Abstract
Vaccines have had a major impact on the reduction of many diseases globally. Vaccines targeted against invasive meningococcal disease (IMD) due to serogroups A, C, W, and Y are used to prevent these diseases. Until recently no vaccine had been identified that could confer broad protection against Neisseria meningitidis serogroup B (MnB). MnB causes IMD in the very young, adolescents and young adults and thus represents a significant unmet medical need. In this brief review, we describe the discovery and development of a vaccine that has the potential for broad protection against this devastating disease.
Introduction
Capsular polysaccharide based vaccines currently exist for prophylaxis of disease caused by meningococcal serogroups A, C, Y and W-135. However, similar approaches to develop a serogroup B Neisseria meningitidis (MnB) vaccine have failed. The serogroup B capsular polysaccharide is composed of polysialic acid repeating units that are similar to structures found on human neuronal cells (particularly during fetal development) and are not suitable as vaccine antigens.Citation1 An alternate vaccine strategy to prevent MnB disease has included vaccines produced from outer membrane vesicles (OMVs) prepared from regional epidemic MnB strains.Citation2 These OMVs have been successfully deployed to control epidemics in Norway, Cuba, New Zealand, and France.Citation3 The immune-dominant antigen in these vesicles is the outer membrane protein porin A (PorA).Citation4 For an OMV vaccine to be effective in an outbreak situation the PorA of the vaccine needs to match that of the epidemic strain. Since PorA proteins show extensive sequence heterogeneity in their cell surface exposed immunogenic loops, many OMVs would be required to assure a broadly protective response against MnB invasive strains, thereby emphasizing the need for alternate approaches for vaccine development. To make a broadly effective vaccine candidate against MnB, vaccine antigens should be: (1) present in the majority of global clinical disease isolates; (2) surface exposed; (3) an important virulence factor; and (4) able to elicit a bactericidal immune response against a high proportion of diverse global invasive disease isolates. Serum bactericidal immune responses as measured in serum bactericidal antibody assays with human complement (hSBA) have been shown to correlate with protection against meningococcal disease.Citation5
Several MnB surface proteins have been considered individually as vaccine antigens but have not satisfied all of the above mentioned criteria. Deficiencies have included; (1) an inability to demonstrate induction of functional immune responses measured in the hSBA (Transferrin Binding Protein, Neisserial Heparin Binding Protein);Citation6,Citation7 (2) low surface expression on MnB invasive clinical isolates (Neisserial Surface Protein A);Citation8 (3) considerable sequence diversity (PorA, meningococcal enterobactin receptor FetA);Citation9,Citation10 and (4) a lack of antigen expression in a significant subset of invasive isolates (Neisserial Adhesin A).Citation11 The development of a multi-antigen vaccine for the prevention of MnB IMD has been described by Giuliani and colleagues.Citation12 In this review, we outline the steps taken for the development of a vaccine candidate that targets a single protein on the meningococcal surface. The vaccine candidate contains two recombinantly expressed factor H binding protein variants and has been shown to elicit broad serum bactericidal activity against diverse MnB clinical isolates.
The Discovery of fHBP
The broadly protective vaccine potential of factor H binding protein (fHBP) was discovered using a combined biochemical and immunological screening approach. The approach was developed to identify MnB surface expressed proteins with broad serogroup B distribution and sufficient amino acid sequence conservation to induce PorA independent hSBA responses against both endemic and epidemic strains. MnB strains were fractionated and the resulting outer membrane protein preparations were differentially solubilized with detergents and separated based on pI and surface charge into protein fractions which were used to immunize mice. The resulting immune sera were assessed in hSBA to identify fractions able to elicit robust bactericidal activity against diverse invasive MnB clinical isolates. The process was repeated until the most active fractions contained only a few proteins. The amino acid sequence of the proteins in the active fractions was determined and the corresponding genes were cloned, expressed in E. coli and purified. Immune sera raised to the recombinant proteins in preclinical species were tested to confirm that these gene products were able to elicit bactericidal activity in hSBA.Citation13 This screening approach, which relied heavily on the ability of the vaccine antigen to elicit broad serum bactericidal activity against diverse MnB strains, resulted in the identification of a single outer membrane lipoprotein, fHBP (also known as Lipoprotein 2086, or LP2086), that had all the prerequisite characteristics of a vaccine antigen as described above.
Human factor H is a negative regulator of the alternative complement pathway and the binding of factor H by fHBP expressed on the cell surface enables the bacteria to avoid attack by the complement system.Citation14 Consistent with a role for fHBP in meningococcal virulence, normal human serum has bactericidal activity against a MnB fHBP knock out strain while an isogenic wild type strain is much less susceptible.Citation15 In addition, the level of fHBP surface expression is inversely correlated with susceptibility to bacterial lysis by normal human serum or whole blood.Citation16 It is conceivable that a vaccine candidate based on fHBP induces protective antibodies mediating complement dependent bactericidal killing but possibly also may indirectly prevent factor H binding to the bacteria thereby limiting bacterial survival in vivo.Citation17,Citation18
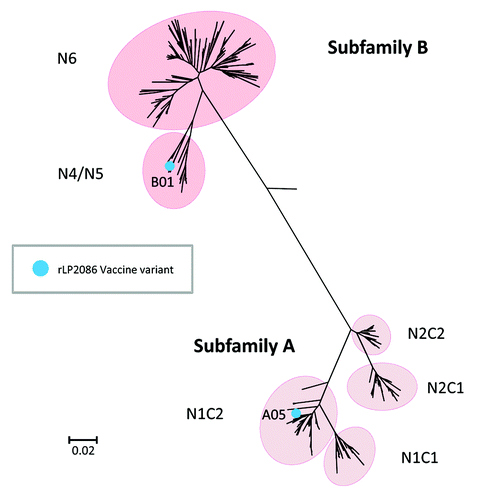
Extensive molecular epidemiology of MnB clinical isolates demonstrated that meningococcal fHBP gene sequences segregate into two subfamilies, designated A and B.Citation13 The fHBP sequence diversity across the two subfamilies was an important contributor to the design of the recombinant LP2086 (rLP2086) vaccine. The lipoproteins of these two subfamilies are immunologically distinct.Citation13,Citation19 Protein variants within subfamilies share ≥83% amino acid sequence identity, but only 60–75% identity between subfamilies ().Citation20 To obtain broad bactericidal immune responses against diverse MnB disease strains, a fHBP based vaccine containing one fHBP from each of the two fHBP subfamilies was required.
Figure 1. Phylogenetic relationship of meningococcal serogroup B fHBP variants. Figure adapted from Murphy et al.Citation20 Phylogenetic tree of fHBP/LP2086 variants based on clustalW alignment and drawn with MEGA 4. Highlighted are subfamily A and B, the subgroups within the respective subfamilies, and the components of the bivalent rLP2086 investigational vaccine, fHBP variants A05 and B01. During the development of bivalent rLP2086, alternative nomenclature systems were used for fHBP in the scientific literature.Citation21,Citation38 The scale bar indicates phylogenetic distance based on protein sequence. The phylogenetic distance between any two variants is the length of the line traced from one branch back to the first common node on the tree and along the branch to the second variant. The end of each branch on the tree represents a specific fHBP variant. The longer the line connecting the two variants, the greater is the amino acid sequence diversity between them.
Since fHBP is a lipoprotein, pre-clinical studies were performed to determine whether the lipidated form of the protein was needed for broad bactericidal activity against MnB invasive disease strains.Citation13 Lipidated and non-lipidated forms of fHBP variants were expressed as recombinant proteins and used to immunize mice.Citation13 In all cases, the lipidated protein antigens induced at least 10-fold higher hSBA titers compared with the non-lipidated forms. In addition to differences in relative potency, immune sera following vaccination with lipidated antigens had bactericidal activity against MnB isolates that expressed fHBP variants that were different from the vaccine antigen. One example, using fHBP variant B01 as antigen in mice and a hSBA strain expressing the heterologous fHBP variant B24, is provided in . The lipidated antigen rLP2086-B01 elicited a 10-fold higher hSBA titer compared with the non-lipidated rP2086-B01 against this heterologous hSBA strain. Similarly, vaccination of rhesus macaques with rLP2086-A05 elicited more robust hSBA responses than non-lipidated rP2086-A05. This was demonstrated using MnB strains that expressed fHBP variant A05, or the heterologous fHBP subfamily A variant A22 (). Based on these data, lipidated forms of fHBP were selected for further development of the final vaccine candidate.
Table 1. Immunization of mice with rLP2086-B01 elicits greater bactericidal activity than rP2086-B01 using a MnB SBA test strain that expresses heterologous (to vaccine antigen) fHBP variant B24
Table 2. Immunization of rhesus macaques with rLP2086-A05 elicits greater hSBA responses than rP2086-A05 using MnB strains that express homologous or heterologous (to vaccine antigen) subfamily B fHBP variants
Consistent with the sequence diversity between subfamily A and B, the breadth of bactericidal activity following immunization with lipidated fHBP antigens was mostly subfamily restricted.Citation13,Citation19 Immunization with lipidated fHBP recombinant antigen from subfamily A elicited high serum bactericidal antibody activity as measured in hSBA against MnB invasive isolates that expressed subfamily A protein variants, while showing much reduced or very low activity against isolates expressing subfamily B variants. Similarly monovalent lipidated fHBP antigen from subfamily B elicited high serum bactericidal antibody activity that was mostly restricted to MnB isolates that express subfamily B protein variants. In preclinical studies, immunization with a bivalent vaccine formulation composed of a lipidated fHBP antigen from both subfamily A and B was able to produce sera with broad spectrum bactericidal activity against most MnB strains tested.Citation19
These preclinical immunogenicity observations together with preliminary epidemiological data supported the development of a vaccine candidate that contains two fully lipidated fHBP proteins, one from each of the two fHBP subfamilies, to assure protection against a broad spectrum of invasive MnB isolates from diverse geographical locations. Therefore, a bivalent vaccine composed of recombinantly expressed lipidated fHBP variants A05 and B01 (bivalent rLP2086) moved forward to clinical development ().
Surveillance of Invasive MnB Isolates and the Epidemiology of fHBP
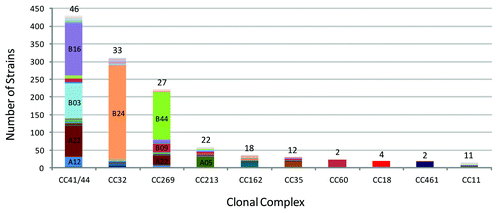
The discovery of the potential for fHBP to be used as a vaccine antigen to confer broad protection against MnB invasive disease isolates required a comprehensive understanding of the epidemiology of MnB isolates. In regards to fHBP gene presence, expression and variant distribution to determine the strategy for identifying MnB isolates for clinical hSBA testing in support of bivalent rLP2086. To support this, the fHBP epidemiology was determined for a collection of invasive MnB disease isolates obtained from the US, UK countries (England, Wales, and Northern Ireland), Norway, Czech Republic, and France was assembled to support this goal. A total of 1263 systematically collected isolates that represent approximately 13% of MnB disease isolates from these countries between 2001 and 2006 (2000 and 2005 from the US) were included, and the resulting collection was called the MnB SBA strain pool (). In addition to characterization of fHBP, the strains were evaluated for other common epidemiological markers such as MLST and PorA subtype. Sequence analysis of the 1263 MnB invasive disease isolates in the MnB SBA strain pool demonstrated that the fHBP gene was present in all of the strains, and that intact sequences capable of coding for full-length proteins were found in all but one isolate. The analysis also revealed that fHBP subfamilies could be further divided into subgroups ().Citation20,Citation21 Subfamily B fHBP variants were more common than subfamily A (), both in the total MnB SBA strain pool (71% compared to 29%) and in each of the component countries, including the US (65% compared to 35%). The subfamily distribution did change when fHBP variants were analyzed as a function of subject age. An increase in disease caused by strains expressing subfamily A variants was observed in the infant and the elderly population ()Citation22, which is consistent with findings from other regions.Citation23 Likewise MnB carriage strains also predominantly harbor fHBP subfamily A variants.Citation24 Despite the identification of 143 unique fHBP variants within the MnB SBA strain pool, a subset of ten fHBP variants is expressed in nearly 80% of all strains (). With one exception, there was little correlation observed between fHBP variants and other common epidemiological genetic markers (i.e., MLST, Clonal Complex, PorA subtype). The single exception noted was that the majority of strains expressing fHBP variant B24 were typed as CC32 ().
Table 3. The composition and fHBP subfamily distribution of the MnB SBA strain pool (2000–2006)
Figure 2. Distribution of fHBP subfamily A or b expressing strains stratified by patient age in the MnB SBA strain pool. Figure adapted from Hoiseth et al.Citation22 The distribution of fHBP subfamily A and subfamily B expressing strains within different age groups in the MnB SBA strain pool (age data available for 1215 subjects). Age groups with significant differences (*P < 0.0005) and with highly significant differences (**P < 0.0001) in subfamily distribution compared with the <1 y age group are marked (Fisher Exact Test).
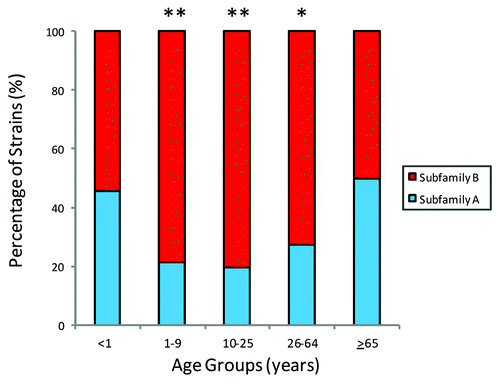
Figure 3. Prevalence of fHBP subfamily A and B variants in the MnB SBA strain pool and the US subset of the MnB SBA strain pool. Data taken from Murphy et al.Citation20 The ten most prevalent fHBP variants (each with ≥20 representative strains) and the cumulative percentage of strains in the MnB SBA strain pool (n = 1263) are pictured. The prevalence of these ten fHBP variants in the US subset of the MnB SBA strain pool (n = 432) and the non-US subset of the MnB SBA strain pool (n = 831) are shown.
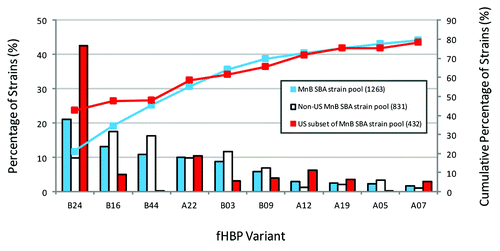
Figure 4. The fHBP variant distribution within the major clonal complexes of the MnB SBA strain pool. Data taken from Murphy et al.Citation20 The relative abundance and fHBP variant distribution within the ten most prevalent CCs in the MnB SBA Strain Pool (n = 1263) is shown. Each color in the respective columns corresponds to an individual fHBP variant and the prominent variants are labeled. Above each of the columns is the number of different fHBP variants that were found associated with the respective CCs.
Assessment of the Potential Breadth of Coverage of Bivalent rLP2086
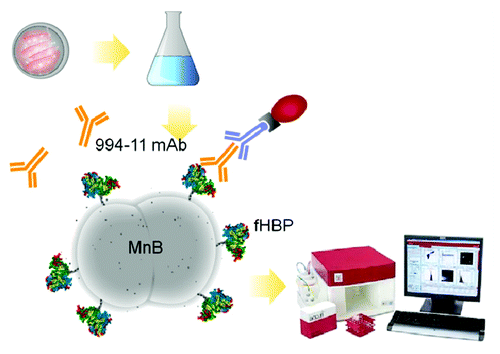
hSBA responses have been correlated with protection from meningococcal disease, and therefore hSBA is the accepted surrogate serological assay for MnB vaccine efficacy.Citation25 To appropriately evaluate the breadth of coverage elicited by vaccination with rLP2086, Pfizer developed robust hSBAs to measure the ability of rLP2086 immune serum samples from nonclinical and clinical studies to kill MnB isolates expressing fHBP variants that are different (heterologous variants) from the vaccine antigen variants (homologous variants). Initial studies evaluated the ability of MnB isolates to be killed in the hSBA using pooled bivalent rLP2086 immune serum.Citation19 Isolates (n = 100) were selected that represented the breadth of fHBP diversity in addition to having diverse geographical and genetic backgrounds. Over 75% of strains were killed in the hSBAs. To explore why a small percentage of strains were resistant in the hSBAs, a large number of genetic factors were analyzed, including fHBP subgroup, fHBP variant and MLST. No correlation was identified between strain susceptibility and any of the markers studied. The hSBA measures antibody mediated, complement dependent bactericidal activity. It is dependent upon the formation of a membrane attack complex (MAC).Citation26 To initiate the complement cascade and MAC formation, complement factor C1q must bind to antibodies bound specifically to fHBP. Activation of the complement cascade is triggered by cross-linking between C1q and multiple antibodies bound to the bacterial surface. Such cross-linking can only occur when the fHBP protein is expressed in sufficient quantities on the surface of bacteria. Therefore, fHBP surface expression of MnB strains grown under hSBA conditions was examined as a variable that could impact susceptibility to hSBA killing. To determine relative surface expression/density of fHBP among MnB invasive disease strains, a flow cytometry assay was developed using a monoclonal antibody that recognized an epitope common to fHBP variants from both subfamily A and B ().Citation27 As only antibody-accessible cell surface antigen (not total cell associated antigen) is relevant to elicit the bactericidal response, the assay was designed to selectively quantify levels of surface expressed fHBP. The distribution of expression levels was independent of fHBP subfamily or subgroup, but relative differences in expression levels from strains expressing one variant compared with another variant were notable. A similar distribution was also observed when comparing the US subset of strains (n = 432 strains) to the entire MnB SBA strain pool (n = 1263 strains).
Figure 5. Overview of the MEASURE assay. Bacteria are grown following standard hSBA procedures. Briefly, MnB bacteria are streaked onto plates and grown overnight before inoculating liquid media with colonies and growing to a specific OD. Bacteria are then stained using a 3-step antibody staining method. Bacteria were stained with the primary anti-LP2086 antibody, MN86–994–11–1, followed by a biotinylated mIgG secondary antibody and tertiary PE-Streptavidin to amplify the fluorescence signal and increase the dynamic range.
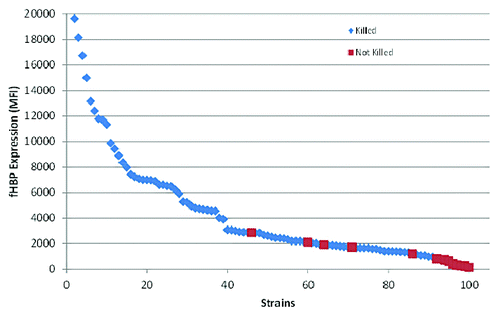
Comparative analysis of in vitro fHBP expression levels and hSBA susceptibility to bivalent rLP2086 immune serum using the 100 isolates tested revealed that there was a threshold level of fHBP expression above which MnB isolates were predictably killed with pooled rabbit sera (). While approximately 38% of strains with expression levels below the threshold level were susceptible to killing in the hSBA, no prediction could be made as to which of these strains with lower expression levels would be susceptible. Since fHBP surface expression level is an important factor in hSBA susceptibility, surface expression was included as a criterion in the unbiased selection of hSBA strains selected for clinical testing.
Figure 6. fHBP surface expression correlates with strain susceptibility in the SBA. Data adapted from Jiang et al.Citation19 Median fluorescence intensity (MFI) was determined using the validated MEASURE assay (McNeil et al., manuscript in preparation). Invasive MnB strains (n = 100) were tested using the Measure assay and the MFI was plotted. Each strain was also run in the hSBA assay. Red squares represent MnB strains that were not killed in the hSBA and blue triangles represent MnB strains that were killed.
While a surface expression level threshold can predict susceptibility of a MnB isolate in hSBA, it cannot predict the percentage of human subjects that will mount protective antibody responses to a vaccine as measured in hSBA and it is the hSBA response rate that correlates with protection.Citation5,Citation25 To determine response rate and assessment of the potential breadth of vaccine coverage of the investigational bivalent rLP2086 vaccine, serum samples from individual human subjects need to be taken prior to and following immunization with bivalent rLP2086 and evaluated in the hSBA. Therefore great care was needed to identify the MnB strains that would be used for clinical testing to confirm that the vaccine can confer broad protection against circulating MnB strains.
The Strategy for Identifying MnB Strains for Clinical Testing
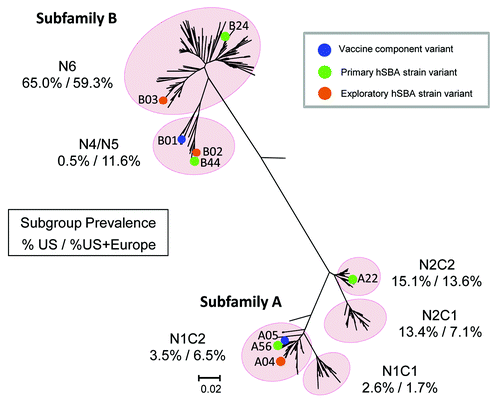
While clinically devastating, the incidence and sporadic occurrence of IMD do not permit the conduct of phase 3 studies based on clinical disease endpoints, and therefore hSBA responses have to be used as a surrogate of efficacy. Nonclinical and exploratory clinical studies established that bivalent rLP2086 elicits a dose-dependent immune response that is capable of killing MnB isolates in hSBA irrespective of fHBP variant sequence or any other epidemiological marker studied.Citation19,Citation28 Considering the diversity of potential MnB strains and the practical limitations for the number of strains that could be tested in hSBA, an unbiased approach was used to select MnB hSBA test strains that represented either fHBP subfamily A or B expressing strains from the MnB SBA strain pool.Citation20 Differences in fHBP surface expression were taken into consideration by randomly selecting strains with low to medium rather than high surface expression levels to reflect more closely the normal distribution of fHBP expression among disease isolates. Furthermore, low baseline positivity rate was an important consideration for test strain identification for use in hSBA, as the populations at risk for meningococcal disease are characterized by non-existing or low baseline bactericidal activity to most strains. In addition, to demonstrate that vaccine induced responses provide broad coverage against IMD strains, the hSBA test strains had to express fHBP variants different (heterologous) from the vaccine antigens and strains that reflected the fHBP diversity of meningococcal disease isolates. Based on these considerations, the 4 primary hSBA test strains selected were PMB2948 (fHBP variant B24), PMB2707 (fHBP variant B44), PMB80 (fHBP variant A22), and PMB2001 (fHBP variant A56).Citation29 These four primary hSBA test strains include representatives of both subfamily A and B and four of the six major fHBP subgroups, representing at least 84% of strains circulating in the US (). These strains were used to estimate vaccine efficacy using hSBA immunogenicity endpoints.
Figure 7. fHBP Phlyogenetic Tree Illustrating MnB hSBA Strain and Vaccine Component Variants. Phylogenetic tree of fHBP/LP2086 variants in the MnB SBA strain pool based on clustalW alignment, and drawn with MEGA 4. Highlighted are the fHBP variant components of bivalent rLP2086 (A05 and B01), the fHBP variants expressed by the primary hSBA strains, and the fHBP variants expressed by the exploratory hSBA strains listed in . The numbers beneath each fHBP subgroup in the figure represent the percentage of isolates in the US subset of the MnB SBA strain pool (on the left) or the MnB SBA strain pool (on the right) that reside in each subgroup. The scale bar indicates phylogenetic distance based on protein sequence.
The Clinical Performance of Bivalent rLP2086
The clinical development of bivalent rLP2086 began in 2006 with 3 early Phase 1 studies in subjects aged 18 to 25 y,Citation30 8 to 14 y,Citation31 and 18 mo to 36 mo.Citation32 These studies were conducted using an initial formulation of bivalent rLP2086, at dose levels of 20 µg, 60 µg, or 200 µg administered on a 0, 1, 6-mo schedule. Overall, bivalent rLP2086 was shown to be well tolerated and immunogenic in these Phase 1 trials. Subsequently, the drug substance manufacturing process and the drug product formulation were enhanced to increase scalability of manufacture and to ensure long-term stability of the vaccine. The resulting final formulation was used for all subsequent clinical studies including a Phase 1 Study (B1971004) and a Phase 1/2 Study (B1971003) conducted in subjects 18 to 40 y of age, where the final selected dose level of 120 µg (60 µg of rLP2086 variant A05 and 60 µg of rLP2086 variant B01) was confirmed.Citation33,Citation34 The potential for bivalent rLP2086 to generate broadly bactericidal antibodies was demonstrated in the Phase 2 proof-of-concept and dose ranging study (B1971005) conducted in subjects 11 to 18 y who received vaccine administered on a 0, 2, 6-mo schedule ().Citation30 Additional Phase 2 and 3 studies with the 120 µg dose level were then initiated to collect safety, immunogenicity, lot consistency and concomitant vaccine use data (). The functional immunogenicity for most of these studies was assessed using the four primary MnB strains that were randomly selected to demonstrate breadth of coverage. Completed phase 1 and 2 studies have demonstrated a robust hSBA response to MnB test strains expressing either subfamily A or B fHBP variants after 2 or 3 vaccine administrations of rLP2086 at a dose level of 120 μg.Citation30 Across the phase 1 and phase 2 studies, bivalent rLP2086 showed consistent and robust immune responses to a panel of MnB test strains expressing fHBP variants that are heterologous to the vaccine, including the four primary MnB test strains that are currently being used to evaluate the immune responses to bivalent rLP2086 in phase 3 clinical studies.
Table 4. The bivalent rLP2086 vaccine induces a broad immune response in adolescents against MnB isolates that express diverse fHBP variants
Table 5. Phase 1, 2, and 3 studies using the final formulation of bivalent rLP2086
Conclusion
A new bivalent vaccine for protection against MnB disease has been designed based on the discovery of a vaccine antigen (fHBP) that meets all the prerequisites of a preferred vaccine antigen. Specifically, fHBP is: (1) present in the vast majority of global clinical disease isolates; (2) surface exposed; (3) an important meningococcal virulence factor; and (4) able to elicit a bactericidal protective immune response against the majority of diverse invasive disease isolates globally. Bivalent rLP2086 hSBA responses which represent and are being used as surrogates of efficacy are robust and have been demonstrated to kill MnB strains that are representative of endemic and epidemic disease causing isolates.
Taken together, the non-clinical and clinical studies with bivalent rLP2086 using hSBA as the critical immunological/efficacy assessment tool, demonstrated the potential for this vaccine to broadly protect against MnB disease. The potential of this vaccine to meet the unmet medical need for prevention of MnB invasive disease has been recognized by having received breakthrough therapy designation from the FDA, with the possibility of accelerated regulatory approval.Citation35,Citation36 Introduction of this vaccine will make it possible to answer additional questions that were not the subject of this review including whether the vaccine has the potential to protect against other meningococcal serogroups that also express fHBPCitation37 and whether the vaccine can impact meningococcal carriage in the nasopharynx. By targeting adolescents and young adults for vaccination, not only does bivalent rLP2086 have the potential to prevent MnB invasive disease in this susceptible population, but it may also limit transmission of MnB in other age populations. Thus, demonstration of prevention of carriage will have the potential to further reduce the burden of disease, especially in the very young infant populations through herd protection.Citation37
| Abbreviations: | ||
| hSBA | = | serum bactericidal assay with human complement |
| OMVs | = | outer membrane vesicles |
| MnB | = | serogroup B Neisseria meningitidis |
| IMD | = | invasive meningococcal disease |
| PorA | = | Porin A |
| fHBP | = | factor H binding protein |
| LP2086 | = | lipoprotein 2086 |
| bivalent rLP2086 | = | bivalent recombinant LP2086 vaccine |
| MLST | = | multi-locus sequence typing |
| CC | = | clonal complex |
| MAC | = | membrane attack complex |
| MFI | = | median fluorescence intensity |
Disclosure of Potential Conflicts of Interest
All authors are employed by Pfizer and as such may own Pfizer stock.
Acknowledgements
The authors would like to thank Julia Li, Han-Qing Jiang, Kathryn Mason, Lynn Miller, Christine Stokes, Jennifer Obregon, and Deanne Illenberger for technical support; all are employees of Pfizer. The authors also thank Michael Pride, Shite Sebastian, Robert Donald, also employees of Pfizer for their insightful comments associated with this work. This work was funded by Pfizer.
References
- Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983; 2:355 - 7; http://dx.doi.org/10.1016/S0140-6736(83)90340-9; PMID: 6135869
- Milagres LG, Ramos SR, Sacchi CT, Melles CE, Vieira VS, Sato H, Brito GS, Moraes JC, Frasch CE. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun 1994; 62:4419 - 24; PMID: 7927704
- Wong SH, Lennon DR, Jackson CM, Stewart JM, Reid S, Ypma E, O’Hallahan JM, Oster P, Mulholland K, Martin DR. Immunogenicity and tolerability in infants of a New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine. Pediatr Infect Dis J 2009; 28:385 - 90; http://dx.doi.org/10.1097/INF.0b013e318195205e; PMID: 19384263
- Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991; 338:1093 - 6; http://dx.doi.org/10.1016/0140-6736(91)91961-S; PMID: 1682541
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine 2005; 23:2222 - 7; http://dx.doi.org/10.1016/j.vaccine.2005.01.051; PMID: 15755600
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A 2010; 107:19490 - 5; http://dx.doi.org/10.1073/pnas.1013758107; PMID: 20962280
- Danve B, Guinet F, Boutry E, Speck D, Cadoz M, Nassif X, et al. Safety and immunogenicity of a Neisseria meningitidis group B transferrin binding protein vaccine in adults. In: Nassif MJQM, Taha MK, ed. Eleventh International Pathogenic Neisseria Conference. Paris, France, 1998; p53.
- Moe GR, Tan S, Granoff DM. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun 1999; 67:5664 - 75; PMID: 10531214
- Sacchi CT, Whitney AM, Popovic T, Beall DS, Reeves MW, Plikaytis BD, Rosenstein NE, Perkins BA, Tondella ML, Mayer LW. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J Infect Dis 2000; 182:1169 - 76; http://dx.doi.org/10.1086/315833; PMID: 10979914
- Urwin R, Russell JE, Thompson EA, Holmes EC, Feavers IM, Maiden MC. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect Immun 2004; 72:5955 - 62; http://dx.doi.org/10.1128/IAI.72.10.5955-5962.2004; PMID: 15385499
- Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, Schmink S, Muzzi A, Bambini S, Rappuoli R, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine 2011; 29:4739 - 44; http://dx.doi.org/10.1016/j.vaccine.2011.04.092; PMID: 21571026
- Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A 2006; 103:10834 - 9; http://dx.doi.org/10.1073/pnas.0603940103; PMID: 16825336
- Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088 - 100; http://dx.doi.org/10.1128/IAI.72.4.2088-2100.2004; PMID: 15039331
- Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 2006; 177:501 - 10; http://dx.doi.org/10.4049/jimmunol.177.1.501; PMID: 16785547
- Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis 2008; 197:1053 - 61; http://dx.doi.org/10.1086/528994; PMID: 18419542
- Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, Aricò B, Rappuoli R, Pizza M. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun 2009; 77:292 - 9; http://dx.doi.org/10.1128/IAI.01071-08; PMID: 18852235
- Konar M, Granoff DM, Beernink PT. Importance of inhibition of binding of complement factor H for serum bactericidal antibody responses to meningococcal factor H-binding protein vaccines. J Infect Dis 2013; 208:627 - 36; http://dx.doi.org/10.1093/infdis/jit239; PMID: 23715659
- Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 2011; 79:3751 - 9; http://dx.doi.org/10.1128/IAI.05182-11; PMID: 21708990
- Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086 - 93; http://dx.doi.org/10.1016/j.vaccine.2010.06.083; PMID: 20619376
- Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200:379 - 89; http://dx.doi.org/10.1086/600141; PMID: 19534597
- Beernink PT, Granoff DM. The modular architecture of meningococcal factor H-binding protein. Microbiology 2009; 155:2873 - 83; http://dx.doi.org/10.1099/mic.0.029876-0; PMID: 19574307
- Hoiseth SK, Murphy E, Andrew L, Vogel U, Frosch M, Hellenbrand W, Abad R, Vazquez JA, Borrow R, Findlow J, et al. A multi-country evaluation of Neisseria meningitidis serogroup B factor H-binding proteins and implications for vaccine coverage in different age groups. Pediatr Infect Dis J 2013; 32:1096 - 101; http://dx.doi.org/10.1097/INF.0b013e31829aa63b; PMID: 23694830
- Bettinger JA, Scheifele DW, Halperin SA, Vaudry W, Findlow J, Borrow R, Medini D, Tsang R, members of the Canadian Immunization Monitoring Program, Active (IMPACT). Diversity of Canadian meningococcal serogroup B isolates and estimated coverage by an investigational meningococcal serogroup B vaccine (4CMenB). Vaccine 2013; 32:124 - 30; http://dx.doi.org/10.1016/j.vaccine.2013.03.063; PMID: 23588089
- Ala’aldeen DA, Flint M, Oldfield NJ, Omer SA, McNeil LK, Jiang Q, Murphy E, Giardina PC, Novikova EG, Dodge-Scully IL, et al. Human antibody responses to the meningococcal factor H binding protein (LP2086) during invasive disease, colonization and carriage. Vaccine 2010; 28:7667 - 75; http://dx.doi.org/10.1016/j.vaccine.2010.09.038; PMID: 20875489
- Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 2009; 27:Suppl 2 B112 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.04.065; PMID: 19464093
- Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344:1058 - 66; PMID: 11287977
- McNeil LK, Murphy E, Zhao XJ, Guttmann S, Harris SL, Scott AA, Tan C, Mack M, DaSilva I, Alexander K, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine 2009; 27:3417 - 21; http://dx.doi.org/10.1016/j.vaccine.2009.01.075; PMID: 19200847
- Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, Jones TR, Arora A. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 2012; 30:6163 - 74; http://dx.doi.org/10.1016/j.vaccine.2012.07.065; PMID: 22871351
- Vesikari T, Diez-Domingo J, Ostergaard L, Beeslaar J, Eiden J, Jiang Q, et al. Safety and immunogenicity of an investigational meningococcal serogroup B bivalent rLP2086 vaccine in healthy adolescents. 32nd Annual ESPID Meeting. Dublin, Ireland, 2014.
- Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garcés-Sánchez M, Martinón-Torres F, Beeslaar J, Szenborn L, Wysocki J, et al, 2001 Study Investigators. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12:597 - 607; http://dx.doi.org/10.1016/S1473-3099(12)70087-7; PMID: 22569484
- Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr Infect Dis J 2013; 32:364 - 71; http://dx.doi.org/10.1097/INF.0b013e31827b0d24; PMID: 23114369
- Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, et al. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18-36 months: a phase 1 randomized-controlled clinical trial. Pediatr Infect Dis J 2012; 31:1061 - 8; PMID: 22718089
- Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, Anderson AS, Jones TR, Harris SL, Jansen KU, et al. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine 2013; 31:1569 - 75; http://dx.doi.org/10.1016/j.vaccine.2013.01.021; PMID: 23352429
- Sheldon EA, Schwartz H, Jiang Q, Giardina PC, Perez JL. A phase 1, randomized, open-label, active-controlled trial to assess the safety of a meningococcal serogroup B bivalent rLP2086 vaccine in healthy adults. Hum Vaccin Immunother 2012; 8:888 - 95; http://dx.doi.org/10.4161/hv.19983; PMID: 22832260
- Pfizer. Press release anouncing the designation of breakthrough therapy by the FDA to bivalent rLP2086, 2014
- Pfizer. Press release anouncing the completion of the submission of the biological license application for the licensure of bivalent rLP2086, 2014
- Bröker M. Indirect effects by meningococcal vaccines: herd protection versus herd immunity. Hum Vaccin 2011; 7:881 - 2; http://dx.doi.org/10.4161/hv.7.8.16273; PMID: 21785283
- Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, et al. Vaccination against NeisZseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 2003; 197:789 - 99; http://dx.doi.org/10.1084/jem.20021911; PMID: 12642606
