Abstract
Abrin toxin (AT) is a highly potent toxin, and is classified as one of the most important biological warfare and bioterrorism agents. There is currently no approved vaccine for AT. Therefore, the development of an effective vaccine is important in the prevention of intoxication by abrin. In this study, five vectors containing different gene of truncated abrin toxin A chain (tATA) fragments were constructed, and two of them (tATA11-126, tATA41-188) were successfully expressed as a soluble form in E.coli strain. Both of the two tATA retained most of their immunogenicity with either low or no toxic effects as determined by both in vitro and in vivo assays. They were used to immunize BALB/c mice three times at an interval of three weeks apart. As a result, the tATA1 can elicite 80% protective efficacy against i.p. challenge of 5 × LD50 of abrin, and the tATA4 provides a better protection, which can elicite 100% protective efficacy against intraperitoneal challenge of 40 × LD50 of abrin. The superior fragment (tATA41-188) should be considered as a promising vaccine candidate for further investigations.
Abbreviations:
- tATA, truncated A chain of abrin toxin
- rATA, recombinant A chain of abrin toxin
- LD50, 50% lethal dose
- E.coli, Escherichia coli
- PCR, polymerase chain reaction
- i.p., intraperitoneal/intraperitoneally
- s.c., subcutaneous/subcutaneously
- i.n., intranasal
- i.g., intragastric
- BSA, bovine serum albumin
- PBS, phosphate–buffered saline solution
- IPTG, isopropyl-1-thio-β-galactopyranoside
- SD, standard deviation
- AU, absorbance unit
- pAb, polyclonal antibody
Introduction
Abrin toxin (AT) is an extremely toxic plant protein derived from the seeds of the plant Abrus precatorius, which originates in Southeast Asia, and is now found in subtropical areas. AT consists of two subunits: an enzymatic A-chain (ATA) and a lectin-active B-chain (ATB). The ATA is a glycosidase that removes an adenine residue from an exposed loop of 28S rRNA, which inhibits protein synthesis to exert its toxic effect. The ATB is the portion that binds β-D-galactopyranoside moieties on the eukaryotic cell membrane, allowing the ATA enter into the cell.Citation1 Due to its availability, potential lethality, and lack of specific post-exposure treatment, the development of an effective vaccine may be a feasible means of countering the potential biothreat for at-risk populations, such as military populations and research scientists working with abrin.
AT shows significant similarities to ricin, both at the sequence level, and the structural level.Citation2 However, the reported LD50 of abrin in the mouse model is 0.04 μg/kg, which is almost 75 times more toxic than ricin.Citation3 Since the A-chain of the A-B structural toxin is ultimately responsible for toxicity and is immunologically protective in toxin challenges,Citation4-6 variants of the A-chain hold promise as vaccine candidates. For ricin, many reported vaccine candidates have used the A-chain rather than the B-chain, because many studies have shown that immunization with the A-chain induces greater protection than immunization with the B-chain.Citation7,8 For abrin, one such vaccine candidate is recombinant mutant abrin A-chain, which had its toxicity eliminated by introducing two point mutations (E164A R167L).Citation4 Earlier study of the abrin vaccine used abrin toxoid in 1995.Citation9 Our laboratory is focused on the development of toxin vaccines, including botulinum neurotoxin,Citation10 epsilon toxin,Citation11 ricin, and abrin.Citation7,12
In this study, two truncated ATA fragments (tATA1Citation1-126, tATA41-188) have been expressed with high immunogenicities and low toxicities, and it is believed that tATA41-188 may become a promising vaccine candidate against the intoxication induced by AT.
Results
Preparation and confirmation of the tATA protein
tATA1 (15kDa) and tATA4 (22kDa) were successfully expressed in E. coli and secreted in the supernatant, while the other three (tATA2, tATA3, and tATA5) were produced as inclusion bodies. Due to the conformational change of inclusion body protein, only soluble proteins (tATA1, 4) were chosen for further study. After one-step purification, the purity of the protein was found to be greater than 98% pure as determined by SDS-PAGE (A).
Figure 1. Description of the 5 truncated abrin A-chains. ATA: the amino acid of the whole abrin toxin A-chain, which contained 251 amino acids. tATA1–5 decribe five truncated fragments of ATA.
Figure 2. SDS-PAGE and western blotting analysis of targeted proteins. (A) SDS-PAGE analysis of rATA, tATA4, and tATA1 proteins. (B) Western blotting analysis of rATA, tATA4, and tATA1 proteins. Lanes M, low molecular weight protein markers. Lane 1: purified rATA (control). Lane 2: purified tATA4. Lane 3: purified tATA1.
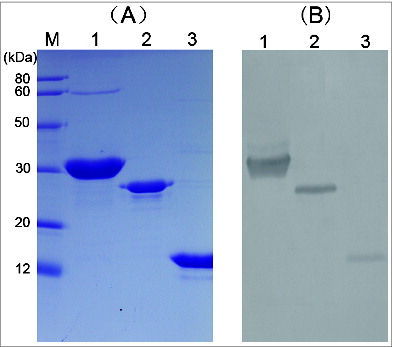
Western blotting showed that in addition to tATA1 and tATA4, that rATA (positive control) was also recognized by rabbit pAb against native abrin (B). The results showed that both proteins were similarly antigenic and as specific as rATA.
Safety tests of tATA1/tATA4 in vitro and in vivo
Cytotoxicity
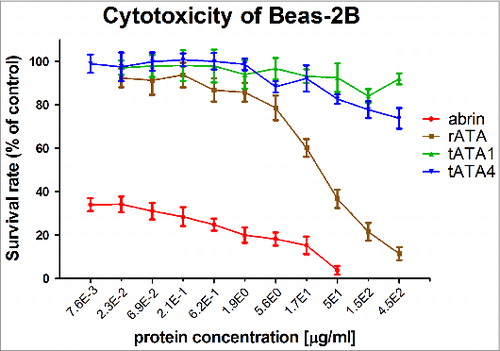
The target proteins were measured for cytotoxicity using the metabolic indicator MTS.13 The results revealed that cytotoxicity of tATA1 and tATA4 was significantly lower in BEAS-2B cells compared with AT and rATA (). At the protein level of 450 μg/ml, the survival rates of the cells in the tATA1 and tATA4 group approximated 80%, but the survival rate of the cells in the rATA group was less than 20%. In contrast to native AT and rATA, both tATA1 and tATA4 were almost non-toxic for BEAS-2B cells. Above all, the rank orders of observed cytotoxicity were: AT > > rATA > tATA4 ≈tATA1.
Figure 3. Cytotoxic effects of four proteins (tATA1, tATA4, rATA, abrin) against the BEAS-2B cell-line model. The toxicities of target proteins were tested using the CellTiter 96® AQueous One Solution Cell Proliferation Assay, by measuring the toxicities in the human bronchial epithelial cell-line BEAS-2B. Each point represents the arithmetic mean ± SD of triplicate determinations.
Toxicity assay in the mouse
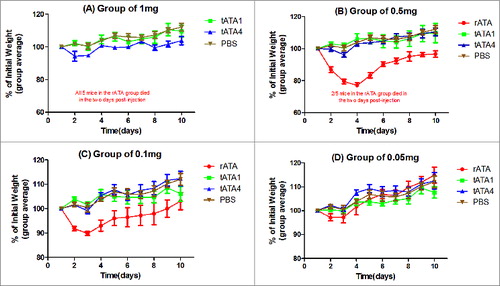
Dose-response relationship: Four different doses (0.05, 0.1, 0.5, and 1 mg) of three distinct proteins (tATA1, tATA4, rATA) were i.p. injected into mice respectively, the same volume of PBS were injected into mice which were used as negative control. Mice in the highest dose group (1 mg per mouse) received approximately 100 times the dose that we proposed to use in the human clinical trial (10 μg). In the rATA group, mice that were injected with the two highest dose of rATA (1 or 0.5 mg/mouse) showed signs of intoxication between 24 h and 48 h post-challenge, including reduced activity, piloerection, and reduced eating of provided food, hunched posture, and labored respiration. In addition, all five mice in the rATA (1mg/mouse) group and two out of five mice in the rATA (0.5 mg/mouse) group died between 48 h and 72 h later. Further, the remaining three mice that survived in the rATA (0.5 mg/mouse) group lost more than 20% of their body weight by day 4 and did not even recover their initial weight by day 10. Whereas, none of the mice, received a single dose of the tATA1 and tATA4 ranged from 0.05mg to 1mg, died or lost weight more than 8% (). This demonstrated that the tATA1 and tATA4 were almost non-toxic even at those levels.
Figure 4. Average weights (as percentage of initial) vs time (days). Thirteen groups of mice (5 in each group) were i.p. injected with four different doses of tATA1 (▪), tATA4 (▴), rATA (•) (positive control), and as a negative control group, mice were challenged with PBS (▾). Weights and survival status of all the mice were recorded 10 d post-injection. Each point represents the change in of the mice as the arithmetic mean ± SD in each group. (A) The injection dose was 1mg. All 5 mice in the rATA group died within two days post-injection. (B) The injection dose was 0.5 mg. further, 2 of the 5 mice in the rATA group also died, and the remaining 3 mice lost more than 20% of their body weight after the third day. (C) The injection dose was 0.1mg. (D) The injection dose was 0.05mg.
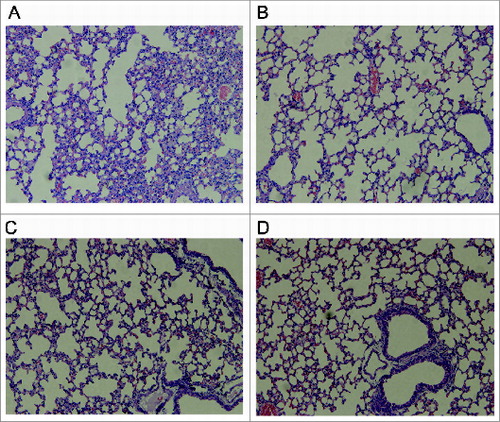
Histopathology: The results showed that severe pathological alterations were only detected in the lungs of mice in the rATA (0.5 mg/mouse) group. Histologically, the lungs of the mice in this group showed epithelial necrosis, generalized interstitial edema, partial consolidation and moderate intra-alveolar hemorrhage (A). The interstitial tissues were inflamed with large numbers of macrophages and lymphocytes. While the mice injected with the highest dose of tATA1 or tATA4 (1mg/mouse) appeared as normal as the mice injected with PBS alone (B–D), the results still indicated that tATA1 and tATA4 provoked no obviously observed toxicity in mice even at the highest doses (1 mg/ml).
Figure 5. Histopathologic alterations in the lungs of mice injected with 0.5 mg of rATA (A), 1 mg of tATA4 (B), 1 mg of tATA1(C), and PBS alone (D). (A) Epithelial necrosis, generalized interstitial edema, partial consolidation and moderate intra-alveolar hemorrhage; (B), (C), and (D) (control): no obvious edema or necrosis.
Vaccination and measurement of antibody titers
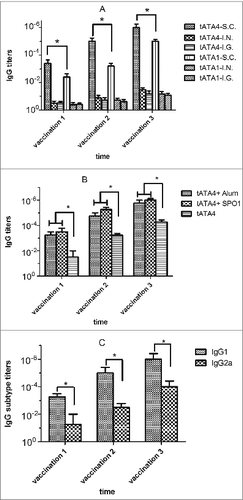
The ELISA results of the anti-rATA antibody titers of sera (mice immunized with tATA1/tATA4 formulated with SPO1 adjuvant in three routes) are shown in A. The antibody titers increased following booster s.c. immunizations, and the highest mean titer was 1:106 (tATA4-SC, at vaccination 3). The antibody titers of mice in tATA4-SC group were significantly higher than that in tATA1-SC group after all the three vaccinations (P < 0.05). The antibody titers did not increased after primary and booster immunizations via the i.n. or i.g. route. The experiment was repeated twice and the results were reliable and showed high reproducibility. The data from two separate experiments are shown (A). The ELISA results of the anti-rATA antibody titers of sera (mice immunized with or without different adjuvants) are also shown (B). The results indicated that antigen mixed with adjuvant was more immunogenic than in the absence of adjuvant (P < 0.05). The anti-rATA IgG1 and IgG2a titers were also detected in the tATA4+SPO1 group (C). Although both IgG1 and IgG2a were detected after each vaccination, it is clear that the average titers of IgG1 were 100 times higher than that found for IgG2a (P < 0.05).
Figure 6. Measurement of antibody titers. All immunization schedules involved mice that were immunized 3 times at 3 wk intervals and serums specimens were collected from the tail veins of mice, which were then used to measure antibody titers by ELISA one week after each immunization respectively. The graphs show arithmetic mean antibody titers of 5 mice per group ± SD. T-test and ANOVA were used to compare treatment group means at given time points. The X-axis “vaccination 1, 2, 3” represents the time point one week following the first, second and third immunization respectively. The Y-axis represents the antibody titers. Titer was calculated as the reciprocal of the highest dilution having an OD450 greater than 0.1 AU (absorbance unit) after correcting for background. *P < 0.05 indicated statistical significance between paired groups. (A) The anti-rATA antibody titers between different groups of immunization routes after each vaccination. S.C.: subcutaneous immunization (15μg/mouse). I.N.: intranasal immunization (20μg/mouse). I.G.: intragastric immunization (200μg/mouse). tATA4 and tATA1 were mixed with the same volume of SPO1 adjuvant to immunize the mice. (B) The anti-rATA antibody titers between groups of different adjuvants after each vaccination. tATA4 (15μg/mouse) alone or mixed with the same volume of SPO1 adjuvant or Imject Alum adjuvant were used to immunize mice subcutaneously. (C) The anti-rATA antibody subtype titers of sera after each vaccination. The sera that were used to detect the antibody titers were obtained from the 5 mice in the “tATA4+Imject Alum” group (B).
Survival rates after abrin challenge
The challenge results of mice immunized by different routes are shown in and the survival rates are those found by at day 10 post challenge. It is clear that s.c. immunization (mice immunized with 15 μg of tATA1 or tATA4 mixed with SPO1 adjuvant three times at three week intervals) was the most efficient route in the three immunization ones. Additionally, the mice in the i.n. instillation immunized group and in the i.g. administration immunized group could not survive, even at the lowest challenge dose of 5 × LD50 of AT. In the group of mice immunized s.c. with tATA4, all animals survived after i.p. challenge of 40 × LD50 of AT, and 80% of the mice survived when the dose increased to 50 × LD50. The survival rates of the mice in the s.c. immunized with tATA1 group were 80% and 40% after i.p. injection of 5 × LD50 and 10 × LD50 of AT respectively, which were considerably lower than that found for tATA4. All the control mice (s.c. immunized with PBS) died within the first 2 d after i.p. injection with 2 × LD50 of AT. From this observation, we conclude that s.c. immunization with tATA4 is the most efficient way to protect mice from AT challenge.
Table 1. The sequences of the five pairs of primers
Table 2. The survival rates of the mice immunized tATA1 or tATA4 with SPO1 adjuvant 3 times at 3 wk intervals in different routes after challenge of different doses of abrin
Due to its superior protection, fragment tATA4 was chosen as the antigen in this different adjutant immunization experiments. The challenge results are shown in . Fourteen groups of mice (ten per group), were s.c. immunized with 15 μg of tATA4 mixed with or without different adjuvant three times at three week intervals, and were then challenged with four doses (20, 30, 40, and 50 × LD50) of AT by i.p. injection one week after the third immunization. The results demonstrated that the survival rates of the mice in the “tATA4+SPO1” group were 100% and 70% when the AT challenge doses were 40 × LD50 and 50 × LD50 respectively. The survival rates of the mice in the “tATA4+Imject® Alum” group and the “tATA4” group were 100% and 80% when the AT challenge dose was 30 × LD50. All the mice in the control group died at the second day after challenge with 20 × LD50 of AT. The results indicated that tATA4 immunized mice with the adjuvant were better than without the adjuvant (P < 0.01), and there was no significant difference between use of either the SPO1 adjuvant or the Imject® Alum adjuvant in this study (P = 0.406).
Table 3. The survival rates of the mice which were immunized subcutaneously with tATA4 mixed with or without adjuvant 3 times at 3 wk intervals after challenge of different doses of abrin
Discussion
Abrin is one of the most potent plant toxins known.Citation14 Although non-infectious, it has a significant potential for misuse by terrorist organizations as a bioweapon in much the same way as ricin. For example, weapons-grade ricin was manufactured and tested in artillery shells in the late 1980s in Iraq,Citation15 and letters containing ricin that were addressed to the US. President Barack Obama, and a US senator and a judge were intercepted by the FBI in April 2013. Until now, there are at least three kinds of ricin vaccine,Citation16 which include two recombinant RTA vaccinesCitation17,18 and a mutant RTA vaccine.Citation6 However, only a few studies have detailed abrin, including one study of a neutralizing antibody to the abrin A-chain, which can protect mice from lethal doses of AT that was reported in 2008,Citation19 and a recombinant mutant abrin A chain vaccine, which can protect mice against 10 × LD50 of AT that was reported in 2011,Citation4 and a recombinant chimeric protein vaccine candidate containing B chain of ricin and abrin, which can protect mice against 4 × LD50 of RT and AT that was reported in 2014.Citation12 There were no further reports regarding an abrin vaccine. Thus, abrin, which is a more dangerous analog, should receive at least equal attention from international sovereign governments.
There is a consensus opinion for vaccine design that a reduction in protein subunits to a minimum level that includes an essential domain containing a neutralizing epitope should be tested on antigens as a strategy.Citation20 It was also previously reported that the B-chain was far less immunogenic than the A-chain in these A-B structural toxins.Citation7,8,21 Based on the two opinions, we constructed five tATA fragments, which contained partial overlap sequences. Only tATA1 and tATA4 were successfully expressed in a soluble form. Fortunately, both were identified as being effective and almost non-toxic when administered to mice even at the highest dose of 1 mg per mouse. Additionally, tATA4 induced improved protection as compared tATA1 in immunized mice. This result is consistent with recent findings that the helix that is composed of amino acids 148–167 is the core of the ATA structure and truncation of the helix might destabilize the structure and result in less protective efficacy as reported by Bagaria, et al.Citation22 This could explain why tATA4 induced improved protection as compared tATA1.
In this case, compared with the whole A chain of abrin, tATA4 lacked C-terminal domain residues (which represents a quarter of the whole chain), which contributed to the elimination of toxic enzymatic activity while preserving most of the antigenicity, and this has been identified in the efficacy and safety analyses both in vitro and in vivo. This study is also consistent with earlier findings on ricin vaccine RTA (1-33/44-198),Citation21 which based on minimum essential folding domains required for protective efficacy.
As a biothreat agent, AT is likely to be disseminated as an aerosol or used to contaminate food products. By either route, the toxin would first contact a mucosal surface in the body. So we immunized mice with tATA4 by three routes (intranasal instillation, i.g. administration or s.c. injection) to evaluate their protective effects. After three immunizations, the sera titers of the mice that were immunized through different routes varied (A). The antibody titers for tATA4 and tATA1 delivered s.c. increased precipitously after each successive immunization. However, the titers that were delivered by either i.n. or i.g. routes increased slowly after each successive immunization. This result indicates that tATA4 immunized s.c. can induce higher IgG antibodies in serums of mice than tATA4 immunized i.n. and i.g. In addition, theoretically, sIgA may exert important protective functions in mucosal immunity,Citation23 but we could not detect any sIgA in the irrigating aspirates either from lung or intestine (no data shown). Furthermore, the challenge observations confirmed that mice s.c. immunized with tATA4 acquired the best protection against a dose of 40 × LD50 of AT challenge (), while the mice in the groups immunized by other routes of immunization did not do as well, with most mice not surviving the challenge with even 2 × LD50 of AT. Our results are in accord with those which demonstrated that the principal mechanism of protection against abrin exposure is via serum IgG antibodies,Citation24,25 though sIgA antibodies may play a role in protecting mucosal surfaces against abrin exposure.Citation8 In addition, we also compared two different adjuvants (SPO1 and Alum-based adjuvant) in our study. It is well-known that alum adjuvant was the first approved adjuvant for use in humans. The SPO1 adjuvant is a novel adjuvant that was invented by Wang Xiliang's group.Citation26 It was reported that a novel oil/water emulsion, SPO1, was a dominant Th1-immune promoting adjuvant when used in influenza vaccines. However, in our study the situation was reverse. Antibody subtype analysis (C) showed that mice developed both IgG1 and IgG2a antibody titers, but IgG1 titers were markedly higher than IgG2a, which supported the dominant expression of a Th2-mediated immune response. The Th2-mediated immune response is associated with humoral immunity and the lack of cellular immunity is a shortcoming in this study. A similar situation occurred in mice immunized with alum adjuvant (data not shown). This demonstrated that the antigen in protein form, and not the adjuvant, was the dominant factor that contributed to the type of immune response.
In conclusion, the purified tATA4 antigen is safe in the mouse. It can induce high titers of anti-abrin antibody to wholly protect the mouse from a dose of 40 × LD50 of AT challenge when administered s.c. with SPO1 as the adjuvant. Taken together, these data suggest that tATA4 is a potential vaccine candidate for abrin and should be further studied for stability prolonged storage and measures of safety and efficiency in human volunteers.
Materials and Methods
Animals
All animal experiments were performed in accordance with the Guideline for Animal Experiments of the Academy of Military and Medical Sciences, and were approved by the Animal Ethics Committee of the Academy of Military and Medical Sciences.
Preparation and confirmation of the recombinant tATA
The sequence of abrin A-chain contains 251 amino acids which were divided into to four parts in average. Five truncated fragments were produced by different combinations of the four parts. Five pairs of primers incorporating the 5′ and 3′ restriction sites of EcoRІ and NheІ respectively, were used to amplify DNA of five truncated fragments of ATA and these fragments were expressed in E. coli: tATA11-126, tATA263-188, tATA3126-251, tATA41-188, and tATA564-251 (). The sequences of the five pairs of primers were listed in . The amplified five tATA genes were then ligated into the pET-His vector to produce the recombinant expression vectors, pET-His-tATA1-5, which were transformed into E.coli strain BL21 (DE3). After a series of expression condition trials, tATA1 and tATA4 were successfully expressed in the form of soluble, and the optimal expression conditions of tATA1 (induction temperature: 20°C, concentration of IPTG: 0.5mM, induction time: 14–16 h) and tATA4 (induction temperature: 20°C, concentration of IPTG: 0.01mM, induction time: 14–16 h) was determined. Next, the protein was purified by nickel-nitrilotriacetic affinity chromatography (GE Healthcare, 17–5248) and the concentration was measured by a BCA assay kit (Novagen, 71285–3). The purity of the protein was confirmed by using BandScan 5.0 software (GlykoPrep). As a positive control, recombinant ATA (rATA) was prepared from an E.coli lysate and purified using the same method described here.
Western blotting was performed to confirm the antigenicity of tATA. The purified tATA protein was transferred onto a nitrocellulose membrane (Pall, 66485). The detail of the procedures were performed according to our laboratory's previously published protocol.Citation11
Safety testing of tATA1 and tATA4 in vitro and in vivo
Cytotoxicity assay of tATA1, tATA4, rATA, and abrin
The cytotoxicity of four different proteins in the human bronchial epithelial cell- line BEAS-2B, was tested using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, G3580).Citation11 The BEAS-2B cells were cultured in RPMI 1640 (Hyclone, SH30809.01B) supplemented with 10% (v/v) fetal bovine serum. Approximately 1 × 104 cells per well in 100 μl medium were seeded into a 96-well plate. Next, 100 μl of each concentration of serial triple diluted tATA or rATA or abrin proteins in complete culture medium were added to the wells. All manipulations were performed according to the manufacturer's instructions. The absorbance was read at a wavelength of 490 nm, and the data obtained was directly proportional to the number of living cells in the culture.
Toxicity assay in the mouse
(1) Dose-response relationship: 65 female BALB/c mice (5–6 wk old and weighing 18- 20 g) were equally divided into 13 groups (5 mice per group). Mice were i.p. injected with 4 different doses (0.05, 0.1, 0.5, and 1 mg) of tATA1, tATA4, rATA (as the positive control), and PBS (negative control) as a single injection. Survival rates and weight changes were monitored daily for 10 d post-administration.
(2) Histopathology of mice post-administration: To determine whether there was evidence of abrin-related tissue damage in the mice, representative living mice were euthanized prior to histopathology experiment at 10 d post-administration. Vital organs (heart, liver and lung) were chosen for histopathological studies.
Vaccination and abrin challenge
Different routes
We used 5–6 wk old female BALB/c mice (weighing 18–20 g), which were divided into groups of ten per group. Mice were immunized three times, and at three week intervals by the s.c. injection (15 μg per mouse), i.n. instillation (20 μg per mouse), or i.g. administration (200 μg per mouse) with tATA1 or tATA4 mixed with SPO1 adjuvant (a novel oil/water emulsion invented and verified by the group of Dr. Wang Xiliang, and its formulation is with two emulsifiers composed of squalene, poly-oxyethylenated castor oil, and poly (ethylene glycol) block-poly (propylene glycol) blockpoly [ethylene glycol]) at a volume ratio of 1:1.Citation26 Mice immunized s.c. with PBS were included as controls in this study. Following challenge of the mice by i.p. injection of 7 distinct doses of AT (2, 5, 10, 20, 30, 40, and 50 × LD50), after one week following the third immunization, the survival rates and body weight changes of the mice were recorded for ten days, which was a period of time necessary for the mice to regain their initial weight.
Different adjuvants
To determine the effect of different adjuvants, 14 groups of mice (ten in each group) were immunized s.c. three times and at three week intervals with tATA4 (15μg per mice) that was mixed with Imject® Alum (at a volume ratio of 1:1) (Thermo Scientific, 77161) or SPO1 adjuvant (at a volume ratio of 1:1) or in the absence of using an adjuvant. As a control, the same numbers of mice were injected s.c. with PBS. Finally, mice were challenged by i.p. injections with 4 different doses (20, 30, 40, and 50 × LD50) of AT one week after the third immunization. The mice were then observed for signs of morbidity, mortality and weight changes for the next 10 d.
Titers measurements of antigen-specific antibody IgG, IgG1, and IgG2a
Serum from the tail vein of mice were collected one week after each immunization, and levels of IgG, and the subtype patterns of IgG1 and IgG2a antibodies specific for rATA antigen were determined by enzyme-linked immune-sorbent assay (ELISA). The rATA protein was used as coating antigen, and anti-mouse antibody serotypes IgG, IgG1, IgG2a (Southern biotech, 98609, 1073, 1080) were used to detect the specific titers. All manipulations were performed according to our laboratory's previously published protocol.Citation11
Statistical analysis
All data are shown as the arithmetic mean ± SD. Student's paired t test, analysis of variance (ANOVA) and logistic regression were used to compare treatment group differences. *P < 0.05 indicated statistical significance between the two groups.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Prof. Wang Xiliang for providing the SPO1 adjuvant, Prof. Zhu Maoxiang for the BEAS-2B cells, and Dr. Kou Zhihua for her suggestions in the experimental design. All are from the Academy of Military Medical Sciences, Beijing.
References
- Jang DH, Hoffman RS, Nelson LS. Attempted suicide, by mail order: Abrus precatorius. J Med Toxicol 2010; 6:427-30; PMID:20563676; http://dx.doi.org/10.1007/s13181-010-0099-1
- Barbieri L, Ciani M, Girbés T, Liu WY, Van Damme EJ, Peumans WJ, Stirpe F. Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett 2004; 563:219-22; PMID:15063752; http://dx.doi.org/10.1016/S0014-5793(04)00286-8
- Olsnes S, Sandvig K, Eiklid K, Pihl A. Properties and action mechanism of the toxic lectin modeccin: interaction with cell lines resistant to modeccin, abrin, and ricin. J Supramol Struct 1978; 9:15-25; PMID:732310; http://dx.doi.org/10.1002/jss.400090103
- Han YH, Gao S, Xin WW, Kang L, Wang JL. A recombinant mutant abrin A chain expressed in Escherichia coli can be used as an effective vaccine candidate. Hum Vaccin 2011; 7:838-44; PMID:21817853; http://dx.doi.org/10.4161/hv.7.8.16258
- Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine 2005; 23:4775-84; PMID:15961194; http://dx.doi.org/10.1016/j.vaccine.2005.04.037
- Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine 2002; 20:3422-7; PMID:12213413; http://dx.doi.org/10.1016/S0264-410X(02)00312-2
- Marsden CJ, Smith DC, Roberts LM, Lord JM. Ricin: current understanding and prospects for an antiricin vaccine. Expert Rev Vaccines 2005; 4:229-37; PMID:15889996; http://dx.doi.org/10.1586/14760584.4.2.229
- Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol 2004; 172:6221-8; PMID:15128810; http://dx.doi.org/10.4049/jimmunol.172.10.6221
- Griffiths GD, Lindsay CD, Allenby AC, Bailey SC, Scawin JW, Rice P, Upshall DG. Protection against inhalation toxicity of ricin and abrin by immunisation. Hum Exp Toxicol 1995; 14:155-64; PMID:7779439; http://dx.doi.org/10.1177/096032719501400201
- Gao YL, Gao S, Kang L, Nie C, Wang JL. Expression of Hc fragment from Clostridium botulinum neurotoxin serotype B in Escherichia coli and its use as a good immunogen. Hum Vaccin 2010; 6:462-6; PMID:20519939; http://dx.doi.org/10.4161/hv.6.6.11709
- Li Q, Xin W, Gao S, Kang L, Wang J. A low-toxic site-directed mutant of Clostridium perfringens ϵ-toxin as a potential candidate vaccine against enterotoxemia. Hum Vaccin Immunother 2013; 9:2386-92; PMID:23835363; http://dx.doi.org/10.4161/hv.25649
- Wang J, Gao S, Zhang T, Kang L, Cao W, Xu N, Liu W, Wang J. A recombinant chimeric protein containing B chains of ricin and abrin is an effective vaccine candidate. Hum Vaccin Immunother 2014; 10:10; PMID:24509607; http://dx.doi.org/10.4161/hv.27870
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 1991; 3:207-12; PMID:1867954
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon 2004; 44:361-70; PMID:15302520; http://dx.doi.org/10.1016/j.toxicon.2004.05.003
- Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA 2005; 294:2342-51; PMID:16278363; http://dx.doi.org/10.1001/jama.294.18.2342
- Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 2007; 25:7459-69; PMID:17875350; http://dx.doi.org/10.1016/j.vaccine.2007.08.018
- Marsden CJ, Knight S, Smith DC, Day PJ, Roberts LM, Phillips GJ, Lord JM. Insertional mutagenesis of ricin A chain: a novel route to an anti-ricin vaccine. Vaccine 2004; 22:2800-5; PMID:15246614; http://dx.doi.org/10.1016/j.vaccine.2004.01.024
- Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein Eng Des Sel 2004; 17:391-7; PMID:15187223; http://dx.doi.org/10.1093/protein/gzh043
- Surendranath K, Karande AA. A neutralizing antibody to the a chain of abrin inhibits abrin toxicity both in vitro and in vivo. Clin Vaccine Immunol 2008; 15:737-43; PMID:18353919; http://dx.doi.org/10.1128/CVI.00254-07
- McHugh CA, Tammariello RF, Millard CB, Carra JH. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci 2004; 13:2736-43; PMID:15340172; http://dx.doi.org/10.1110/ps.04897904
- Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, Smith LA. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine 2007; 25:4149-58; PMID:17408819; http://dx.doi.org/10.1016/j.vaccine.2007.03.011
- Bagaria S, Ponnalagu D, Bisht S, Karande AA. Mechanistic insights into the neutralization of cytotoxic abrin by the monoclonal antibody D6F10. PLoS One 2013; 8:e70273; PMID:23922965; http://dx.doi.org/10.1371/journal.pone.0070273
- Uren TK, Wijburg OL, Simmons C, Johansen FE, Brandtzaeg P, Strugnell RA. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol 2005; 35:180-8; PMID:15593123; http://dx.doi.org/10.1002/eji.200425492
- Peek LJ, Brey RN, Middaugh CR. A rapid, three-step process for the preformulation of a recombinant ricin toxin A-chain vaccine. J Pharm Sci 2007; 96:44-60; PMID:16998874; http://dx.doi.org/10.1002/jps.20675
- Hewetson JF, Rivera VR, Creasia DA, Lemley PV, Rippy MK, Poli MA. Protection of mice from inhaled ricin by vaccination with ricin or by passive treatment with heterologous antibody. Vaccine 1993; 11:743-6; PMID:8342322; http://dx.doi.org/10.1016/0264-410X(93)90259-Z
- Yu S, Tang C, Shi X, Yang P, Xing L, Wang X. Novel Th1-biased adjuvant, SPO1, enhances mucosal and systemic immunogenicity of vaccines administered intranasally in mice. Vaccine 2012; 30:5425-36; PMID:22709954; http://dx.doi.org/10.1016/j.vaccine.2012.05.085
