Study Group. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89 - 99; http://dx.doi.org/10.1016/S1470-2045(11)70286-8; PMID: 22075171
- Verstraeten T, Descamps D, David M-P, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.09.049; PMID: 18845199
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009; 5:332 - 40; http://dx.doi.org/10.4161/hv.5.5.7211; PMID: 19221517
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al, GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 65; http://dx.doi.org/10.1016/S0140-6736(04)17398-4; PMID: 15541448
- Harper DM, Franco EL, Wheeler CM, Moscicki A-B, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G, HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247 - 55; http://dx.doi.org/10.1016/S0140-6736(06)68439-0; PMID: 16631880
- The GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6·4 years. Lancet 2009; 374:1975 - 85; http://dx.doi.org/10.1016/S0140-6736(09)61567-1; PMID: 19962185
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al, HPV-010 Study Group. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705 - 19; http://dx.doi.org/10.4161/hv.5.10.9518; PMID: 19684472
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David M-PM, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425 - 34; http://dx.doi.org/10.4161/hv.4.6.6912; PMID: 18948732
- David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, Van Damme P. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009; 115:Suppl S1 - 6; http://dx.doi.org/10.1016/j.ygyno.2009.01.011; PMID: 19217149
- Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, Barr E, Ault KA. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine 2007; 25:4324 - 33; http://dx.doi.org/10.1016/j.vaccine.2007.02.069; PMID: 17445955
- Herrero R, Wacholder S, Rodríguez AC, Solomon D, González P, Kreimer AR, Porras C, Schussler J, Jiménez S, Sherman ME, et al, Costa Rica Vaccine Trial Group. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov 2011; 1:408 - 19; http://dx.doi.org/10.1158/2159-8290.CD-11-0131; PMID: 22586631
- Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al, CVT Vaccine Group. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; a 103:1444 - 51; http://dx.doi.org/10.1093/jnci/djr319; PMID: 21908768
- Angelo M-G, David M-P, Zima J, Baril L, Dubin G, Arellano F, Struyf F. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014; a 23:466 - 79; http://dx.doi.org/10.1002/pds.3554; PMID: 24644063
- Angelo M-G, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf 2014; b 23:456 - 65; http://dx.doi.org/10.1002/pds.3593; PMID: 24644078
- Wacholder S, Chen BE, Wilcox A, Macones G, Gonzalez P, Befano B, Hildesheim A, Rodríguez AC, Solomon D, Herrero R, et al, CVT group. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ 2010; 340:c712; http://dx.doi.org/10.1136/bmj.c712; PMID: 20197322
- van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol 2006; 44:3292 - 8; http://dx.doi.org/10.1128/JCM.00539-06; PMID: 16954263
- Pastrana DV, Buck CB, Pang Y-YS, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 2004; 321:205 - 16; http://dx.doi.org/10.1016/j.virol.2003.12.027; PMID: 15051381
